From Magic Mountain to Table Mountain
DOI: https://doi.org/10.4414/smw.2012.13665
Andreas
Diacon, Florian
von Groote-Bidlingmaier, Peter R.
Donald
Summary
Prior to the introduction of chemotherapy, tuberculosis management relied upon aerotherapy, heliotherapy and good nutrition. This “treatment”, exemplified by the regimen applied in Swiss and other European mountain resorts, is described by Thomas Mann in the book “The Magic Mountain”. Tuberculosis chemotherapy began in 1944 with the introduction of streptomycin and para-amino-salicylic acid, later augmented by isoniazid. Early experience taught physicians that treatment must be given with multiple drugs to prevent emergence of resistance and that prolonged treatment adherence for 18–24 months was needed for a permanent cure of tuberculosis. Between 1970 and 1980 rifampicin was introduced and with pyrazinamide it made “short-course” treatment possible. For 30 years, a 6-month directly observed treatment short-course (DOTS) based on the three compounds isoniazid, rifampicin and pyrazinamide was the foundation of tuberculosis control strategies world-wide, and in recent years this was supplemented with ethambutol in view of increasing rates of isoniazid resistance. However, even 6 months of treatment is too long to easily ensure the compliance necessary to permanently cure tuberculosis. The recent spread of the HIV/AIDS epidemic has placed tuberculosis programmes under severe pressure and is accompanied by an increase in drug-resistance making tuberculosis virtually untreatable in some instances. In 2004 the first of a new generation of anti-tuberculosis drugs entered clinical evaluation. A series of clinical trials, often conducted at sites in Cape Town, South Africa, has shown them to be efficacious and hold promise of being able to shorten tuberculosis treatment and treat drug-resistant tuberculosis. Development and assessment of these drugs is ongoing but there is renewed hope that these new drugs and regimens will assist in finally conquering tuberculosis, preventing a return to Magic Mountain where sunshine and fresh air was all that could be offered to patients.
It is now approaching 70 years since the introduction of chemotherapy for the treatment of tuberculosis. However, tuberculosis still remains to be one of the world’s most important health problems. At least 6 months of treatment are required to treat most forms of tuberculosis and the spread of drug resistance threatens our ability to successfully manage this devastating disease. Within the last 5 years a number of new drugs have been discovered and some have already entered clinical evaluation. This paper reviews the history of anti-tuberculosis chemotherapy and draws lessons from man’s experience in treating and managing this formidable disease (fig. 1).
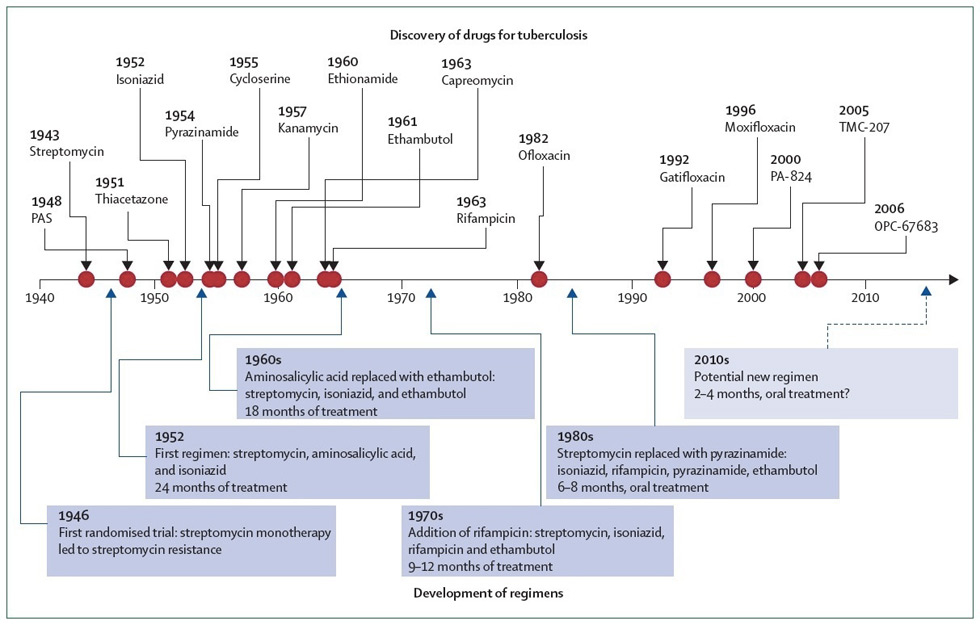
Figure 1
History of drug discovery and development of treatment regimens for tuberculosis. Compounds that are in the early-stage of development, but for which there are no published human proof-of-concept data, are not shown. Arrow with dashed line represents a possible future regimen. Red dots represent when the drugs were first reported. (From [51]: Ma Z, Lienhardt C, McIlleron H, Nunn AJ, Wang X. Global tuberculosis drug development pipeline: the need and the reality. Lancet. 2010;375(9731):2100–9. Copyright 2012, with permission from Elsevier, Oxford, UK.)

Figure 2
Typical architecture of a tuberculosis sanatorium. All patient rooms were supplied with balconies for prescribed doses of exposure of patients to sunlight and fresh air, which was believed to be beneficial at the time (Sanatorium Schatzalp, Davos, Switzerland). (Copyright by Davos Tourismus, Switzerland [swiss-image.ch/Christof Schuerpf], with permission.)

Figure 3
Mechanisms of action of new compounds in clinical development for tuberculosis. (From [51]: Ma Z, Lienhardt C, McIlleron H, Nunn AJ, Wang X. Global tuberculosis drug development pipeline: the need and the reality. Lancet. 2010;375(9731):2100–9. Copyright 2012, with permission from Elsevier, Oxford, UK.)

Figure 4
First tuberculosis patient treated with TMC207. TMC207 was the first of a series of new compounds to enter clinical testing. The image shows the patient sitting among the study team that dispensed TMC207 in Cape Town on 28 June 2005 for the first time to a tuberculosis patient. In 2011 TMC207 became available for compassionate use for patients with highly resistant tuberculosis. (The patient has given written informed consent for publication.)

Figure 5
A The Magic Mountains. The promise for cure in the pre-chemotherapy area was a stay in a sanatorium at altitude such as the Sanatorium Schatzalp, which was set prominently atop Davos, Switzerland, in this painting by tuberculosis sufferer Ernst Ludwig Kirchner (Blick auf Davos, 1924. Courtesy of Bündner Kunstmuseum Chur, Switzerland. Reprinted with permission).
B Today, drug research carried out at the foot of Table Mountain carries the authors’ hopes for curbing the epidemic of drug resistant tuberculosis.
It must first be appreciated that, even in the absence of chemotherapy, a proportion of tuberculosis patients will recover. Thus in patients with sputum microscopy smear-positive pulmonary tuberculosis it was reported that, although approximately 50% of patients would die within two years of diagnosis, 25% would be chronically ill and 25% would recover. With less serious forms and degrees of disease the propensity to recover would be greater. Against this background it is not surprising that, before the age of the controlled clinical trial, several forms of therapy were looked upon as efficacious. Sancrysin, a form of gold therapy, was, for example, used for a number of years before being shown to be useless [1].
In the absence of chemotherapy, heliotherapy, aerotherapy, bed rest and attention to nutrition were looked upon as being able to contribute to successful recovery from tuberculosis for decades. This approach created a sanatorium industry located in mountain resorts where patients could receive carefully titrated doses of sunlight, fresh air, bed rest and exercise; the nurses’ cookbook and disciplined adherence to the timetable was as important as any prescription chart (fig. 2). The activities in such institutions were graphically described by the winner of the Nobel Prize for literature Thomas Mann in his novel “The Magic Mountain” [2]. Today it is believed that better nutrition and gradual exposure to sunlight could have contributed to restore vitamin D deficiency [3–5], which is essential for macrophage function, and bed rest and moderate exercise would improve perfusion to areas of the lung most affected by cavitary tuberculosis [6–8].
The first indication of change came when the pioneering work of Gerhard Domagk, assisted by chemists from Bayer in Germany, discovered the sulphonamides [9]. Domagk was later awarded the Nobel Prize for medicine. Some sulphonamides and their derivatives where shown in vitro and in vivo to inhibit and kill mycobacteria [10], and the use of promizole, a sulphonamide derivative, in nine children with miliary tuberculosis, usually considered fatal, led to the recovery of six [11]. The path to acceptance of anti-tuberculosis agents as a valid medical concept was a thorny one, however, as antibiotic therapy was not a well established concept at that time and prevailing medical wisdom considered tuberculosis a disease best approached by modifying the host's immune response with agents such as tuberculin. The story of the search for the cure for tuberculosis is graphically summarised in a documentary novel entitled “The Greatest Story Never Told” [12].
Two researchers working in very different circumstances were responsible for the discovery of the first two drugs that had an undisputed effect in patients with tuberculosis that was demonstrated in controlled clinical trials. Jorgen Lehman was a Danish doctor working in Gothenburg, Sweden. He already had some experience with competitive inhibition of enzyme systems when in 1940 he received from his friend Frederick Bernheim, who was working in the USA, a paper published in Science detailing how the oxygen uptake of the bacterium accelerated when salicylate was added to a culture of Mycobacterium tuberculosis[13]. Lehman immediately saw the opportunity to design a drug that would inhibit the relevant enzyme system. The resulting para-aminosalicylic acid (PAS) was synthesised with the help of the Swedish Ferosan pharmaceutical company and in October 1944 PAS was given to a patient with severe pulmonary and abdominal tuberculosis [14]. It was soon apparent that PAS was having a significant beneficial effect in patients with tuberculosis [15] and later in 1950 the first placebo-controlled clinical trial conducted by the Therapeutic Trials Committee of the Swedish National Association against Tuberculosis showed beyond any doubt that PAS was efficacious in the treatment of tuberculosis [16].
Simultaneously Selwyn Waksman, a soil scientist working at Rutgers Agricultural College in New Jersey USA was conducting research into the potential of soil microbes to produce antibiotic substances. In 1944 Arnold Schatz, a doctoral student with Waksman as supervisor, described the discovery of streptomycin (SM) and noted its activity against M. tuberculosis [17]. The anti-tuberculosis activity of SM was confirmed in both animal experiments [18] and in patients, and in 1948 the British Medical Research Council [19] conducted a controlled clinical trial in which the efficacy of SM was compared to that of hospitalisation with bed rest. Although patients receiving SM recovered more frequently than those receiving bed rest, a five-year follow-up failed to demonstrate a significant difference between the groups [20]. The main factor causing this unexpected finding was the emergence of resistance to SM, which occurred as early as one month after starting treatment. The next important step was the combination of PAS and SM and, although this did not eliminate the development of resistance, it did control it to a significant degree. At five year follow-up, 31% of SM patients had died as had 36% of the PAS patients but only 19% of the SM and PAS patients [21].
The next important step forward was the discovery of the anti-tuberculosis activity of isoniazid (INH). INH arose indirectly from the earlier discovery of the thiosemicarbazone, thioacetazone (known initially as Conteben), by Gerhard Domagk and his team of researchers [22, 23]. This drug was not highly active, but proved to be a suitable companion drug for more efficacious drugs, assisting in preventing the emergence of resistance. Due to the risk of severe Steven’s Johnson syndrome in HIV-infected patients this drug is no longer freely available today. The discovery of INH was reported in 1952 by three groups of researchers at the pharmaceutical companies of Bayer in Germany and Hoffman La Roche and Squibb in the United States of America [24, 25]. After initial scepticism INH proved to be the most efficacious drug yet discovered and miraculous responses to treatment were enthusiastically reported by the lay press and confirmed in various clinical trials. However as with all other anti-tuberculosis drugs, INH rapidly elicited resistance if given as monotherapy. Combining INH, PAS and streptomycin resulted in the best regimen yet and by 1952 this was established as the gold standard for anti-tuberculosis treatment [26].
The main uncertainty was the length of treatment required to ensure a permanent relapse-free cure. A treatment duration of 6 months for cavitary pulmonary tuberculosis was followed by relapse in 62% of cases of pulmonary tuberculosis, after 12 months of treatment 19% relapsed and 4% relapsed after 24–36 months of treatment [27]. Ultimately many clinicians settled on a compromise of 18 months treatment, but some experienced clinicians expressed the opinion that at least two years treatment or even life-long treatment was necessary [28].
Before the end of the 1950’s two critical facts had thus been identified regarding tuberculosis chemotherapy: the emergence of resistance to any drug was a serious threat in the presence of overt or covert monotherapy and secondly, a prolonged period of treatment was needed to ensure final sterilisation of all tuberculosis lesions and to prevent relapse. During this decade research into anti-tuberculosis agents continued and a number of compounds were identified. These included pyrazinamide (PZA), which was later to play an important part in short-course sterilising regimens, viomycin, cycloserine and terizidone, kanamycin from which amikacin was later developed, capreomycin and the thioamides ethionamide and prothionamide. While all these were shown to have anti-tuberculosis efficacy this was not sufficiently strong to challenge the gold standard of INH, streptomycin and PAS, or their dose was limited by the existence of toxicity.
The manner in which unanticipated toxicity can limit the action of a drug is well illustrated by ethambutol (EMB) introduced by Lederle in 1961 [29]. Early studies found good anti-tuberculosis efficacy, but optic toxicity was encountered which was shown to be dose related [30]. Consequently the dose was eroded to the point where the efficacy of EMB was severely compromised. EMB does not contribute to sterilisation of tuberculosis lesions [31] and its use with INH in a 6-month continuation phase of treatment led to worse results than a 4-month continuation phase with INH and rifampicin (RMP) [32]; the role of EMB is now limited to assisting in prevention of resistance in companion drugs.
In 1957 a new Streptomyces species, S. mediterranei (now Amycolatopsis mediterranei), was isolated by Lepetit, Milan, from a soil sample from a pine forest near St Raphaël, France [33]. This bacterium produced a number of compounds with good activity against a wide range of bacteria including M. tuberculosis which were termed “rifamycins” in analogy to the then fashionable film noir “Rififi” [34]. [28] Modification of these compounds produced an orally active agent, RMP [35]. In animal experiments the outstanding characteristic of RMP was exceptional sterilising activity [36] and moderate bactericidal activity that was dose-related [37, 38]; this sterilising activity was confirmed in a brilliant series of clinical trials [39] and RMP is now an essential element in modern short-course anti-tuberculosis chemotherapy and one of 5 drugs identified by the World Health Organization (WHO) as essential.
Possibly the most interesting of the agents developed in the 1950’s was PZA, but in early clinical trials when given at a dose of 50 mg/kg body weight in retreatment patients hepatotoxicity was encountered and further research on the drug was stopped for the time being. In vivo animal experiments, however, showed that when given together with INH sterilisation of lesions appeared to be achieved [40]. The use of PZA was re-examined by the East African and British Medical Research Councils and when given with INH and streptomycin low relapse rates were achieved, and when PZA was combined with INH and RMP the relapse rates following 6 months therapy fell even lower [39]. Confirmation of these findings was provided by further studies conducted in Singapore and Hong Kong which culminated in the acceptance of 6-month short-course chemotherapy as the new standard of treatment [41, 42]. Following the acclaimed success of this “short-course” regimen [43], which was equally effective when given to hospitalised or ambulatory patients, interest in tuberculosis drug research, and indeed research into all aspects of tuberculosis, went into decline along with the then extensive infrastructure for stationary and ambulatory tuberculosis treatment and control. Scientific interest in tuberculosis was resuscitated only in approximately 1990 under the impact of the devastating interaction of HIV and tuberculosis. The consequences of this “cursed duet” [44] caused an explosion in the incidence of tuberculosis throughout the world which has overwhelmed health services in developing countries. Rising rates of drug resistance were also a reason for grave concern. In 2008, 11.5 million people fell ill from tuberculosis, 1.9 million died and 0.44 million were resistant to INH and RMP. More than 50 countries have reported cases of extensively resistant tuberculosis that are also resistant to fluoroquinolones and aminoglycosides, and thus are very hard to cure with the remaining drugs that are relatively toxic and ineffective.
Fluoroquinolones, which were developed as antibiotics for other infections, unobtrusively entered the area of tuberculosis chemotherapy, at first as agents for the management of drug-resistant tuberculosis [45]. Later studies of early bactericidal activity showed fluoroquinolones, with the exception of ciprofloxacin, to have excellent bactericidal activity [46, 47], while animal studies hinted at equally interesting sterilising activity. A study from India found low relapse rates following the addition of ofloxacin to a standard regimen of INH, RMP and PZA given for 4 or 5 months [48]; a Cochrane review, however, concluded that the addition of fluoroquinolones to the usual standard regimens did not confer any additional benefit [49].
In 2005 a new era dawned for tuberculosis chemotherapy with the publication in Science of a report of the discovery by researchers at the pharmaceutical company Tibotec, led by Dr Koen Andries, of a new agent designated TMC207 (bedaquiline), an inhibitor of the mycobacterial ATP synthase [50]. TMC207 was the first of a series of novel compounds that have entered clinical evaluation. Other agents include the nitroimidazoles PA-824 and OPC-67683 (delamanid), the ethylenediamine SQ109 (related to ethambutol), and the oxazolidines PNU-100480 and AZD-5847 (fig. 3) [51]. During mouse studies many of these new agents appeared to have good sterilising activity implying that they may aid in shortening the treatment duration [52]. Conventional anti-tuberculosis drugs are also receiving renewed interest either for dose-optimisation (RMP, INH), evaluation of different compounds within a drug class (rifapentine, rifabutin, RMP) or as potential synergistic agents when combined with novel compounds (PZA, RMP, clofazimine).
The first clinical studies of these new agents evaluated their early bactericidal activity over the first one or two weeks of therapy and were conducted in Cape Town (fig. 4) [53–56]. Interestingly these ground breaking studies found several of the new agents to have significant anti-mycobacterial activity, but with a slow onset, as is the case with PZA. Despite this slow onset of activity further studies in patients with multidrug-resistant (MDR) tuberculosis over a longer duration have provided confirmation of the good activity of these new agents [57, 58]. There is every hope now that it may be possible to construct totally new regimens for the treatment of tuberculosis. Instead of testing each novel compound separately, the current strategy relies on data derived from a mouse model to identify the most promising drugs and combinations, and these are then tested clinically over 2-weeks in dose-ranging studies that aid the selection of the most appropriate doses and regimens [59]; these regimens can then be advanced to longer studies over 8 weeks guiding further evaluation and finally for the full duration of treatment. Testing of combinations rather than adding new drugs to old combinations, if successful, can reduce the time required for registration of a new regimen by many years. The underlying idea is to create new combination regimens that are short and safe and composed only of novel drugs to which natural resistance is virtually non-existent and that do not interfere with anti-retroviral agents. Such a “universal regimen” would be suitable to treat all conventionally drug-sensitive or drug-resistant tuberculosis patients alike, whether HIV-infected or not, and thus obviate the need for susceptibility testing altogether.
Threats to the successful control of tuberculosis in developing countries remain, perhaps the most important problem being dysfunctional health systems; the introduction of new agents or regimens into control programmes that cannot carry out directly observed treatment of tuberculosis could rapidly lead to the loss of these agents through the development of resistance. To control tuberculosis under these circumstances will require not only new drugs and regimens, but also dedication of health systems to continual training, retraining and accurate documentation of results.
Our patients with extensively resistant tuberculosis, for whom there are no effective treatment options, find themselves in a situation not dissimilar to that in the pre-antibiotic era. The idea of sanatoria has resurfaced as an aid to prevent the further spread of such virtually untreatable tuberculosis [60]. Despite the enormity of the task facing tuberculosis control services and programmes, there is now fresh hope that, with the help of new agents and renewed efforts by the partnership of researchers and health services, tuberculosis may be controlled and finally eliminated as a serious health problem. Perhaps it is appropriate that many of the most recent studies of these new anti-tuberculosis agents are being carried out at the Cape of Good Hope; perhaps Table Mountain will prove to be the Magic Mountain of tuberculosis control in the 21st century (fig. 5)?
Acknowledgments:Our thanks go the Swiss Lung League for supporting the drug research facility at Brooklyn Chest Hospital, Cape Town, South Africa.
References
1 Amberson J, McMahon B, Pinner M. A clinical trial of sanocrysin in pulmonary tuberculosis. Am Rev Tuberculosis. 1931;24:401–35.
2 Mann T. Der Zauberberg. Berlin: S Fischer Verlag; 1924.
3 McMurray DN. Impact of nutritional deficiencies on resistance to experimental pulmonary tuberculosis. Nutr Rev. 1998;56(1 Pt 2):S147–52.
4 Green M. Cod liver oil and tuberculosis. BMJ. 2011;343:d7505.
5 Chocano-Bedoya P, Ronnenberg AG. Vitamin D and tuberculosis. Nutr Rev. 2009;67(5):289–93.
6 McMurray DN. Hematogenous reseeding of the lung in low-dose, aerosol-infected guinea pigs: unique features of the host-pathogen interface in secondary tubercles. Tuberculosis (Edinb). 2003;83(1–3):131–4.
7 Murray JF. Bill Dock and the location of pulmonary tuberculosis: how bed rest might have helped consumption. Am J Respir Crit Care Med. 2003;168(9):1029–33.
8 Balasubramanian V, Wiegeshaus EH, Taylor BT, Smith DW. Pathogenesis of tuberculosis: pathway to apical localization. Tuber Lung Dis. 1994;75(3):168–78.
9 Domagk G. Ein Beitrag zur Chemotherapie der bakteriellen Infektionen. Deutsche Medizinische Wochenschrift. 1935;61:250.
10 Feldman WH, Hinshaw HC, Mann FC. Promizole in tuberculosis. Am Rev Tuberculosis. 1944;50:418–40.
11 Lincoln EM, Stone S, Hoffman OR. The treatment of miliary tuberculosis with promizole. Bull Johns Hopkins Hosp. 1948;82(1):56–75.
12 Ryan F. Tuberculosis: The greatest story never told. Bromsgrove, Worcestershire, UK: Swift Publishers; 1992.
13 Bernheim F. The effect of salicylate on the oxygen uptake of the tubercle bacillus. Science. 1940;92(2383):204.
14 Dubovsky H. Correspondence with a pioneer, Jurgen Lehmann (1898–1989), producer of the first effective antituberculosis specific. S Afr Med J. 1991;79(1):48–50.
15 Lehmann J. Twenty years afterward: Historical notes on the discovery of the antituberculosis effect of paraaminosalicylic acid (PAS) and the first clinical trials. Am Rev Respir Dis. 1994;90:953–6.
16 The Therapeutic Trials Committee of the Swedish National Association against Tuberculosis. Para-aminosalicylic acid treatment in pulmonary tuberculosis. Am Rev Tuberc. 1950;61(5):597–612.
17 Schatz A, Bugie E, Waksman SA. Streptomycin, a substance exhibiting antibiotic activity against gram-positive and gram-negative bacteria. Proc Soc Exp Biol Med. 1944;55:66–9.
18 Feldman WH, Hinshaw HC. Effects of streptomycin on experimental tuberculosis in guinea pigs: a preliminary report. Proc Staff Meet Mayo Clin. 1944;19:593–9.
19 Medical Research Council. Streptomcyin treatment of pulmonary tuberculosis. Br Med J. 1948;2(4582):769–82.
20 Fox W, Sutherland I, Daniels M. A five-year assessment of patients in a controlled trial of streptomycin in pulmonary tuberculosis; report to the Tuberculosis Chemotherapy Trials Committee of the Medical Research Council. Q J Med. 1954;23(91):347–66.
21 Fox W, Sutherland I. A five-year assessment of patients in a controlled trial of streptomycin, para-aminosalicylic acid, and streptomycin plus para-aminosalicylic acid, in pulmonary tuberculosis. Q J Med. 1956;25(98):221–43.
22 Domagk G. Die experimentellen Grundlagen einer Chemotherapie der Tuberkulose. Beitr Klin Tuberk Spezif Tuberkuloseforsch. 1948;101(4):365–94.
23 Heilmeyer L. Die Chemotherapie der Tuberkulose. Dtsch Med Wochenschr. 1949;74(6):161–7.
24 Fox HH. The chemical attack on tuberculosis. Trans N Y Acad Sci. 1953;15(7):234–42.
25 McDermott W. The story of INH. J Infect Dis. 1969;119(6):678–83.
26 Mitchison DA. The diagnosis and therapy of tuberculosis during the past 100 years. Am J Respir Crit Care Med. 2005;171(7):699–706.
27 Medical Research Council. Long-term chemotherapy in the treatment of chronic pulmonary tuberculosis with cavitation: a report to the Medical Research Council by their Tuberculosis Chemotherapy Trials Committee. Tubercle. 1962;43:201–67.
28 McDermott W. Antimicrobial therapy of pulmonary tuberculosis. Bull World Health Organ. 1960;23(4–5):427–61.
29 Shepherd RG, Wilkinson RG. Antituberculous agents II. N,N'-diisopropylethylenediamine and analogs. J Med Pharm Chem. 1962;91:823–35.
30 Carr RE, Henkind P. Ocular manifestations of ethambutol, toxic amblyopia after administration of an experimental antituberculous drug. Arch Ophthalmol. 1962;67:566–71.
31 Mitchison DA. Role of individual drugs in the chemotherapy of tuberculosis. Int J Tuberc Lung Dis. 2000;4(9):796–806.
32 Jindani A, Nunn AJ, Enarson DA. Two 8-month regimens of chemotherapy for treatment of newly diagnosed pulmonary tuberculosis: international multicentre randomised trial. Lancet. 2004;364(9441):1244–51.
33 Sensi P, Margalith P, Timbal MT. Rifomycin, a new antibiotic; preliminary report. Farmaco Sci. 1959;14(2):146–7.
34 van Ingen J, Aarnoutse RE, Donald PR, Diacon AH, Dawson R, Plemper van Balen G, et al. Why do we use 600 mg of rifampicin in tuberculosis treatment? Clin Infect Dis. 2011;52(9):e194–9.
35 Maggi N, Pasqualucci CR, Ballotta R, Sensi P. Rifampicin: a new orally active rifamycin. Chemotherapy. 1966;11(5):285–92.
36 Grumbach F, Canetti G, Le Lirzin M. Rifampicin in daily and intermittent treatment of experimental murine tuberculosis, with emphasis on late results. Tubercle. 1969;50(3):280–93.
37 Sirgel FA, Fourie PB, Donald PR, Padayatchi N, Rustomjee R, Levin J, et al. The early bactericidal activities of rifampin and rifapentine in pulmonary tuberculosis. Am J Respir Crit Care Med. 2005;172(1):128–35.
38 Verbist L, Gyselen A. Antituberculous activity of rifampin in vitro and in vivo and the concentrations attained in human blood. Am Rev Respir Dis. 1968;98(6):923–32.
39 Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946–1986, with relevant subsequent publications. Int J Tuberc Lung Dis. 1999;3(10 Suppl 2):S231–79.
40 McDermott W, McCune RM, Jr., Tompsett R. Dynamics of antituberculous chemotherapy. Am Rev Tuberc. 1956;74(2 Part 2):100–8.
41 Hong Kong Chest Service/British Medical Research Council. Five-year follow-up of a controlled trial of five 6-month regimens of chemotherapy for pulmonary tuberculosis. Hong Kong Chest Service/British Medical Research Council. Am Rev Respir Dis. 1987;136(6):1339–42.
42 Hong Kong Chest Service/British Medical Research Council. Controlled trial of 2, 4, and 6 months of pyrazinamide in 6-month, three-times-weekly regimens for smear-positive pulmonary tuberculosis, including an assessment of a combined preparation of isoniazid, rifampin, and pyrazinamide. Results at 30 months. Am Rev Respir Dis. 1991;143(4 Pt 1):700–6.
43 Iseman MD, Sbarbaro JA. Short-course chemotherapy of tuberculosis. Hail Britannia (and friends)! Am Rev Respir Dis. 1991;143(4 Pt 1):697–8.
44 Chretien J. Tuberculosis and HIV. The cursed duet. Bull Int Union Tuberc Lung Dis. 1990;65(1):25–8.
45 Tsukamura M, Nakamura E, Yoshii S, Amano H. Therapeutic effect of a new antibacterial substance ofloxacin (DL8280) on pulmonary tuberculosis. Am Rev Respir Dis. 1985;131(3):352–6.
46 Johnson JL, Hadad DJ, Boom WH, Daley CL, Peloquin CA, Eisenach KD, et al. Early and extended early bactericidal activity of levofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2006;10(6):605–12.
47 Sirgel FA, Donald PR, Odhiambo J, Githui W, Umapathy KC, Paramasivan CN, et al. A multicentre study of the early bactericidal activity of anti-tuberculosis drugs. J Antimicrob Chemother. 2000;45(6):859–70.
48 Tuberculosis Research Centre (Indian Council of Medical Research). Shortening short course chemotherapy: A randomised clinical trial for treatment of smear positive pulmonary tuberculosis with regimens using ofloxacin in the intensive phase. Ind J Tub. 2002;49:27–38.
49 Ziganshina LE, Squire SB. Fluoroquinolones for treating tuberculosis. Cochrane Database Syst Rev. 2008(1):CD004795.
50 Andries K, Verhasselt P, Guillemont J, Gohlmann HW, Neefs JM, Winkler H, et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science. 2005;307(5707):223–7.
51 Ma Z, Lienhardt C, McIlleron H, Nunn AJ, Wang X. Global tuberculosis drug development pipeline: the need and the reality. Lancet. 2010;375(9731):2100–9.
52 Tasneen R, Li SY, Peloquin CA, Taylor D, Williams KN, Andries K, et al. Sterilizing activity of novel TMC207- and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob Agents Chemother. 2011;55(12):5485–92.
53 Rustomjee R, Diacon AH, Allen J, Venter A, Reddy C, Patientia RF, et al. Early bactericidal activity and pharmacokinetics of the diarylquinoline TMC207 in treatment of pulmonary tuberculosis. Antimicrob Agents Chemother. 2008;52(8):2831–5.
54 Diacon AH, Dawson R, Hanekom M, Narunsky K, Venter A, Hittel N, et al. Early bactericidal activity of delamanid (OPC-67683) in smear-positive pulmonary tuberculosis patients. Int J Tuberc Lung Dis. 2011;15(7):949–54.
55 Diacon AH, Dawson R, Hanekom M, Narunsky K, Maritz SJ, Venter A, et al. Early bactericidal activity and pharmacokinetics of PA-824 in smear-positive tuberculosis patients. Antimicrob Agents Chemother. 2010;54(8):3402–7.
56 Diacon AH, Dawson R, du Bois J, Narunsky K, Venter A, Donald PR, et al. Phase II dose-ranging trial of the early bactericidal activity of PA-824. Antimicrob Agents Chemother. 2012;56(6):3027–31.
57 Diacon AH, Pym A, Grobusch M, Patientia R, Rustomjee R, Page-Shipp L, et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med. 2009;360(23):2397–405.
58 Gler MT, Skripconoka V, Sanchez-Garavito E, Xiao H, Cabrera-Rivero JL, Vargas-Vasquez DE, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med. 2012;366(23):2151–60.
59 Diacon AH, Dawson R, Von Groote-Bidlingmaier FT, et al. A randomized trial of the 14-day bactericidal activity of combinations of PA-824, bedaquiline, pyrazinamide and moxifloxacin. Lancet. 2012; in press.
60 Dheda K, Migliori GB. The global rise of extensively drug-resistant tuberculosis: is the time to bring back sanatoria now overdue? Lancet. 2012;379(9817):773–5.





