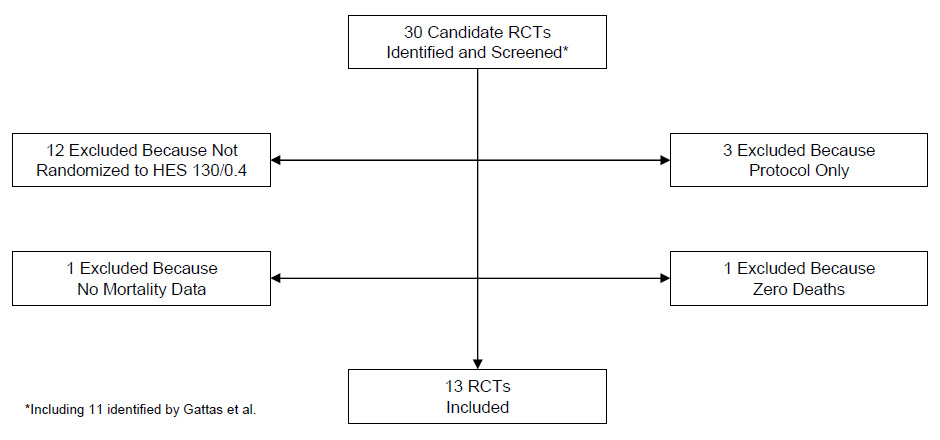
Figure 1
Process of RCT selection for the updated meta-analysis. Abbreviations: HES, hydroxyethyl starch; RCT, randomised controlled trial.
DOI: https://doi.org/10.4414/smw.2012.13656
Despite its widespread use, concerns about possible detrimental effects on kidney function were already raised by a randomised controlled trial (RCT) in 2001 [1] for hydroxyethyl starch (HES) with molecular weight of ≥200 kDa and/or degree of substitution >0.4 in the setting of severe sepsis. Recently, the retraction of numerous studies claiming excellent tolerance of lower molecular weight HES solutions (6% HES 130/0.4) also raised suspicion about possible adverse effects due to this newer group of HES [2].
Gattas and co-workers presented a meta-analysis of RCTs evaluating the effect of HES 130/0.4 on mortality in the January 2012 issue of Anesthesia & Analgesia [3]. The pooled relative risk (RR) of death was computed with its 95% confidence interval (CI) under a random effects model for 11 unretracted trials [4–14]. HES 130/0.4 showed no significant effect on mortality (RR, 0.95; CI 0.64–1.42). However, trials reported in 2011 and 2012 have not been not included in that meta-analysis whose search strategy was confined to 24 December 2010.
RCTs were sought in which HES 130/0.4 was compared with crystalloid or colloid control fluid for intravascular volume expansion in patients >18 years of age suffering acute illness or undergoing major surgery. Mortality was the endpoint of the meta-analysis, and only RCTs with available mortality data were eligible for inclusion. At least one death must have occurred so that relative mortality risk could be computed. Both published and unpublished trials were sought. Publication bias is a recognised threat to the validity of meta-analyses, and inclusion of unpublished data when available can help reduce this threat [15].
The search strategy was generally similar to that described by Gattas et al. [3]. Briefly, computer searches were performed in MEDLINE, EMBASE, the Cochrane Library, controlled-trials.com, ClinicalTrials.gov and the abstract databases of major meetings in surgery, anaesthesiology and intensive care. Search terms included: fluid therapy; volume expansion; resuscitation; rehydration; blood substitutes; colloids; hetastarch; hydroxyethyl starch; pentastarch; Voluven; tetrastarch; randomised controlled trial; and random allocation. Roots and variants of these terms were also used.
However, whereas Gattas et al. [3] included 6 retracted RCTs of Boldt et al. in their computation of RR, those trials were excluded from the present update. Retraction of the 6 RCTs and 83 other publications of Boldt et al. was based upon lack of Institutional Review Board approval [16]. Of those 6 RCTs, two in cardiac surgery [17, 18] are known to have been fabricated [2]. Other available information casts further doubt upon the reliability of any trial reported by Boldt et al. Specifically, at least one other cardiac surgery RCT by those investigators is already known to have been fabricated [2], results from 5 of their RCTs in patients with trauma or sepsis showed extreme homogeneity consistent with fraud [19] and data in 4 of their publications were found to have been manipulated [20]. Investigations into the work of Boldt et al. remain ongoing. In a sensitivity analysis, Gattas et al. [3] demonstrated that mortality results in the 6 retracted RCTs of Boldt et al. were much more favorable to HES 130/0.4 (pooled RR, 0.73) than in the other included trials of their meta-analysis (pooled RR, 0.95).
Computer searches were supplemented by examining reference lists and online contents of major surgery, anaesthesiology and intensive care journals and consulting intravenous fluid suppliers. Both investigators shared in determining the eligibility of candidate trials.
The pooled RR for mortality was computed under a random effects model. Heterogeneity was evaluated by Cochran Q test and the I2 statistic. Publication bias was assessed by rank correlation test [21]. The pooled RR was adjusted for publication bias by the trim and fill method [22]. That method has been widely used in meta-analyses, and in an empirical study utilising the FDA trial registry database as an unbiased gold standard, the adjusted effect size computed by trim and fill more closely coincided with the true value than did the unadjusted effect size [23]. The analysis was performed with Comprehensive Meta Analysis version 2.2.048 (Biostat, Inc., Englewood, NJ, USA).
The RCT selection process is outlined in (fig. 1). Two additional clinical trials were identified by this search [24, 25]: The first one was a double-blind randomised trial of 115 trauma patients at a single centre in South Africa (FIRST) [24]. Mortality data for that study [24] appeared in a separate report [26]. The second study was a double-blind randomised trial of 196 patients with severe sepsis at 21 intensive care units in France and 3 in Germany (CRYSTMAS) [25]. The hypothesis that 6% HES 130/0.4 would allow earlier enteral nutrition and require significantly less resuscitation fluid than 0.9% NaCl was refuted in this study. There was also a trend towards higher incidence of renal failure in sepsis patients. Both studies reported a trend towards higher mortality in the HES groups.

Figure 1
Process of RCT selection for the updated meta-analysis. Abbreviations: HES, hydroxyethyl starch; RCT, randomised controlled trial.
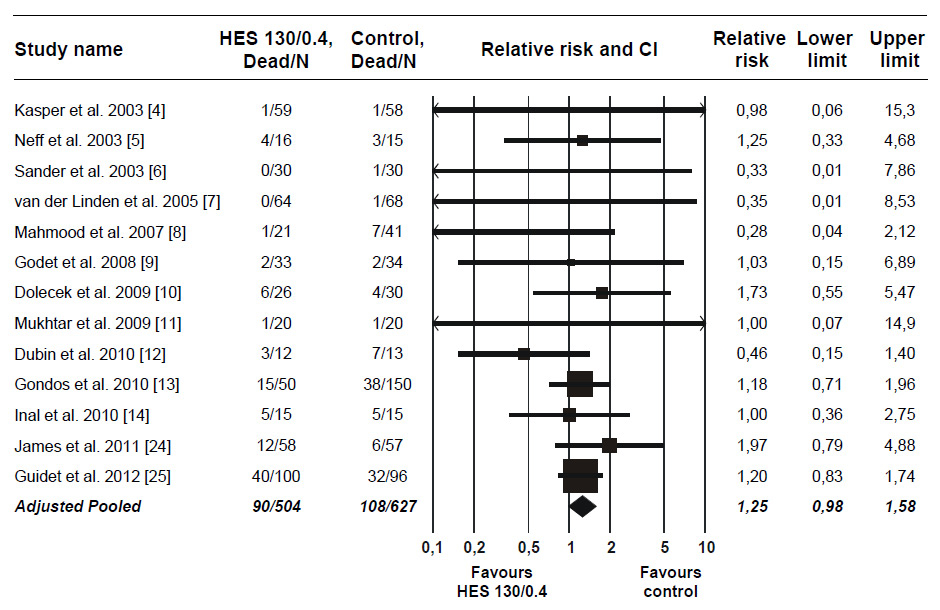
Figure 2
Relative risk of mortality after HES 130/0.4 infusion in RCTs. Pooled relative risk adjusted for publication bias. For trials with more than one control group, data for the different control groups were pooled [8, 13]. In one trial with mortality reported separately for the first 24 hours and thereafter, the data for the two time periods were pooled [12]. Error bars indicate CI. Data points scaled according to meta-analytic weight. Abbreviations: CI, 95% confidence interval; HES, hydroxyethyl starch; RCT, randomised controlled trial.
An updated meta-analysis adding those two trials [24, 25] to the 11 unretracted trials [4–14] included by Gattas et al. [3] appears in (fig. 2). More than half the total weight of evidence in the updated meta-analysis (51.3%) was contributed by the two new trials. Thus, those trials more than double the available evidence on mortality after HES 130/0.4 infusion. The pooled RR for mortality increased to 1.14 (CI, 0.89 to 1.46).
Additionally, publication bias favoring HES 130/0.4 was present (p = 0.038). Such bias typically signifies exaggeration of benefit or underestimation of harm among smaller, less reliable trials. Adjustment for the observed publication bias by the trim and fill method further increased the RR for mortality to 1.25 (CI, 0.98 to 1.58; p = 0.069). One limitation of trim and fill is poor performance in the presence of significant heterogeneity. This, however, was not an issue with the updated meta-analysis as no heterogeneity was found (I2, 0%; CI, 0% to 32%; p = 0.81).
One limitation of the meta-analysis is that many included studies were not designed to assess mortality, and the possibility of ascertainment bias cannot be dismissed. Furthermore, among the included trials different clinical indications such as surgery, sepsis and trauma were represented, and diverse crystalloids and colloids served as control fluids. Such differences are the rule rather than the exception in meta-analysis, however, and some degree of clinical heterogeneity is expected [27]. The lack of observed statistical heterogeneity suggests that the meta-analytic findings are robust and generalisable. Furthermore, by borrowing strength from multiple small trials this meta-analysis affords increased statistical power in evaluating an endpoint such as mortality with a relatively low event rate.
The quite strong indication of possible harm by use of 6% HES 130/0.4 resulting from our analysis is also reflected by a recently published report from a task force group of the European Society of Intensive Care Medicine [28]. In addition to the strong recommendation against the use of HES with molecular weight of ≥200 kDa and/or degree of substitution >0.4 (“older” HES solutions) in patients with severe sepsis or risk of acute kidney injury, they also suggested not to use 6% HES 130/0.4 (“newer” HES solutions) or gelatin in these populations. Their suggestion not to use 6% HES 130/0.4 was based on two meta-analyses and a recent systematic review that found inadequate and controversial clinical data to address the hypothesis that safety differences exist between ‘‘older’’ and ‘‘newer’’ HES solutions with different molecular weight and substitution ratio regarding renal function or blood loss [29–31]. In their consensus statement, the results of the two most recently published RCTs also identified by our search had been taken into consideration.
HES 130/0.4 has achieved very widespread clinical acceptance. In one survey, for instance, HES 130/0.4 was found to be the colloid preferred by 81% of Scandinavian intensive care units [32]. Yet, according to a systematic review, the safety of HES 130/0.4 has not thus far been adequately assessed [33]. Based on their meta-analysis, Gattas et al. [3] concluded that the poor quality and small size of available studies do not allow the benefits and risks of HES 130/0.4 to be reliably estimated. The present update suggests that concerns about the safety of HES 130/0.4 may increase with the accumulation of further evidence. Indeed, adding to the concern are supplementary data from the CRYSTMAS study [25] included in new Prescribing Information for HES 130/0.4 available from the US Food and Drug Administration ( http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/NewDrugApplicationsNDAs/UCM083138.pdf ). A trend toward increased need for renal replacement therapy was observed in patients receiving HES 130/0.4, and in a Kaplan-Meier analysis the difference nearly reached statistical significance (p = 0.064). Additionally, the mean duration of renal replacement therapy was more than twice as long in HES 130/0.4 recipients as in the control group.
The large-scale Scandinavian Starch for Severe Sepsis/Septic Shock Trial (6S) [34] and the Crystalloid versus Hydroxyethyl Starch Trial (CHEST) [35] in intensive care unit patients should help determine more precisely the effect of HES 130/0.4 on mortality. In the interim, best current evidence suggests a trend toward higher mortality among HES 130/0.4 recipients.
1 Schortgen F, Lacherade JC, Bruneel F, Cattaneo I, Hemery F, Lemaire F, et al. Effects of hydroxyethylstarch and gelatin on renal function in severe sepsis: a multicentre randomised study. Lancet. 2001;357(9260):911–6.
2 Shafer SL Shadow of doubt. Anesth Analg. 2011;112(3):498–500.
3 Gattas DJ, Dan A, Myburgh J, Billot L, Lo S, Finfer S. Fluid resuscitation with 6% hydroxyethyl starch (130/0.4) in acutely ill patients: an updated systematic review and meta-analysis. Anesth Analg. 2012;114(1):159–69.
4 Kasper SM, Meinert P, Kampe S, Görg C, Geisen C, Mehlhorn U, et al. Large-dose hydroxyethyl starch 130/0.4 does not increase blood loss and transfusion requirements in coronary artery bypass surgery compared with hydroxyethyl starch 200/0.5 at recommended doses. Anesthesiology. 2003;99(1):42–7.
5 Neff TA, Doelberg M, Jungheinrich C, Sauerland A, Spahn DR, Stocker R. Repetitive large-dose infusion of the novel hydroxyethyl starch 130/0.4 in patients with severe head injury. Anesth Analg. 2003;96(5):1453–9.
6 Sander O, Reinhart K, Meier-Hellmann A. Equivalence of hydroxyethyl starch HES 130/0.4 and HES 200/0.5 for perioperative volume replacement in major gynaecological surgery. Acta Anaesthesiol Scand. 2003;47(9):1151–8.
7 van der Linden PJ, De Hert SG, Deraedt D, Cromheecke S, De Decker K, De Paep R, et al. Hydroxyethyl starch 130/0.4 versus modified fluid gelatin for volume expansion in cardiac surgery patients: the effects on perioperative bleeding and transfusion needs. Anesth Analg. 2005;101(3):629–34.
8 Mahmood A, Gosling P, Vohra RK. Randomized clinical trial comparing the effects on renal function of hydroxyethyl starch or gelatine during aortic aneurysm surgery. Br J Surg. 2007;94(4):427–33.
9 Godet G, Lehot J-J, Janvier G, Steib A, de Castro V, Coriat P. Safety of HES 130/0.4 (Voluven®) in patients with preoperative renal dysfunction undergoing abdominal aortic surgery: a prospective, randomized, controlled, parallel-group multicentre trial. Eur J Anaesthesiol. 2008;25(12):986–94.
10 Dolecek M, Svoboda P, Kantorová I, Scheer P, Sas I, Bíbrová J, et al. Therapeutic influence of 20% albumin versus 6% hydroxyethylstarch on extravascular lung water in septic patients: a randomized controlled trial. Hepatogastroenterology. 2009;56(96):1622–8.
11 Mukhtar A, Aboulfetouh F, Obayah G, Salah M, Emam M, Khater Y, et al. The safety of modern hydroxyethyl starch in living donor liver transplantation: a comparison with human albumin. Anesth Analg. 2009;109(3):924–30.
12 Dubin A, Pozo MO, Casabella CA, Murias G, Pálizas F Jr, Moseinco MC, et al. Comparison of 6% hydroxyethyl starch 130/0.4 and saline solution for resuscitation of the microcirculation during the early goal-directed therapy of septic patients. J Crit Care. 2010;25(4):659.e1–.e8.
13 Gondos T, Marjanek Z, Ulakcsai Z, Szabó Z, Bogár L, Károlyi M, et al. Short-term effectiveness of different volume replacement therapies in postoperative hypovolaemic patients. Eur J Anaesthesiol. 2010;27(9):794–800.
14 Inal MT, Memis D, Karamanlioglu B, Sut N Effects of polygeline and hydroxyethyl starch solutions on liver functions assessed with LIMON in hypovolemic patients. J Crit Care. 2010;25(2):361.e1–.e5.
15 Cook DJ, Guyatt GH, Ryan G, Clifton J, Buckingham L, Willan A, et al. Should unpublished data be included in meta-analyses? Current convictions and controversies. JAMA. 1993;269(21):2749–53.
16 Rasmussen LS, Yentis SM, Van Aken H, Shafer SL, Eisenach JC, Edmunds LH, et al. Editors-in-chief statement regarding published clinical trials conducted without IRB approval by Joachim Boldt. Minerva Anestesiol. 2011;77(5):562–3.
17 Boldt J, Brosch C, Ducke M, Papsdorf M, Lehmann A. Influence of volume therapy with a modern hydroxyethylstarch preparation on kidney function in cardiac surgery patients with compromised renal function: A comparison with human albumin. Crit Care Med. 2007;35(12):2740–6.
18 Boldt J, Brosch C, Röhm K, Lehmann A, Mengistu A, Suttner S. Is albumin administration in hypoalbuminemic elderly cardiac surgery patients of benefit with regard to inflammation, endothelial activation, and long-term kidney function? Anesth Analg. 2008;107(5):1496–503.
19 Ioannidis JPA, Trikalinos TA, Zintzaras E Extreme between-study homogeneity in meta-analyses could offer useful insights. J Clin Epidemiol. 2006;59(10):1023–32.
20 Martin E. Ein Tsunami der besonderen Art. Anästhesiol Intensivmed Notfallmed Schmerzther. 2011;46:149.
21 Schwarzer G, Antes G, Schumacher M. A test for publication bias in meta-analysis with sparse binary data. Stat Med. 2007;26(4):721–33.
22 Duval SJ, Tweedie RL. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63.
23 Moreno SG, Sutton AJ, Turner EH, Abrams KR, Cooper NJ, Palmer TM, et al. Novel methods to deal with publication biases: secondary analysis of antidepressant trials in the FDA trial registry database and related journal publications. BMJ. 2009;339:b2981.
24 James MF, Michell WL, Joubert IA, Nicol AJ, Navsaria PH, Gillespie RS. Resuscitation with hydroxyethyl starch improves renal function and lactate clearance in penetrating trauma in a randomized controlled study: the FIRST trial (Fluids in Resuscitation of Severe Trauma). Br J Anaesth. 2011;107(5):693–702.
25 Guidet B, Martinet O, Boulain T, Philippart F, Poussel JF, Maizel J, et al. Assessment of hemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 versus 0.9% NaCl fluid replacement in patients with severe sepsis: The CRYSTMAS study. Crit Care. 2012;16(3):R94.
26 James MF, Michell WL, Joubert IA, Nicol AJ, Navsaria PH, Gillespie RS. Hydroxy ethyl starch in patients with trauma: reply from the authors. Br J Anaesth. 2012;108(1):160–1.
27 Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
28 Reinhart K, Perner A, Sprung CL, Jaeschke R, Schortgen F, Johan Groeneveld AB, et al. Consensus statement of the ESICM task force on colloid volume therapy in critically ill patients. Intensive Care Med. 2012;38(3):368–83.
29 Dart AB, Mutter TC, Ruth CA, Taback SP. Hydroxyethyl starch (HES) versus other fluid therapies: effects on kidney function. Cochrane Database Syst Rev. 2010;1:CD007594.
30 Groeneveld ABJ, Navickis RJ, Wilkes MM. Update on the comparative safety of colloids: a systematic review of clinical studies. Ann Surg. 2011;253(3):470–83.
31 Zarychanski R, Turgeon AF, Fergusson DA, Cook DJ, Hébert P, Bagshaw SM, et al. Renal outcomes and mortality following hydroxyethyl starch resuscitation of critically ill patients: systematic review and meta-analysis of randomized trials. Open Med. 2009;3(4):E196–209.
32 Fluids study investigators for the Scandinavian Critical Care Trials Group. Preferences for colloid use in Scandinavian intensive care units. Acta Anaesthesiol Scand. 2008;52(6):750–8.
33 Hartog CS, Kohl M, Reinhart K. A systematic review of third-generation hydroxyethyl starch (HES 130/0.4) in resuscitation: safety not adequately addressed. Anesth Analg. 2011;112(3):635–45.
34 Perner A, Haase N, Wetterslev J, Åneman A, Tenhunen J, Guttormsen AB, et al. Comparing the effect of hydroxyethyl starch 130/0.4 with balanced crystalloid solution on mortality and kidney failure in patients with severe sepsis (6S – Scandinavian Starch for Severe Sepsis/Septic Shock trial): Study protocol, design and rationale for a double-blinded, randomised clinical trial. Trials. 2011;12(1):24.
35 Crystalloid versus Hydroxyethyl Starch Trial (CHEST) Management Committee. The Crystalloid versus Hydroxyethyl Starch Trial: protocol for a multi-centre randomised controlled trial of fluid resuscitation with 6% hydroxyethyl starch (130/0.4) compared to 0.9% sodium chloride (saline) in intensive care patients on mortality. Intensive Care Med. 2011;37(5):816–23.
Funding / potential competing interests: C. J. Wiedermann has received fees for speaking and travel reimbursements from manufacturers of plasma-derived therapies (CSL Behring, Kedrion, Baxter and PPTA). M. Joannidis received speaker fees from Fresenius-Kabi, Baxter and CSL Behring.