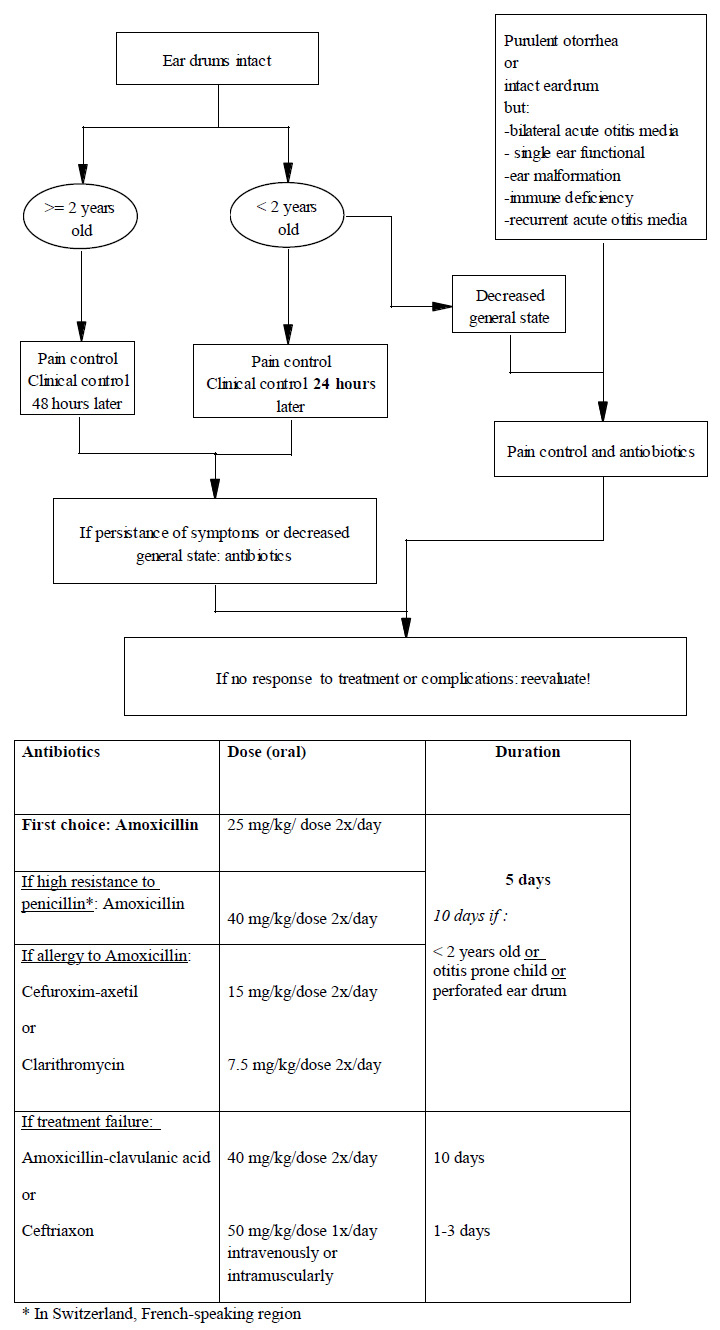
Figure 1
Current recommendations for the treatment of acute otitis media by the pediatric infectious diseases specialists of Switzerland ( http://www.pigs.ch ).
DOI: https://doi.org/10.4414/smw.2012.13654
Infectious diseases have been, since the beginning of time, closely linked to paediatrics. The reason is simple and unfortunately still prevails in developing countries; infections were and are major causes of childhood morbidity and mortality [1]. While Hippocrates (4th century B.C.), Celsus (in the 1st century A. D.), Oribasius (4th century), or Ibn Sina (11th century) described with astounding accuracy paediatric infections, such as mumps, roundworms, infant rashes, cough, throat inflammation, or tetanus, the first “paediatric infectious disease” printed book is attributed to the Italian Paolo Bagellardo in 1472. During this period, however, theories about diseases hadn’t evolved and clinical examination was not valued. It is only with Guillaume de Baillou’s work that clinical observation was used to describe a whooping cough epidemic (1578). By the end of the 17th century, the concept of contagious nature was suggested concerning diphtheria [2], and specific paediatric infectious diseases were recognised. For example, Thomas Sydenham carefully described chorea in 1686, as well as measles and scarlet fever.
The public health burden of infectious diseases in childhood was possibly first studied by Hugh Smith, who showed that from 1762 to 1771 approximately 2/3 of children born in London died before the age of 5 and that most deaths occurred before the age of 2 [2, 3].
However, three major steps changed the landscape of paediatric infectious diseases. First, when in 1798 Edward Jenner published his report on smallpox vaccine, which started a new fantastic era of protecting children against several (fatal) diseases before they even get sick. Second, was the discovery of bacteria, and later viruses, and other infectious organisms. Semmelweis, a Hungarian obstetrician, who, after observing that puerperal death rates were much higher in wards managed by physicians compared to midwives, suggested that doctors disinfect their hands between patients, and wear clean coats for the ward and different clothing for the room where post mortems were carried out. Although he was ferociously criticised, a simple measure such as hand hygiene achieved an unbelievably important drop in perinatal death rates. Louis Pasteur and Robert Koch in the 1860s and 1870s showed that microorganisms grew in broth in a sealed tube, but no growth or spoiling of the broth occurred if it was boiled first. The aetiologic agents of many infectious diseases where then identified at the turn of the century. Pasteurisation significantly changed the outcome of paediatric infectious diseases, because a safe milk supply effectively reduced mortality from diarrheal illness in young children. Third, the discovery of antimicrobials, such as penicillin, first discovered in 1928 by Fleming and then developed by Chain, Florey and Heatley completely changed the perspective from a preventive to a curative approach in paediatric infectious diseases.
In 2012, new challenges await the physician taking care of children with infectious diseases. Some old diseases are forgotten, but re-emerge, some “new” diseases have appeared, and new “hosts” change the way diseases arise or endanger these children. Some of these aspects will be reviewed below.
Most common infectious diseases in general paediatric practice – in developed countries – include acute otitis media, pharyngitis and gastroenteritis. Diagnosis for all three is not usually a problem, but treatment recommendations have changed over time for several reasons.

Figure 1
Current recommendations for the treatment of acute otitis media by the pediatric infectious diseases specialists of Switzerland ( http://www.pigs.ch ).
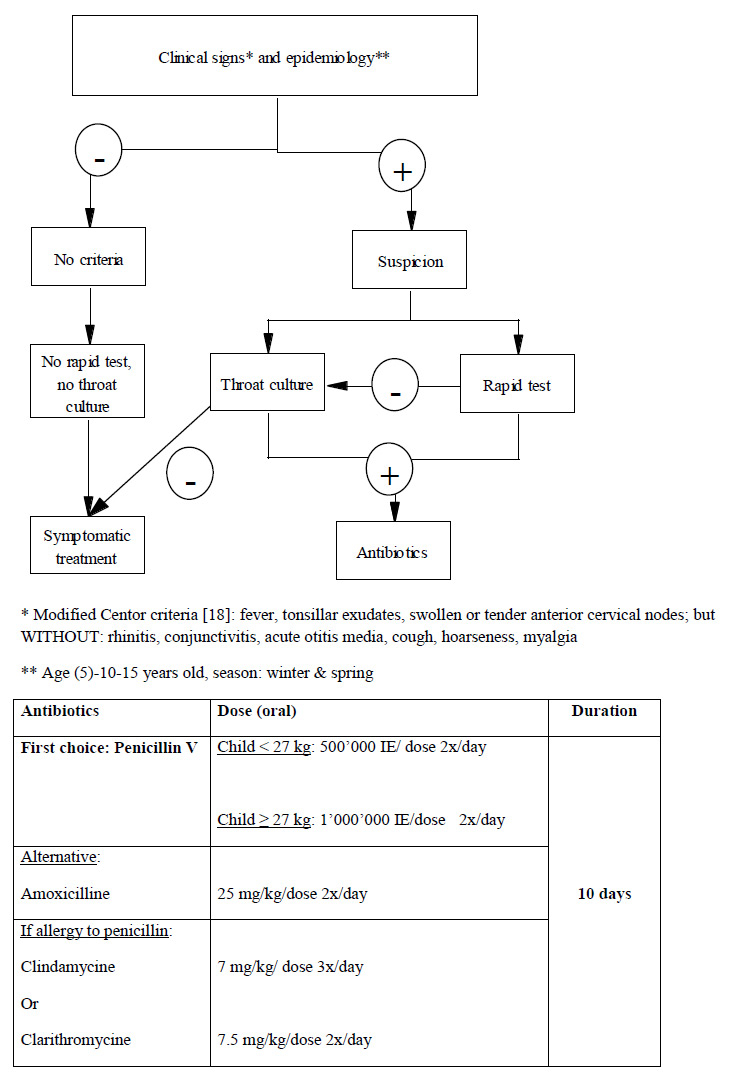
Figure 2
Current recommendations for the management of acute pharyngitis by the Pediatric Infectious Diseases Specialists of Switzerland ( http://www.pigs.ch ).
Acute otitis media is extremely common during childhood: 90% of children have at least one episode by 2 years of age [4]. Risk factors include young age or exposure to young children through daycare for example, and family history. Exposure to smoke has also been reported as a risk factor [5]. Tympanocenteses during an acute episode can recover viruses, such as respiratory syncytial virus, parainfluenza (types 1–3), influenza (A and B), enterovirus, rhinovirus, but also bacteria, such as Streptococcus pneumoniae, Haemophilus influenzae(usually non-typable), Streptococcus pyogenes (= group A Streptococcus), orMoraxella catarrhalis [6, 7]. The proportion of different microorganisms varies according to age and region. Two recent events have changed the approach to acute otitis media: first the introduction of universal pneumococcal vaccination, and the emergence of penicillin-resistant pneumococcal strains.
In the pre-licensure trial, pneumococcal conjugate vaccine had a 7% efficacy in preventing AOM with an increased efficacy in children with recurrent episodes [8]. In a Cochrane review vaccination showed a pooled relative reduction of AOM between 6% and 7% in children <12 years, but had no clear effect on recurrent AOM [9]. In another study, vaccination decreased pneumococcal AOM by 48%, with a 38% decrease in otorrhea visits as a collateral benefit, and possibly an economic impact on public health [10, 11]. Further epidemiological studies are needed to evaluate the effect of serotype replacement to non-vaccine serotypes on the incidence of otitis media. Increasing penicillin and multidrug resistance among S. pneumoniae strains preceded the introduction of pneumococcal vaccines. The 7 serotypes originally included in the conjugate vaccine were among the strains most commonly found in invasive pneumococcal diseases and with known antibiotic resistance. Since then, serotype replacement, especially the emergence of serotype 19A, has been observed and has been the basis of increasing the pneumococcal conjugate vaccine’s valence to contain 13 serotypes, including 19A. In Switzerland, in 2009, two thirds of pneumococcal strains found in nasopharyngeal swabs were serotypes included in the 13-valent pneumococcal conjugate vaccine (77%; 95% confidence interval: 66%–88%) [12]. Furthermore, 16% of the strains had an intermediate or complete resistance to one antibiotic, while 6% showed several resistances.
According to the Pediatric Infectious Diseases Group of Switzerland ( http://www.pigs.ch ), which includes all paediatric infectious diseases specialists of Switzerland, recommendations for the treatment of acute otitis media in this country are summarised in (fig. 1).
Complications of acute otitis media include perforation of the tympanic membrane, or less commonly facial palsy, mastoiditis, labyrinthitis, and hearing loss.
Pharyngitis, an inflammation of the mucous membrane and underlying structures of the pharynx, is also a very common disease, and accounts for approximately 5% of all visits [13]. It can be found in all age groups, but is most common among school-aged children and adolescents. Many viruses and bacteria can cause pharyngitis, but most cases are viral and self-limited. Streptococcus pyogenes, also called group A beta-hemolytic Streptococcus (GAS), is the most important bacterial cause of pharyngitis. The challenge of pharyngitis is to identify properly the minority of patients with GAS (around 15–30%) who need antibiotic treatment among the majority with viral pharyngitis who do not. The reason to treat GAS pharyngitis- a self-limited disease- is to shorten the clinical course of the disease, to avoid suppurative complications, such as lymphadenitis, peritonsillar abscess, mastoiditis, but mostly nonsuppurative sequelae such as acute rheumatic fever, acute post-streptococcal glomerulonephritis, or reactive arthritis, among others [14]. Diagnosis is usually made with either rapid antigen detection tests, or regular throat culture. The specificity of both tests is high (between 90–95%), but their sensitivity varies (between 55–99%) depending on the test used, and the skill of the person doing the swab [15, 16]. Chiappini et al. recently reviewed noticeably different recommendations from international guidelines for the management of acute pharyngitis [17]. Twelve national guidelines were reviewed and showed substantial differences in the management of pharyngitis, although some used Centor’s score to evaluate the risk of GAS, and all recommended penicillin as the first choice for treating GAS pharyngitis [18]. In Switzerland, management of acute pharyngitis, as recommended by the Pediatric Infectious Diseases Group of Switzerland ( http://www.pigs.ch ) is summarised in (fig. 2). Patients are no more contagious after 24 hours of antibiotics. However, patients can have GAS in their pharynx after an appropriate antibiotic treatment. This bacteriologic failure should be divided in clinical failure, when patients still have pharyngitis after taking the antimicrobial, and carrier state. Carrier state can last several months during which the patient may have viral pharyngitis and still test positive for GAS. Eradication of carrier state is suggested when there is a family history of rheumatic disease, when there is an outbreak of acute rheumatic fever or acute post-streptococcal glomerulonephritis in the community, when there is a “ping-pong” effect in the family, and when despite reassurance the parent’s anxiety level is unbearable. Eradication is usually obtained with oral clindamycin for 10 days (20 mg/kg/day in 3 doses) [19]. Unfortunately, chronic carriage can be re-acquired after a successful eradication. Preventing GAS infection and its sequelae through vaccination would be essential. Several vaccine models- reviewed recently by Pandey et al- are currently being investigated [20]. Some target the M-protein, others antigens critical for the bacteria’s infectiousness. These vaccines are not yet available for clinical use.
After respiratory illnesses, diarrhea is the most common disease among children, occurring at a rate of approximately 3 per year in children younger than 3 years of age going to childcare [21]. It remains the leading cause of paediatric morbidity and mortality worldwide. Infectious diarrhea can be caused by viral, bacterial, or parasitic microorganism, most acquired through the fecal-oral route from person-to-person transmission or from contaminated water or food. In childcare centres and in most developed countries, rotavirus, enteric adenovirus, astrovirus, caliciviruses are the most frequent causes of acute gastroenteritis. In mild cases, the aetiologic agent is not always searched for, as treatment is usually symptomatic, and is based on rehydration and electrolyte replacement. In very young children, in children with risk factors such as immune deficiency, in children returning from a trip, or in children with a severe presentation, the pathogen should be explored to provide- if necessary- additional treatment, such as antibiotics. Before the late 1960s, rehydration in hospitals was usually recommended intravenously. Since then, several studies have shown the benefit of oral rehydration compared with intravenous therapy [22, 23]: it reduces hospitalisation rates, costs, and does not require much equipment. It does not however decrease the frequency or volume of stools, nor the duration of diarrhea. When the child refuses to breastfeed or to drink the formulation of oral rehydration therapy, it can be administered through a nasogastric tube. Because of the important burden of disease in young children, vaccines against rotavirus were developed. The first widely distributed vaccine (Rotashield®, Wyeth Laboratories) was withdrawn in 1999, after an association with an increased risk for intussusception was demonstrated [24]. Since then two oral vaccines are available to children between 6 weeks and 6 months of age and have not been associated with this complication: Rotarix® (2008; GlaxoSmithKline) and Rotateq® (2006; Merck). Their efficacy is estimated around 85–95% against severe disease and hospital admission.
In Switzerland, it is estimated that rotaviral infection is responsible for approximately 6,000 consultations per year (1.6 per 100 children <5 years old). However, secondary hospital admissions only concern 1.6 per 1,000 children <5 years old, to which nosocomial disease should be added. Cost-effect analyses in Switzerland by the Federal Commission of Vaccination have decided against introducing rotavirus vaccination in the routine immunisation schedule, but kept it as an optional, individually decided vaccination [25].
| Table 1: Recommendations for adult vaccination against diphtheria, tetanus, and pertussis in Switzerland (2012). Adapted from: OFSP. Optimisation des rappels vaccinaux contre la diphtérie, le tétanos et la coqueluche (dT/dTpa) chez l’adulte. Bull OFSP. 2011;51:1161–71 [31]. | ||||||||
| Age | 16 to 24 years old | 25 to 29 years old | 30 to 64 years old | ≥65 years old | ||||
| Time since last dose of tetanus vaccine | <10 years | ≥10 years | <2 years | ≥2 years | < 20 years | ≥20 years | <10 years | ≥10 years |
| Previously fully vaccinated against tetanus | 0* | 0* | 0* | 1 x dTpa | 0* | 1 x dT* | 0* | 1 x dT* |
| Previously partially vaccinated against tetanus | 1–3 x dT* | 1 x dTpa / 0–2 x dT | 1–3 dT* | 1–3 x dT* | ||||
| d: diphtheria vaccine; T: tetanus vaccine; pa: acellular pertussis vaccine; * 1 x dTpa : if regular contact (professional or personal) with infants younger than 6 months old if never vaccinated as an adult and if more than 10 years since last pa. Minimum time since last T: 4 weeks. | ||||||||
Some “old” paediatric infectious diseases were almost successfully eradicated through vaccination but have re-emerged following lower public awareness of their danger and subsequent lower vaccination rates. Physicians should therefore learn to recognise these diseases and encourage their patients (or their children) to get immunised to attain eradication.
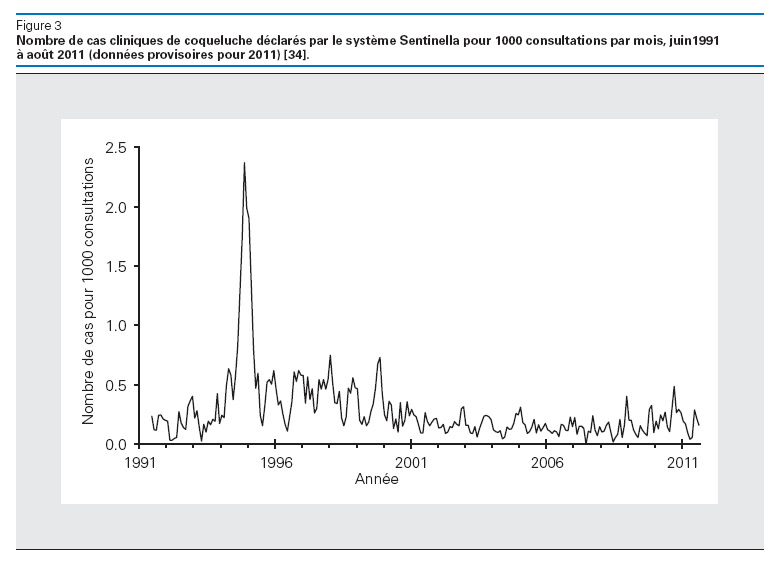
Figure 3
Clinical cases of pertussis declared by the Sentinella system per 1,000 consultations, by month (June 1991–August 2011). Source: OFSP. Optimisation des rappels vaccinaux contre la diphtérie, le tétanos et la coqueluche (dT/dTpa) chez l’adulte. Bull OFSP. 2011;51:1161–71 [31]. © Bulletin OFSP, reprinted with permission).
Whooping cough, or pertussis, is a ubiquitous, acute respiratory tract infection, which starts usually as a mild upper airway disease (catarrhal stage), progresses to coughing spells characterised by a inspiratory whoop and commonly followed by vomiting (paroxysmal stage), and ends over several weeks (convalescent stage). In its first stage, pertussis is highly communicable, with a secondary attack rate of up to 90% among non immune household contacts. Untreated patients may be contagious for 3 weeks or more, although communicability diminishes rapidly after the catarrhal stage. Until recently, Bordetella pertussis was believed to be the only cause of pertussis; however, it is now known that B. parapertussis, especially in Europe, and possibly other members of the Bordetella family (possibly B. holmesii for example) can cause similar symptoms, although probably less severe [26]. Estimates from World Health Organization (WHO) suggest that, in 2008, about 16 million cases of pertussis occurred worldwide, 95% of which were in developing countries, and that about 195 000 children died from the disease [27]. A serological study from the US showed that 21% (95% confidence interval [CI], 13–32%) of adults with prolonged cough defined as lasting >2 weeks had pertussis [28]. Adolescents and adults are significant sources of transmission of B. pertussisto unvaccinated young infants. A study conducted in Canada, France, Germany and the US showed that when pertussis occurred in infants, household members – primarily parents – were the source of B. pertussisin 76–83% of cases [29].
Polymerase chain reaction (PCR) on a nasopharyngeal specimen is usually used to confirm the diagnosis. It is important to recognise, that the calcium alginate swabs used for throat cultures inhibit the PCR and should not be used for pertussis, only a Dacron swab (or nasal wash).
Recommended treatments of pertussis include macrolides, such as azithromycin (3–5 days) or erythromycin (14 days), or trimethoprim-sulfamethoxazole (14 days). For children younger than 6 months old, paediatricians should be consulted for appropriate treatment, as infantile hypertrophic pyloric stenosis has been reported in young children. Treatment is most effective during the catarrhal stage [30].
Chemoprophylaxis is recommended for all household contacts, regardless of age and immunisation status to limit secondary transmission and/ or repeated infection of the infant. Antibiotics, doses, and duration are the same as for treatment of pertussis. Close contacts who are unimmunised or underimmunised should be vaccinated [30].
In 2008, about 82% of all infants worldwide received 3 doses of pertussis vaccine. In Switzerland, 95% of children <2 years old had received 3 doses, and 87% 4 doses, as recommended in the national vaccination schedule [31]. WHO estimates that in 2008 global vaccination against pertussis averted about 687,000 deaths. In Switzerland, approximately 50 children with pertussis are admitted to the hospital every year, mostly infants, and 4 deaths have been directly linked with pertussis in the last 15 years (fig. 3) [32]. However, the number of cases is probably critically underestimated, especially among (vaccinated or not) teenagers and adults, who have been repeatedly identified as infectious sources for the infants, too young to be vaccinated while most at risk for severe outcome [33, 34]. Vaccine efficacy is estimated between 75% and 90%, but protection following a full vaccination schedule is known to be waning. For this reason, several countries, including Switzerland in 2012, introduced a booster pertussis vaccination in young adults between 25 and 19 years old [31, 35, 36]. Hopefully, this measure will decrease morbidity and mortality among the infants and decrease the burden of disease in older children and adults.
The WHO pledged to get Europe measles-free by 2015. Switzerland however became sadly worldwide famous during the 2008 Euro soccer tournament because of a measles outbreak that started in 2006, and since then several outbreaks have been reported in this country [37, 38]. The main reason is that to be eliminated, the population’s vaccine coverage should be higher than the current 82–85%. In 2011, still more than 600 cases have been reported in Switzerland, which is probably an underestimation of the true number of cases (Federal Office of Public Health data).
Measles is an acute viral disease characterised by fever, cough, conjunctivitis, runny nose and an erythematous maculopapular rash. Other manifestations such as otitis media, bronchopneumonia (5–15%), croup or diarrhea can also occur in younger children. Encephalitis and death from respiratory failure or neurologic complications occurs in about 1 of every 1,000 cases in previously healthy patients, and more frequently in younger (<5 years old) and immunocompromised patients. Treatment is symptomatic and does not prevent long-term complications such as subacute sclerosing panencephalitis 7–10 years later.
Outside of an outbreak situation, diagnosis can be tricky, not because diagnostic tests aren’t available (serologies are routinely performed in most laboratories), but because physicians haven’t seen measles during their training, or do not think of it when seeing a child with an exanthema.
Two doses of measles live-attenuated vaccine develop a long-lasting serological protection in >99% of patients. In Finland, for example, where a two doses schedule has been introduced since 1982 and vaccination coverage reaches 98%, indigenous measles has been eliminated since 1995 [39]. It is therefore within reach to have a measles-free Europe, and subsequently a measles-free world if recommendations are followed and physicians emphasise the individual and public health impact of vaccinating all children or to catch-up the unvaccinated adults.
With the advent of modern neonatology care, survival of very low birth weight (≤1500g; VLBW) or extremely low birth weight (≤1000 g; ELBW) premature infants is common. These very young patients’ immune system is immature: they are therefore particularly vulnerable to invasive infections [40]. In Switzerland, approximately 1% of all births were born before 32 weeks gestational age, with an average birth weight of 1194 g [41]. Neonatal bacterial infections-related mortality has declined in recent years from 30–40% to 5–10% thanks to awareness of maternal and infant factors and earlier treatment with antibiotics. This decrease is mostly secondary to the decrease in early-onset sepsis, while late-onset (7–30 days) or late-late-onset (> 30 days) have slightly increased over the last decades [42]. Early-onset infections are mostly due to Gram-negative organisms, such as Escherichia coli [43]. Common bacteria for late-onset sepsis include coagulase-negative staphylococci (almost always associated with a medical device), but also Staphylococcus aureus, Enterococcus spp. or Gram-negative enteric bacilli. In Germany, almost 91% of neonates were treated with antibiotics, this proportion being even higher (96.2–100%) in preterm infants below 30 weeks’ gestation [44]. In Switzerland, Zingg et al have recently shown that 26.7% of neonates received systemic antibiotics during their stay in neonatology [45]. Amoxicillin in combination with gentamicin was the most frequent antibiotic regimen (47.0%), followed by vancomycin combined with gentamicin (10.2%), amoxicillin alone (9.0%), vancomycin alone (6.1%), and a cephalosporin alone (5.0%, mostly third generation). Infection-associated mortality was 1.3%.
One major difficulty in treating infections in (premature) neonates is the availability of antibiotics that are licensed in this age group, and for which safety studies are available. Pandolfini et al. reported for example that 14–63% of prescriptions were off-label in neonates, and that the proportion of children given at least one unlicensed or off-label drug was 80–97% neonates [46]. Abraham Jacobi, the father of American paediatrics wrote: “Paediatrics does not deal with miniature men and women with reduced doses and the same class of disease in smaller bodies, but… has its own independent range and horizon” [47]. Thus, it is clear that the capacity of absorption, distribution, metabolism, and elimination of antibiotics is different in children and adults, but also more vividly in premature infants [48].
Another new category of pediatric hosts are the immunocompromised patients, which represent a new challenge for paediatric infectious diseases specialists. These include primary immune deficiency patients, which in the past often died during infancy, and who, thanks to antibiotic prophylaxis, intravenous immunoglobulins, and, sometimes, bone marrow transplants have increased their survival. They also include HIV positive children who, thanks to antiretroviral therapy and, again, antibiotic prophylaxis, not only reach adulthood, but can lead “normal” lives. Finally, there is the large group of iatrogenically immunocompromised patients, such as oncological patients, transplant recipients, and all patients treated with immunosuppressive drugs, such as patients with rheumatological diseases, or severe asthma. Pediatric infectious diseases specialists often have to transpose knowledge from similar patients with adult diseases, and find solutions using antibiotics compatible with children.
Emerging pathogens are continuously reported in the literature, thanks to better detection techniques and spread of information. One recent example is the impressive rapidity with which the pandemic influenza A/H1N1 was recognised as a new pathogen, and information was transmitted. Since spring 2009, more than 10,000 peer-reviewed articles have been published on H1N1, and have identified this virus as putting children especially at high risk for infection and severe disease, unlike seasonal influenza which can severely affect young children [49, 50]. Thanks to these early reports, immunisation of children was recommended by the majority of national health authorities even before the pandemic arrived [51].
Three other viruses have played a recent important role in paediatric infectious diseases. The first, human metapneumovirus (HMPV), was discovered in 2001, and was quickly recognised as one of the main players in Respiratory Syncytial Virus (RSV)-negative respiratory tract infections in young children worldwide. It typically affects children between 2 and 3 years of age, and can be associated with coryza, cough, or severe respiratory disease [52]. The duration of symptoms prior to medical evaluation is usually less than a week, and limited data suggest that children shed virus for 1 to 2 weeks [53]. It can be found year long, but is most frequently detected 1–2 months after RSV season. HMPV was classified as the first human member of the Metapneumovirus genus, of the family Paramyxoviridae. At least two genetic lineages of HMPV are circulating in humans, with two sublineages each [54]. Antibody responses are elicited against the highly conserved F protein of HMPV may provide significant cross-protection against different HMPV lineages in animal studies [55]. It is however possible that antigenic variation explains the co-circulation of multiple genetic sublineages of HMPV in humans.
The second, part of the human coronavirus (HCoV) family discovered in 1965 and famous for one of its other members (SARS) was only identified in 2004 as a frequent cause for upper and lower respiratory tract infections in children [56]. HCoV-NL63 typically affects children younger than one year of age, but older children can be infected as well; it can also cause croup, and febrile convulsions. In a birth cohort followed during their first year of life, Kaiser et al showed that coronaviruses were identified in 16% of children with lower respiratory tract symptoms, at a median age of 5.7 months (range 0.7–11.4 months), and mostly during the cold months [57]. Although its incidence seemed lower in other studies, HCoV-NL63 still is one of the most frequently found viruses in nasal aspirates of symptomatic children [58].
Finally, the third virus, discovered in 2005, is the human bocavirus (HBoV), which is famous for being the first virus identified by “molecular virus screening”, a procedure based on DNase treatment of nasopharyngeal samples, random nucleic acid amplification and cloning, followed by large scale sequencing and bioinformatic analyses [59]. Human bocaviruses have been detected worldwide in respiratory and stool samples, but also in other biological samples, as well as in water. All age groups can be infected, but it is most commonly found in young children aged 6–24 months with respiratory tract infections or gastroenteritis. The transmission routes of HBoV are unknown. However, many similar viruses are transmitted by inhalation or contact with infectious sputum, feces, or urine. Clear causality, presentation modes and clinical relevance of HBoV are still debated and more evidence should be gathered in the coming years [60]. Other “new” viruses have been described recently, including KI and WU polyomaviruses, or the torque teno virus, increasing significantly the rate of identifiable viral causes for paediatric respiratory diseases [61].
It is likely that in the near future new techniques, such as molecular screening will identify new pathogens that cause diseases in children. It is also probable that new diseases will emerge, or that new treatment strategies will be available to paediatric patients thanks to clinical research and incentives to cross the gap in paediatric drug development [62]. Finally, research is investigating how to connect diseases recognised in adults with infections acquired during childhood, such auto-immune diseases, and viral infections. It is therefore foreseeable that paediatric infectious diseases will remain a sentinel for public health issues, and for individual healthcare.
1 Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–61.
2 Still GF. The history of paediatrics. London: Oxford University Press; 1931.
3 Colón A, Colón P. Nurturing children, a history of pediatrics. Press G (editor). Westport; 1999.
4 Paradise JL, Rockette HE, Colborn DK, Bernard BS, Smith CG, Kurs-Lasky M, et al. Otitis media in 2253 Pittsburgh-area infants: prevalence and risk factors during the first two years of life. Pediatrics. 1997;99(3):318–33.
5 DiFranza JR, Lew RA. Morbidity and mortality in children associated with the use of tobacco products by other people. Pediatrics. 1996;97(4):560–8.
6 Chonmaitree T. Viral and bacterial interaction in acute otitis media. Pediatr Infect Dis J. 2000;19(5 Suppl):S24–30.
7 Jacobs MR, Dagan R, Appelbaum PC, Burch DJ. Prevalence of antimicrobial-resistant pathogens in middle ear fluid: multinational study of 917 children with acute otitis media. Antimicrob Agents Chemother. 1998;42(3):589–95.
8 Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19(3):187–95.
9 Jansen AG, Hak E, Veenhoven RH, Damoiseaux RA, Schilder AG, Sanders EA. Pneumococcal conjugate vaccines for preventing otitis media. Cochrane Database Syst Rev. 2009(2):CD001480.
10 Stamboulidis K, Chatzaki D, Poulakou G, Ioannidou S, Lebessi E, Katsarolis I, et al. The impact of the heptavalent pneumococcal conjugate vaccine on the epidemiology of acute otitis media complicated by otorrhea. Pediatr Infect Dis J. 2011;30(7):551–5.
11 Huang SS, Johnson KM, Ray GT, Wroe P, Lieu TA, Moore MR, et al. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine. 2011;29(18):3398–412.
12 OFSP. [Maladies à pneumocoques]. Bull OFSP. 2010;47:1121–7.
13 Shaikh N, Leonard E, Martin JM. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta-analysis. Pediatrics. 2010;126(3):e557–64.
14 Randolph MF, Gerber MA, DeMeo KK, Wright L. Effect of antibiotic therapy on the clinical course of streptococcal pharyngitis. J Pediatr. 1985;106(6):870–5.
15 Needham CA, McPherson KA, Webb KH. Streptococcal pharyngitis: impact of a high-sensitivity antigen test on physician outcome. J Clin Microbiol. 1998;36(12):3468–73.
16 Pickering LK, Baker CJ, Kimberlin DW, Long SS. Group A streptococcal infections. In: Pediatrics AAP, editor. Red Book: 2009 Report of the Committee on Infectious Diseases 28th ed. Elk Grove Village, IL; 2009. p. 616–28.
17 Chiappini E, Regoli M, Bonsignori F, Sollai S, Parretti A, Galli L, et al. Analysis of different recommendations from international guidelines for the management of acute pharyngitis in adults and children. Clin Ther. 2011;33(1):48–58.
18 Centor RM, Allison JJ, Cohen SJ. Pharyngitis management: defining the controversy. J Gen Intern Med. 2007;22(1):127–30.
19 Kaplan EL, Johnson DR, Del Rosario MC, Horn DL. Susceptibility of group A beta-hemolytic streptococci to thirteen antibiotics: examination of 301 strains isolated in the United States between 1994 and 1997. Pediatr Infect Dis J. 1999;18(12):1069–72.
20 Pandey M, Batzloff MR, Good MF. Vaccination against rheumatic heart disease: a review of current research strategies and challenges. Curr Infect Dis Rep. 2012;14(4):381–90.
21 Sullivan P, Woodward WE, Pickering LK, DuPont HL. Longitudinal study of occurrence of diarrheal disease in day care centers. Am J Public Health. 1984;74(9):987–91.
22 Duggan C, Lasche J, McCarty M, Mitchell K, Dershewitz R, Lerman SJ, et al. Oral rehydration solution for acute diarrhea prevents subsequent unscheduled follow-up visits. Pediatrics. 1999;104(3):e29.
23 Tamer AM, Friedman LB, Maxwell SR, Cynamon HA, Perez HN, Cleveland WW. Oral rehydration of infants in a large urban U.S. medical center. J Pediatr. 1985;107(1):14–9.
24 Prevention CfDC. Withdrawal of Rotavirus Vaccine Recommendation. MMWR. 1999;48(43).
25 OFSP. Vaccination contre les rotavirus: pas d’introduction dans le Plan suisse de vaccination. Bull OFSP. 2008;28:492–5.
26 Mastrantonio P, Stefanelli P, Giuliano M, Herrera Rojas Y, Ciofi degli Atti M, Anemona A, et al. Bordetella parapertussis infection in children: epidemiology, clinical symptoms, and molecular characteristics of isolates. J Clin Microbiol. 1998;36(4):999–1002.
27 World Health Organization (WHO). Pertussis vaccine: WHO position paper. Weekly Epidemiological Record. 2010;40(85):6.
28 Wright SW, Edwards KM, Decker MD, Zeldin MH. Pertussis infection in adults with persistent cough. JAMA. 1995;273(13):1044–6.
29 Wendelboe AM, Hudgens MG, Poole C, Van Rie A. Estimating the role of casual contact from the community in transmission of Bordetella pertussis to young infants. Emerg Themes Epidemiol. 2007;4:15.
30 Pickering LK, Baker CJ, Kimberlin DW, Long SS. Pertussis (Whooping Cough). In: Pediatrics AAP, editor. Red Book: 2009 report of the Committee on Infectious Diseases 28th ed. Elk Grove Village, IL; 2009;504–20.
31 OFSP. Optimisation des rappels vaccinaux contre la diphtérie, le tétanos et la coqueluche (dT/dTpa) chez l’adulte. Bull OFSP. 2011;51:1161–71.
32 OFSP. Coqueluche/Pertussis. Journal [serial on the Internet]. 2012 last accessed July 11, 2012: Available from: http://www.bag.admin.ch/themen/medizin/00682/00684/01082/index.html?lang=fr.
33 Halperin SA, Bortolussi R, MacLean D, Chisholm N. Persistence of pertussis in an immunized population: results of the Nova Scotia Enhanced Pertussis Surveillance Program. J Pediatr. 1989;115(5 Pt 1):686–93.
34 Wendelboe AM, Njamkepo E, Bourillon A, Floret DD, Gaudelus J, Gerber M, et al. Transmission of Bordetella pertussis to young infants. Pediatr Infect Dis J. 2007;26(4):293–9.
35 Ward JI, Cherry JD, Chang SJ, Partridge S, Lee H, Treanor J, et al. Efficacy of an acellular pertussis vaccine among adolescents and adults. N Engl J Med. 2005 ;353(15):1555–63.
36 Halperin SA, Sweet L, Baxendale D, Neatby A, Rykers P, Smith B, et al. How soon after a prior tetanus-diphtheria vaccination can one give adult formulation tetanus-diphtheria-acellular pertussis vaccine? Pediatr Infect Dis J. 2006;25(3):195–200.
37 OFSP. L’épidémie de rougeole se poursuit en Suisse. Bull OFSP. 2008;24:430–2.
38 OFSP. Nouvelle vague de l’épidémie de rougeole en début d’année 2009: description et mesures. Bull OFSP. 2009;27:484–91.
39 Peltola H, Heinonen OP, Valle M, Paunio M, Virtanen M, Karanko V, et al. The elimination of indigenous measles, mumps, and rubella from Finland by a 12-year, two-dose vaccination program. N Engl J Med. 1994;331(21):1397–402.
40 Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7(5):379–90.
41 Bajwa NM, Berner M, Worley S, Pfister RE. Population based age stratified morbidities of premature infants in Switzerland. Swiss Med Wkly. 2011;141:w13212.
42 Bizzarro MJ, Raskind C, Baltimore RS, Gallagher PG. Seventy-five years of neonatal sepsis at Yale: 1928-2003. Pediatrics. 2005;116(3):595–602.
43 Stoll BJ, Hansen N. Infections in VLBW infants: studies from the NICHD Neonatal Research Network. Semin Perinatol. 2003;27(4):293–301.
44 Neubert A, Lukas K, Leis T, Dormann H, Brune K, Rascher W. Drug utilisation on a preterm and neonatal intensive care unit in Germany: a prospective, cohort-based analysis. Eur J Clin Pharmacol. 2010;66(1):87–95.
45 Zingg W, Pfister R, Posfay-Barbe KM, Huttner B, Touveneau S, Pittet D. Secular trends in antibiotic use among neonates: 2001-2008. Pediatr Infect Dis J. 2011;30(5):365–70.
46 Pandolfini C, Bonati M. A literature review on off-label drug use in children. Eur J Pediatr. 2005;164(9):552–8.
47 Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology-drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–7.
48 Zingg W, Posfay-Barbe KM. Antibiotic use in children - off-label use. Curr Drug Targets. 2012 Apr 18.
49 Kwan-Gett TS, Baer A, Duchin JS. Spring 2009 H1N1 influenza outbreak in King County, Washington. Disaster Med Public Health Prep. 2009;3 Suppl 2:S109–16.
50 Launes C, Garcia-Garcia JJ, Martinez-Planas A, Moraga F, Astigarraga I, Aristegui J, et al. 2009 H1N1: risk factors for hospitalization in a matched case-control study. Eur J Pediatr. 2012;21.
51 OFSP. [Office Fédéral de la Santé Publique. Grippe saisonnière 2008/2009. Epidémiologie, virologie, approvisionnement en vaccins et composition des vaccins]. Bull OFSP. 2009;2009:523–9.
52 Baer G, Schaad UB, Heininger U. Clinical findings and unusual epidemiologic characteristics of human metapneumovirus infections in children in the region of Basel, Switzerland. Eur J Pediatr. 2008;167(1):63–9.
53 van den Hoogen BG, van Doornum GJ, Fockens JC, Cornelissen JJ, Beyer WE, de Groot R, et al. Prevalence and clinical symptoms of human metapneumovirus infection in hospitalized patients. J Infect Dis. 2003;188(10):1571–7.
54 van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7(6):719–24.
55 Skiadopoulos MH, Biacchesi S, Buchholz UJ, Riggs JM, Surman SR, Amaro-Carambot E, et al. The two major human metapneumovirus genetic lineages are highly related antigenically, and the fusion (F) protein is a major contributor to this antigenic relatedness. J Virol. 2004;78(13):6927–37.
56 van der Hoek L, Pyrc K, Jebbink MF, Vermeulen-Oost W, Berkhout RJ, Wolthers KC, et al. Identification of a new human coronavirus. Nat Med. 2004;10(4):368–73.
57 Kaiser L, Regamey N, Roiha H, Deffernez C, Frey U. Human coronavirus NL63 associated with lower respiratory tract symptoms in early life. Pediatr Infect Dis J. 2005;24(11):1015–7.
58 van der Hoek L, Sure K, Ihorst G, Stang A, Pyrc K, Jebbink MF, et al. Croup is associated with the novel coronavirus NL63. PLoS Med. 2005;2(8):e240.
59 Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A. 2005;102(36):12891–6.
60 Jartti T, Hedman K, Jartti L, Ruuskanen O, Allander T, Soderlund-Venermo M. Human bocavirus-the first 5 years. Rev Med Virol. 2012;22(1):46–64.
61 Jartti T, Jartti L, Ruuskanen O, Soderlund-Venermo M. New respiratory viral infections. Curr Opin Pulm Med. 2012;18(3):271–8.
62 Steinbrook R. Testing medications in children. N Engl J Med. 2002;347(18):1462–70.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.