DOI: https://doi.org/10.4414/smw.2012.13641
The number of heart valve procedures performed annually in Western countries is continuously increasing. In the 20th century, introduction of extracorporeal circulation for aortic valve replacement simplified this procedure down to a routine manoeuvre. The initial prosthesis was very simple, containing one ball in a cage, resulting in satisfactory results for this era. Further developments in mechanical as well in biological valves were in demand from then on. Today the aortic valve prosthesis is sophisticated, and has been modified and adapted to provide major function, minimal complication and maximal durability. Although advantages have been achieved there still remain numerous prosthesis related complications. In light of this numerous research groups have been working on the amelioration of aortic valve prosthesis, as we still do not have ideal aortic valve replacement prosthesis.
The selection of a suitable device for aortic valve replacement (AVR) must be individualised through consideration of relative advantages and disadvantages. The following has to be considered when selecting a suitable device: durability of prosthesis, flow characteristics, risk of thromboembolism and need for anticoagulation, technical ease of insertion, infectibility, availability, and valve-related noise. In general, for isolated aortic valve replacement the current clinical practice and recommendation is as follows: patients aged over 65 years should have a bio-prosthesis implanted [1, 2]; in patients younger than 65 years of age, most would opt for a mechanical device.
Both therapy modalities have their advantages and disadvantages. The mechanical prosthesis exposes patients to the cumulative risk of lifelong anticoagulation, restricted hemodynamics and elevated risk for thromboembolism and endocarditis in favour of the defined long-term function of the prosthesis. In comparison, the biological valve has better hemodynamic performance than mechanical devices and does not require life-long anticoagulation therapy. A major disadvantage is structural deterioration which may result in dysfunction and new intervention. This latter consideration is the main limitation to implanting a biological valve in younger patients [3, 4].
In fact, the perfect aortic valve substitute has yet to be developed. It is well known, however, that that the pulmonary root is closer to an ideal substitute than any other current constructed prosthesis. With an almost identical morphology and function compared to the aortic valve, the pulmonary allograft meets almost all conditions for an ideal aortic valve substitute, such as: freedom from thromboembolism, superior hemodynamics over other current commercial devices, and potential for growth with time [5].
In 1967, Ross [6] implanted a pulmonary valve into the aortic root. The pulmonary sinuses of the pulmonary root were partially excised, and the pulmonary valve was secured in the recipient’s aortic root with two suture lines, one below and one above the aortic annulus, leaving the coronary artery orifices intact in their origin from the aortic root. This technically demanding sub-coronary implantation did not gain widespread popularity. At end of 1980s, however, the Ross procedure had a renaissance when the technique of aortic root replacement was reviewed [7]. In this modality of the Ross procedure, after the aortic root is removed, the pulmonary root is implanted into the aortic position including the re-implantation of coronary arteries into the neo-aortic root. Due to its relative simplicity compared to the sub-coronary implantation and consequent more predictable early outcomes, this technique gained broad acceptance among cardiac surgeons. Nowadays, about 97% of surgeons use this root replacement technique and only 3% use the initial sub-coronary implantation technique.
Ross first reported his results of the sub-coronary implantation technique in 1991 [8]. Follow-up of 339 patients up to 24 years reported 80% survival and 85% freedom from reoperation. A decade after the first report, more recent results of this initial patient cohort included 131 patients with a mean follow-up of 20 years [9] where freedom from reoperation was 76 and 62% at 10 and 20 years, respectively. Freedom from autograft replacement was 88 and 75% at 10 and 20 years, respectively. The main indication for reoperation was severe regurgitation of neo-aorta in 28/30 patients. At 25 years, the pulmonary homograft was free of replacement in 69% of patients. Late autograft dysfunction is a well-known phenomenon following the root replacement technique. According to Elkins et al. autograft dysfunction in the first six months is a rare phenomenon [5]. At a mid-term follow up of 2.47 years, echocardiographic evaluations of the pulmonary autograft revealed mild aortic insufficiency (graded 1/4) in 39.2 to 53.6% of patients [10]. A total of 3% of patients had moderate insufficiency early after surgery, increasing to 14.3% at 5 years. Over the long term [11], freedom from pulmonary autograft failure was 86 ± 2% at 10 years and 74 ± 5% at 16 years and was similar for children and young adults. Preoperative predictive factors for late valve dysfunction were female gender, age, and dilated aortic valve annulus [11]. It appears that the Ross procedure is a good option to treat aortic valve disease in young adults, but less so in children and neonates [12]. This was confirmed in the sole meta-analysis of the Ross procedure where both surgical techniques were compared [13], reporting that failure of the autograft was higher in children than in young adults. The pooled linearised rates were 1.15%/y in a consecutive series of children and adults, 0.78%/y in adult patients, and 1.38%/y in a paediatric series [13].
It seems that the key issue around why pulmonary root is not considered as an ideal substitute for aortic valve comes from valve insufficiency due to neo-root dilatation which, after the first decade, may be present in more than one third of patients [11]. Unfortunately, in the past the pathophysiology of neo-aorta dilatation was not explored in full detail in order to determine all the information necessary which may prevent this pathology and to position the pulmonary root so that it can be a “for everyone substitute” for aortic valve replacement.

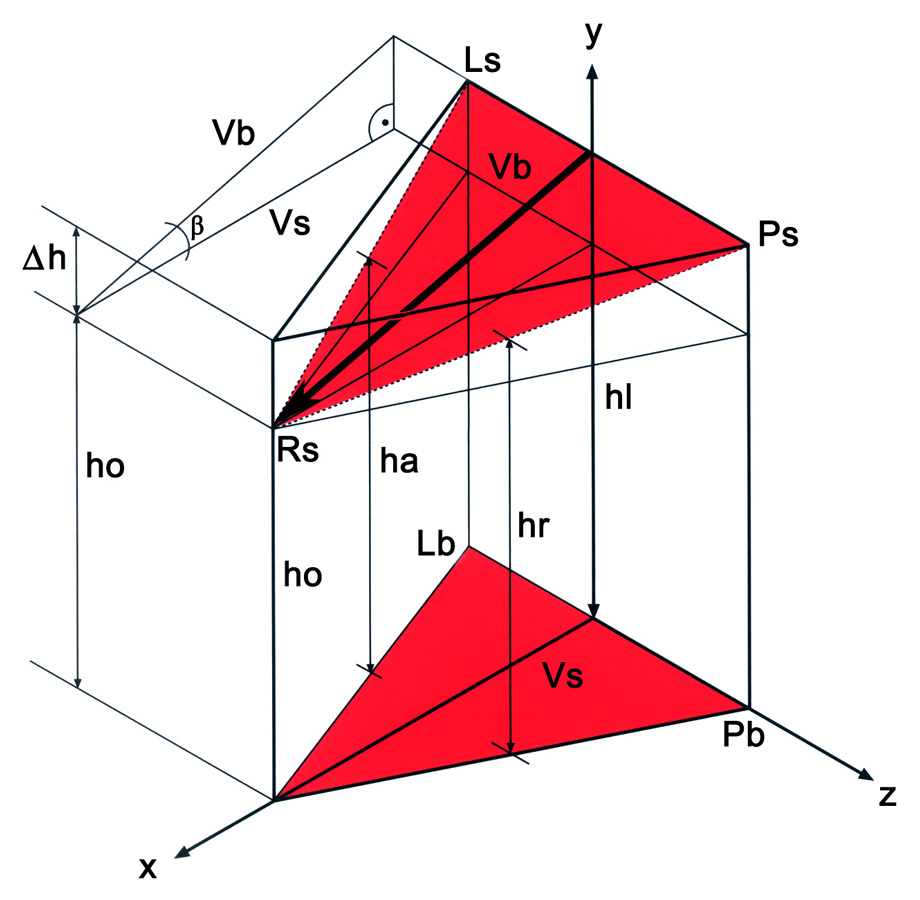
Figure 1
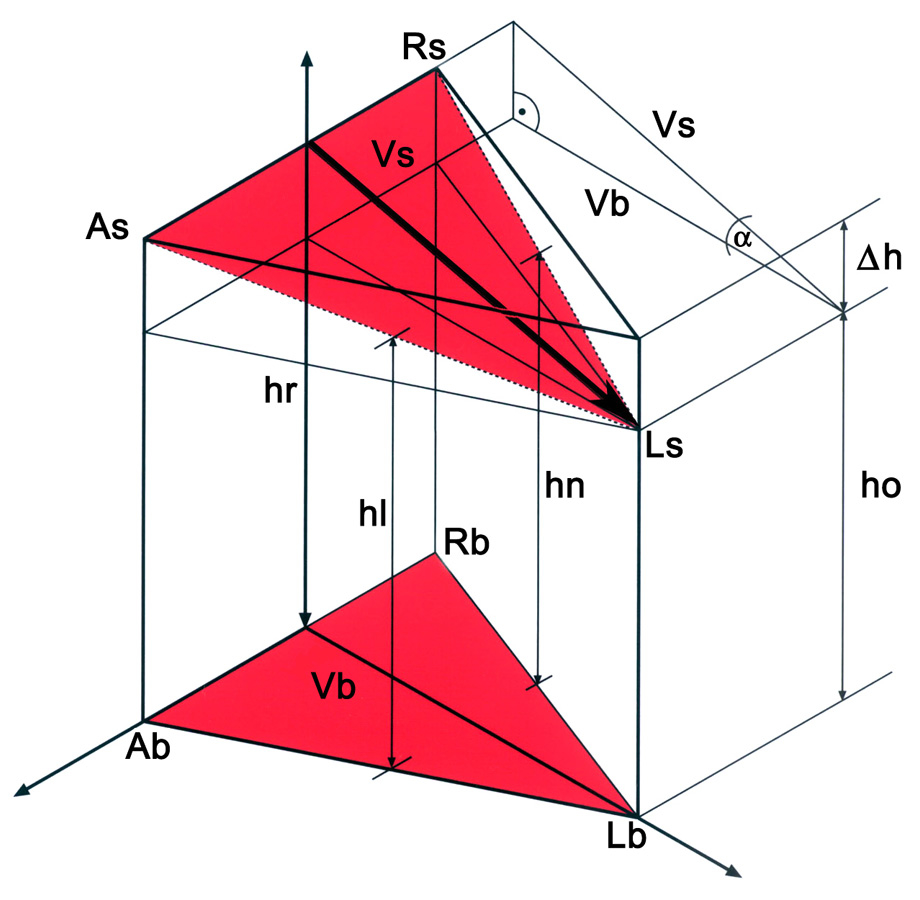
1/A Geometric model of the aortic root. The ‘AsRsLs’ triangle is positioned in the sinutubular junction. The three edges correspond to the anterior (As), right (Rs) and left (Ls) commissures. The triangle at the aortic root base is marked by the ‘AbRbLb’ triangle, where the basal points correspond to the vertical projection of the three commissures into the root base. By interconnecting the points of both triangles, a model of the aortic root is obtained. ‘hr’ corresponds to the height measured in the right sinus; ‘hl’ and ‘hn’ to the heights obtained in the left and non-coronary sinuses, respectively. ‘ho’ is the distance between the ‘Ls’ and ‘Lb’ respectively. ‘∆h’ is calculated as the difference between ‘hr’ and ‘ho’. The ‘Vs’ height of the ‘AsRsLs’ triangle also was calculated. One can observe that the tilt angle between the basal and sinutubular triangles corresponds to the ‘α’ angle in the triangle, which is defined by ‘∆h’ and ‘Vs’. The ‘α’ angle is calculated according to the rules of a right-angle triangle. The tilt angle of the aortic root is 5.47°
1/B Geometric model of the pulmonary root. The ‘PsLsRs’ triangle is found at the level of the sinutubular junction and is defined by the posterior (Ps), left (Rs) and right (Rs) commissures. The triangle at the aortic root base is marked by the ‘PbLbRb’ triangle, where the basal points correspond to projection of the commissures onto the inter-valvular triangles. By interconnecting the mentioned points, a 3-D model of the pulmonary root is obtained. ‘hl’ corresponds to the height measured in the left sinus; ‘ha’ and ‘hr’ to the heights obtained in the anterior and in the right pulmonary sinuses, respectively. ‘ho’ is the distance between ‘Rs’ and ‘Rb’. ‘∆h’ is calculated as the difference between ‘hl’ and ‘ho’. ‘Vs’ is the height of the ‘PsLsRs’ triangle, which also was calculated. One can observe that the tilt angle between the basal and sinutubular triangles corresponds to the ‘β’ angle in the triangle, which is defined by ‘∆h’ and ‘Vs’. The ‘β’ angle is calculated according to the rules of a right-angle triangle. The tilt angle of the pulmonary root is 16.26°.
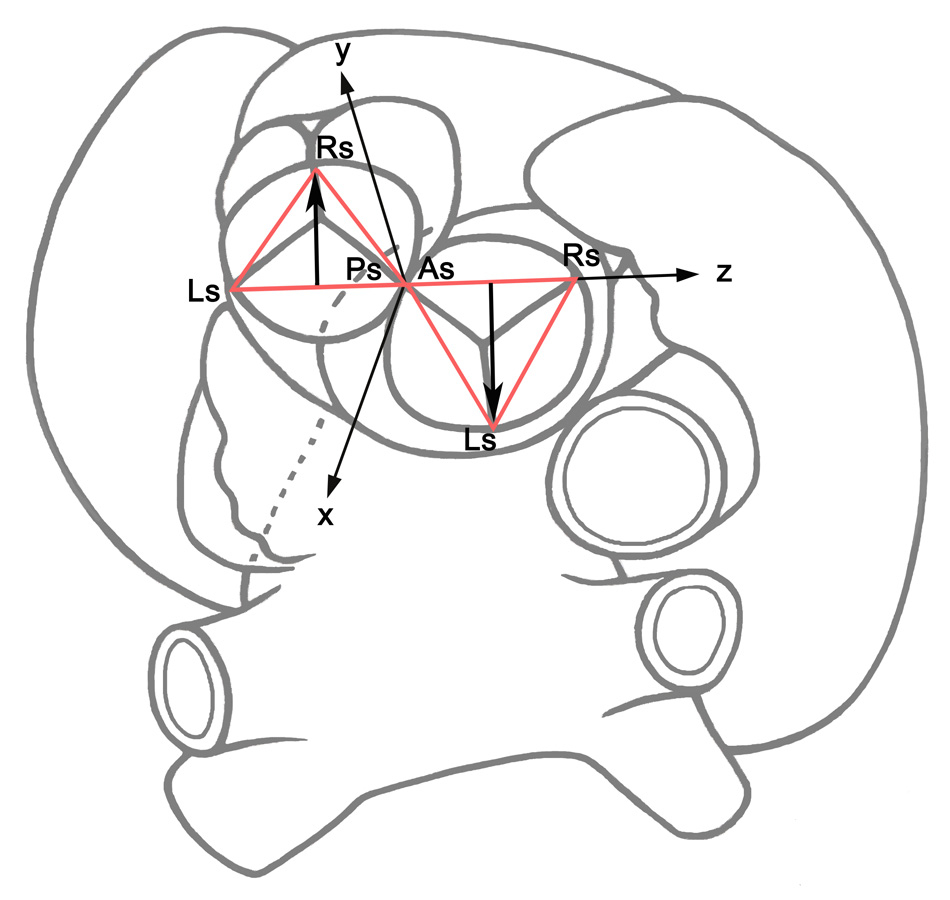
Figure 2
Superior view of the aortic and pulmonary roots. The sinutubular triangles in the aortic (‘AsRsLs’) and pulmonary roots (‘PsLsRs’) are indicated. The root vectors were positioned parallel to each other in opposite directions. The common frame of reference was positioned with its origin just between the posterior commissure of the pulmonary root and the anterior commissure of the aortic root. The x-axis runs toward the left atrium, the z-axis toward the right auricle, and the y-axis is perpendicular to the ‘x-z’ plane. Note that the stretch ‘LsPs’ of the pulmonary root and the stretch ‘AsRs’ of the aortic root are positioned along the z-axis. Consequently, the directions of the root vectors are opposite, but parallel.
At this point of our understanding, since the pulmonary valve is a perfect copy of the aortic valve, it is more than obvious that perfection of the surgical procedure may be the final and most ideal solution for most of our patients needing an aortic valve replacement. Some suggestions do exist in the literature which advocate searching for a solution to prevent long-term neo-aorta dilatation in the morphology of the aortic and pulmonary root [14, 15]. In my personal opinion, we may be on right path to establishing the pulmonary root as an ideal aortic root substitution once we are able to understand the morphological-functional approach in both roots.
The assumption that the geometry and anatomy of aortic root and pulmonary root may play a role in the hemodynamic properties of neo-aorta was first described by David et al. [14]. They postulated that dilatation of the pulmonary autograft is a result of a mismatch between the diameters of the annulus and the sinutubular junction of the aortic and pulmonary roots. Even addressing this discrepancy in diameter, via surgical corrections at the annulus and sinutubular junction, has not prevented late dilatation of the homograft. Despite there being no morphological or physiological data, the authors believed that spatial geometry may influence the dilatation of the neo-aortic root and suggested positioning the left pulmonary sinus into the position of the left coronary sinus as the most functional implantation [14]. In recent literature, there is no evidence of superior hemodynamic performance of homografts associated with implantation of the left pulmonary sinus into the position of the left coronary sinus. We have to note that the suggested orientation does not follow the natural asymmetry of the aortic and pulmonary roots. I agree with the idea that respecting the natural orientation of both roots may influence the function of the pulmonary root in aortic position in order to avoid long-term complications of the Ross procedure.
Lansac went one step further than David, when he integrated the idea of compliant sinuses as the most important pillar of aortic root hemodynamics in his investigations of the Ross procedure [15]. The dynamic differences observed between the pulmonary and the aortic roots at the level of the commissures and sinotubular junction were considered as a probable predicting factor for later dilatation. These might explain global pulmonary root dilatation when subjected to systemic pressure. It is true that one would conclude that these findings of Lansac et al. reinforce the importance of a supra-aortic ridge for proper valve competency and the need for surgical manoeuvres designed to support this area of the autograft.
The authors have suggested implant of the right pulmonary sinus in the position of the right coronary sinus, with the left pulmonary sinus in the position of the non-coronary sinus and the anterior pulmonary sinus in the position of the left coronary sinus [15]. Although these results were suggested to be important in influencing the surgical orientation, there were no hemodynamic measurements performed to test this new implantation mode. Additionally, Lansac’s neo-orientation does not correspond to the orientation of natural pulmonary and aortic roots.
In my previous research, I intensively studied the morphology of the aortic and pulmonary roots. Based on observations, measurements and dissections, I can state that both roots are asymmetric structures and are a mirror image of each other. This is actually not surprising, as one should consider that both roots originate from one common arterial trunk during embryological development and are a result of rotation and septation.
In the normal, healthy aortic root, there are three sinuses: the left coronary sinus (LCS), the right coronary sinus (RCS) and the non-coronary sinus (NCS). The largest is the RCS followed by the NCS and LCS. The difference between the three sinus parameters clearly conveys the asymmetrical shape of the aortic root [16]. Analogue to this in the pulmonary root there are also three sinuses: anterior pulmonary sinus (APS), right pulmonary sinus (RPS) and left pulmonary sinus (LPS). The largest is the LPS followed by RPS and APS [19].
The natural asymmetry of the three sinuses may be expressed in 3-D space as a model with its own direction and orientation [16]. In the case of the aortic root, the vector runs from the highest point of the right coronary sinus towards the left commissure and consequently, the direction may be described as left-posterior (fig. 1A and B). In the pulmonary root, the root vector also begins in the highest point of the left coronary sinus, but it runs towards the right pulmonary commissure. In contrast to the aortic root, the pulmonary root vector exhibits a right-anterior position. Considering this situation, and positioning both vectors in one common orientation, one can note that the directions of the aortic and pulmonary root vectors are opposite [17–19]. Furthermore, positioning the two geometric models into a common x-y-z coordinate system, I observed that the two vectors are parallel to each other and have opposite directions (fig. 2). Translating all these morphological-mathematical findings in the Ross procedure, it is clear that the pulmonary root should be implanted in such a way as to match to the natural geometry of the aortic root.
Namely, the largest pulmonary sinus (the left pulmonary sinus) should be in the position of the largest sinus of the aortic root (the right coronary sinus); the anterior pulmonary sinus should be located in the position of the non-coronary sinus; and the smallest sinus of the pulmonary root, that is, the right pulmonary sinus in the position of the smallest sinus of the aortic root, the left coronary sinus. Doing this the larger neo-sinus will be in direct continuation of convexity of the ascending aorta and the smallest sinus would be positioned on concavity of the ascending aorta. This positioning may contribute to synchrony expansion and dilatation of ascending aorta and of neo-aortic root during the cardiac cycle and may be perhaps the first contribution to avoid later dilatation of neo-root at the level of the sinutubular junction.
As such, this modified Ross procedure would be adapted to the natural geometric and functional conditions ruling in the aortic root and would mean an adjustment feasible for all patient ages with aortic valve disease. Further research is necessary to prove this hypothesis, and it is a long way of experimental and clinical trials.
1 Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): Circulation. 2006;114(5):e84–231.
2 Akins CW, Buckley MJ, Daggett WM, et al. Risk of reoperative valve replacement for failed mitral and aortic bioprostheses. Ann Thorac Surg. 1998;65:1545.
3 Hammermeister K, Sethi GK, Henderson WG, et al. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol. 2000;36:1152.
4 Gallo I, Ruiz B, Duran CM. Clinical experience with the Carpentier-Edwards porcine bioprosthesis: Short-term results (from 2 to 4.5 years). Thorac Cardiovasc Surg. 1983;31:277.
5 Elkins RC, Lane MM, McCue C. Pulmonary autograft reoperation: incidence and management. Ann Thorac Surg. 1996;62:450.
6 Ross DN. Replacement of aortic and mitral valves with a pulmonary autograft. Lancet. 1967;4:2:956–8.
7 Stelzer P, Jones DJ, Elkins RC. Aortic root replacement with pulmonary autograft. Circulation. 1989;80(pt 2):III-209–III-213.
8 Ross D, Jackson M, Davies J. Pulmonary autograft aortic valve replacement: long-term results. J Card Surg. 1991;6:529.
9 Kouchoukos NT, Masetti P, Nickerson NJ, Castner CF, Shannon WD, Dávila-Román VG. The Ross procedure: long-term clinical and echocardiographic follow-up. Ann Thorac Surg. 2004;78:773–81.
10 Takkenberg JJM, Klieverik LMA, Schoof PH, van Suylen RJ, van Herwerden LA, Zondervan PE, et al. The Ross procedure: a systematic review and meta-analysis. Circulation. 2009;119:222–8.
11 Elkins RC, Thompson DM, Lane MM, Elkins CC, Peyton MD. Ross operation: 16-year experience. J Thorac Cardiovasc Surg. 2008;136:623–30.
12 David TE, Omran A, Ivanov J, et al. Dilation of the pulmonary autograft after the Ross procedure. J Thorac Cardiovasc Surg. 2000;119:210.
13 Pasquali SK, Cohen MS, Shera D, Wernovsky G, Spray TL, Marino BS. The relationship between neo-aortic root dilation, insufficiency, and reintervention following the Ross procedure in infants, children, and young adults. J Am Coll Cardiol. 2007;49:1806–12.
14 David TE, Omran A, Webb G, Rakowski H, Armstrong S, Sun Z. Geometrical mismatch of the aortic and pulmonary roots causes aortic insufficiency after the Ross procedure. J Thorac Cardiovascular Surg. 1996;112:1231–9.
15 Lansac E, Lim HS, Shomura Y, et al. Aortic and pulmonary root: are their dynamics similar? Eur J Cardiothorac Surg. 2002;21:268–75.
16 Berdajs D, Turina MI; Operative Anatomy of the Heart Springer Verlag 2011; p 246–251, p; 359–362.
17 Berdajs D, Zünd G, Schurr U, Camenisch C, Turina MI, Genoni M. Geometric models of the aortic and pulmonary roots: suggestions for the Ross procedure. Eur J Cardiothorac Surg. 2007;31(1):31–5. Epub 2006 Nov 28.
18 Berdajs D, Patonay D, Zünd G, Turina MI. Geometrical model of the pulmonary root. J Heart Valve Disease. 2005;14(2):257–60.
19 Berdajs D, Patonay L, Turina M. Clinical anatomy of the aortic root: Cardiovasc Surg. 2002;10(4):320–7.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article were reported.