Carotid artery stenting
DOI: https://doi.org/10.4414/smw.2012.13619
Leo H.
Bonati, Stefan T
Engelter, Philippe A
Lyrer
Summary
Atherosclerotic narrowing (stenosis) of the internal carotid artery accounts for about 10–15% of ischaemic strokes. Carotid endarterectomy (CEA) reduces the risk of stroke in patients with symptomatic stenosis and – to a lesser degree – with asymptomatic carotid stenosis. Endovascular treatment including balloon angioplasty and carotid artery stenting (CAS) has emerged as an alternative to CEA to treat carotid stenosis. The present review summarises the existing evidence on risks and benefits of CAS in comparison with CEA, with a focus on evidence from randomised clinical trials. Across all randomised trials, CEA was associated with a lower risk of peri-procedural stroke or death than CAS, while CAS had lower risks of myocardial infarction, cranial nerve palsy and access site haematoma. The increased stroke risk with CAS is mainly observed in elderly patients; therefore, CAS appears to be a safe option to CEA in younger patients. In the first few years following treatment, both procedures are equally effective in preventing ipsilateral recurrent strokes. Nevertheless, long-term follow-up of ongoing trials must be awaited to investigate whether a potential increase in recurrent stenosis following CAS might limit the long-term effectiveness in stroke prevention. The optimal treatment for asymptomatic carotid stenosis remains to be determined in ongoing clinical trials.
Introduction
In the industrialised world, stroke is the third most common cause of death, the second most common cause of dementia, and the most common reason for acquired disability in adulthood. About 10–15% of ischaemic strokes are caused by focal atherosclerosis and consecutive narrowing (stenosis) of the internal carotid artery. Carotid stenosis is more frequent in men than in women and its prevalence increases with age. Severe asymptomatic carotid stenosis is present in 0.1% of men under the age of 50 years, but in as many as 3.1% of men and 0.9% of women 80 years or older [1]. Impairment of blood flow was long considered to be mainly responsible for the occurrence of cerebral ischaemia in patients with carotid stenosis (haemodynamic hypothesis). Current evidence however, suggests that rupture of the atherosclerotic plaque with embolism of locally formed thrombus or plaque debris, and consecutive occlusion of arteries in the eye or in the brain, is the most important mechanism (embolic hypothesis).
The present review summarises the evidence on endovascular treatment for symptomatic and asymptomatic carotid artery stenosis, including balloon angioplasty and stenting, and ends with the authors’ treatment recommendations. Risks and benefits of endovascular treatment must be compared with the effects of carotid endarterectomy (CEA), the standard procedure to treat carotid stenosis. Therefore, a brief outline of the evidence on CEA is given first.
Endarterectomy versus medical treatment for symptomatic carotid stenosis
Among patients with recent ischaemic symptoms associated with carotid stenosis, the risk of stroke is very high. In clinical trials, symptomatic carotid stenosis is commonly defined as stenosis having caused ischaemic events in the ipsilateral eye (transient monocular blindness: so called amaurosis fugax, or retinal infarcts) or cerebral hemisphere (transient ischaemic attack or stroke) in the past 6 months. Randomised controlled trials which enrolled patients in the 1980ies and early 1990ies clearly established the benefit of CEA for symptomatic carotid stenosis. In The North American Symptomatic Carotid Endarterectomy Trial (NASCET), the cumulative incidence of any ipsilateral stroke (including peri-operative events) in patients with severe symptomatic stenosis (defined as 70% or more luminal narrowing) was reduced from 26% under medical therapy alone to 9% with CEA, after two years (p <0.001) [2]. Marginal benefit was also evident in patients with moderate symptomatic stenosis (50–69% narrowing) after 5 years, in whom surgery reduced the ipsilateral stroke risk from 22.2% to 15.7% (p = 0.045) [3]. In the European Carotid Surgery Trial (ECST), benefit of CEA was only reported in patients with symptomatic carotid stenosis of 80% or more luminal narrowing [4]. However, this discrepancy was largely explained by differences in measurement of the degree of stenosis on angiography between the trials. In the NASCET trial, degree of stenosis was determined by comparing the most narrow vessel diameter at the site of the stenosis with the diameter of the distal normal artery [5]. In the ECST trial, the presumed diameter of the normal artery at the site of stenosis – most often the carotid bulb – was taken as the reference diameter. The method used in the NASCET trial is nowadays the most widely used method for describing the degree of stenosis. In a pooled analysis of the endarterectomy trials, where ECST angiograms were reanalysed using the NASCET method, CEA reduced the combined outcome of peri-procedural stroke or death, or ipsilateral ischaemic stroke up to 5 years after treatment by an absolute difference of 15.9% in patients with severe (≥70%) stenosis and 4.6% in patients with moderate (50–69%) stenosis [6]. The risk of peri-procedural stroke or death (which by definition includes outcome events occurring up to 30 days after treatment) was 7.1% in this pooled analysis, and did not vary significantly with the degree of stenosis.
Endarterectomy versus medical treatment for asymptomatic carotid stenosis
CEA also prevents strokes among patients with asymptomatic carotid disease. In the Asymptomatic Carotid Atherosclerosis Study (ACAS), the 5-year risk of ipsilateral stroke (including any peri-operative stroke or death) was reduced from 11% to 5.1% [7]. In the Asymptomatic Carotid Surgery Trial (ACST), the rate of any stroke up to ten years follow-up (including perioperative death) was reduced from 17.9% to 13.4% [8]. Thus, the absolute reduction in stroke risk achieved with CEA in patients with asymptomatic carotid stenosis was similar to the risk reduction in patients with symptomatic, moderate stenosis. The risks of peri-operative stroke or death in the surgical arms of these trials, 2.3% in ACAS and 2.8% in ACST, were substantially lower than in symptomatic patients.
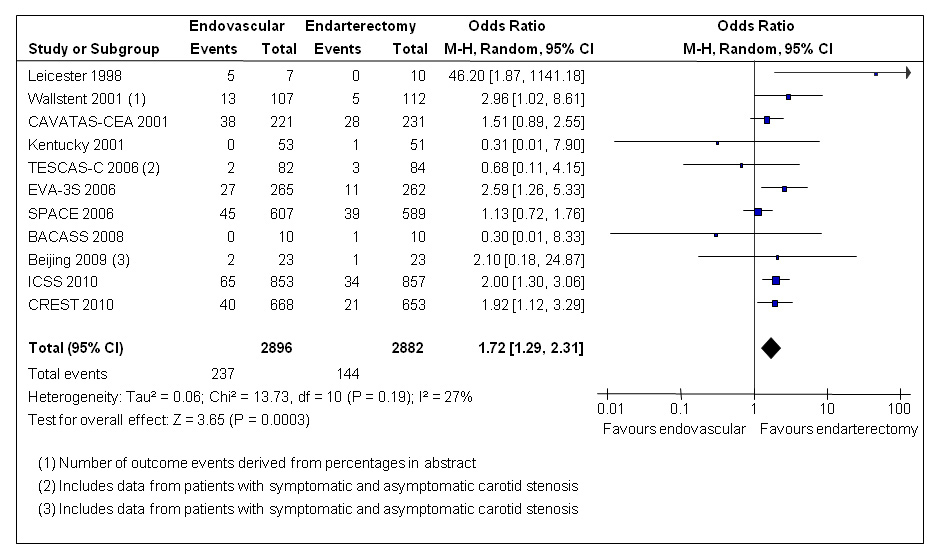
Figure 1
Meta-analysis of randomised trials comparing endovascular treatment with endarterectomy for symptomatic carotid stenosis: any stroke or death between randomisation and 30 days after treatment. Data are numbers of patients with events, total numbers of patients and Mantel-Haenszel random-effects odds ratios including 95% confidence intervals (CI) with endarterectomy as the reference treatment. Squares on the right represent point estimates of odds ratios at trial level, with 95% CI as horizontal bars. The diamond at the bottom represents the summary OR and 95% CI. Data from the following trials are included: Leicester [46], Wallstent [47], CAVATAS [13], Kentucky [48], TESCAS-C [49], EVA-3S [14], SPACE [16], BACASS [50], Beijing [51], ICSS [17], CREST [18].
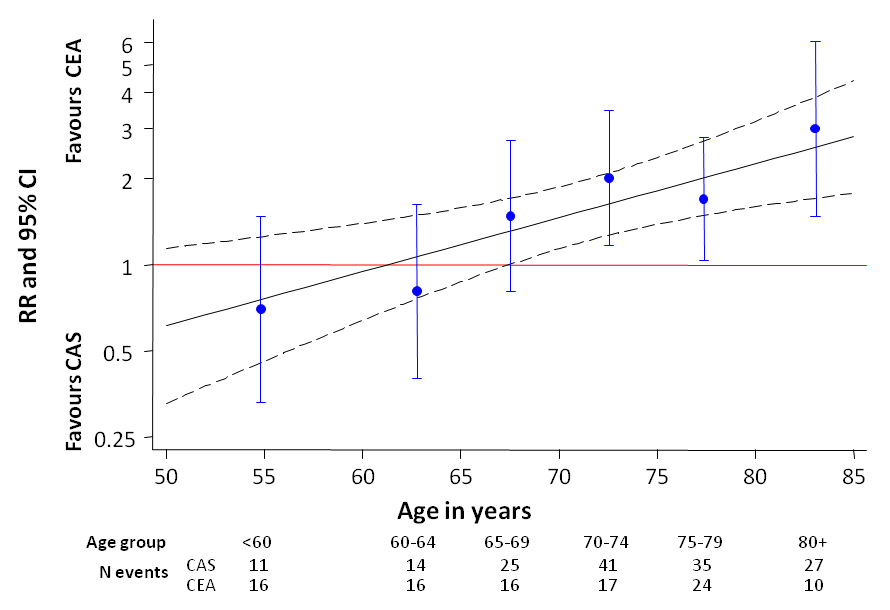
Figure 2
Pooled Individual patient-data meta-analysis of the EVA-3S, SPACE and ICSS trials, comparing primary carotid stenting with endarterectomy for symptomatic carotid stenosis: risk ratio of any stroke or death within 120 days of randomisation according to patient age. (Reproduced from [20]: Bonati LH, Dobson J, Algra A, Branchereau A, Chatellier G, Fraedrich G, et al. Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet. 2010;376(9746):1062–73. © Elsevier, Oxford, UK. Reprint with kind permission.)
CAS =carotid artery stenting; CEA = carotid endarterectomy; RR = risk ratio CAS versus CEA; 95% CI = 95% confidence interval
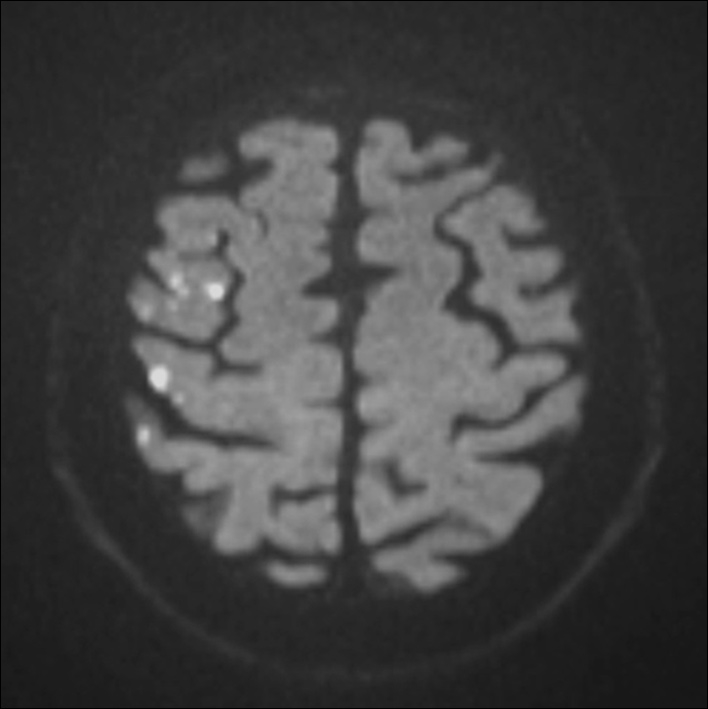
Figure 3
Diffusion weighted MRI of a patient following stenting of the right carotid artery in the MRI substudy of the International Carotid Stenting Study. Multiple hyperintense signals representing acute ischaemic lesions in the territory of the right middle cerebral artery are present. The patient did not experience any symptoms. © Department of Radiology, University Hospital Basel. Reprinted with kind permission.
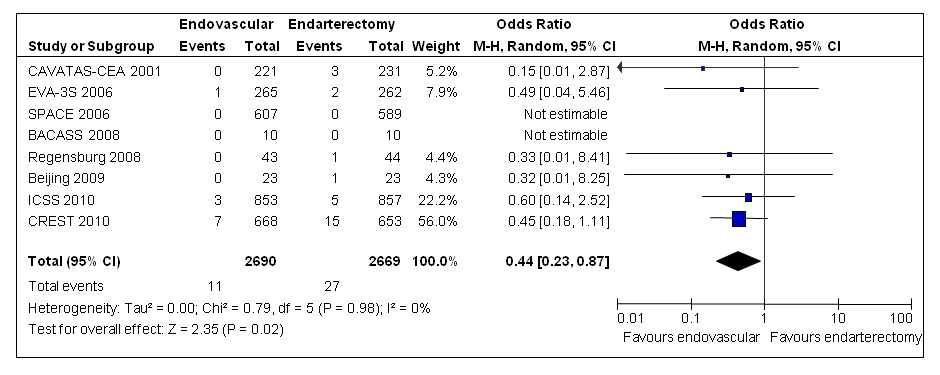
Figure 4
Meta-analysis of randomised trials comparing endovascular treatment with endarterectomy for symptomatic carotid stenosis: myocardial infarction between randomisation and 30 days after treatment. Data are numbers of patients with events, total numbers of patients and Mantel-Haenszel random-effects odds ratios including 95% confidence intervals (CI) with endarterectomy as the reference treatment. Squares on the right represent point estimates of odds ratios at trial level, with 95% CI as horizontal bars. The diamond at the bottom represents the summary OR and 95% CI. Data from the following trials are included: CAVATAS [13], EVA-3S [14], SPACE [15], BACASS [50], Regensburg [52], Beijing [51], ICSS [17], CREST [18].
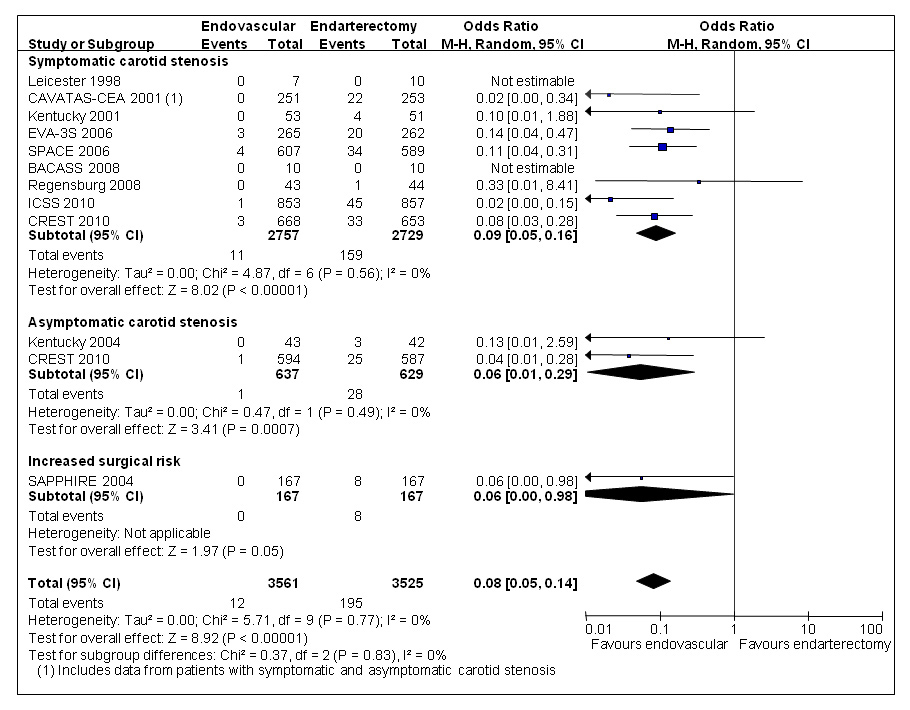
Figure 5
Meta-analysis of randomised trials comparing endovascular treatment with endarterectomy for symptomatic or asymptomatic carotid stenosis: cranial nerve palsy between randomisation and 30 days after treatment. Data are numbers of patients with events, total numbers of patients and Mantel-Haenszel random-effects odds ratios including 95% confidence intervals (CI) with endarterectomy as the reference treatment. Squares on the right represent point estimates of odds ratios at trial level, with 95% CI as horizontal bars. The diamond at the bottom represents the summary OR and 95% CI. Data from the following trials are included: Leicester [46], CAVATAS [13], Kentucky [48, 53], SAPPHIRE [43], EVA-3S [14], SPACE [15], BACASS [50], Regensburg [52], ICSS [17], CREST [18].
Endovascular treatment: balloon angioplasty and stenting
In recent years, endovascular treatment of carotid stenosis has been developed as an alternative to CEA with several potential advantages. Endovascular treatment avoids an incision in the neck with the risk of cranial or cutaneous nerve damage, and potentially reduces general surgical complications, such as myocardial infarction. Surgically inaccessible lesions distant to the carotid bifurcation can be treated, and hospital stay is usually shorter than for surgery. On the other hand, endovascular treatment does not remove the atherosclerotic plaque and may dislodge thrombus or plaque material during the procedure, causing embolic stroke. Another concern has been that residual stenosis after incomplete dilation or recurrence of carotid stenosis might limit the long-term effectiveness of endovascular treatment in preventing strokes.
Endovascular treatment involves insertion of a catheter in the femoral artery at the groin under local anaesthesia, which is then advanced to the site of the stenosis in the internal carotid artery. In the early years of endovascular treatment, the stenosis was dilated by inflating a balloon mounted to the catheter tip (balloon angioplasty), without routine insertion of stents. Primary insertion of stents (carotid artery stenting [CAS]) has since replaced balloon angioplasty as the endovascular technique of choice. Primary stenting has several advantages over balloon angioplasty: the risk of peri-procedural carotid dissection is reduced, and if dissection occurs, adverse consequences such as carotid occlusion or thrombo-embolism might be minimised, because the stent maintains laminar flow across the stenosis and seals the site of dissection [9, 10]. Superior dilation achieved by stenting is also likely to lower the rate of residual or recurrent stenosis compared with balloon angioplasty.
Carotid stenting versus endarterectomy for symptomatic carotid stenosis
Peri-procedural stroke or death
As with CEA, the most frequent major adverse event in CAS is peri-procedural stroke. A vast number of non-randomised single-centre case series and multi-centre registries have reported on the safety of CAS. However, the interpretation of many of these studies is limited by the fact that outcomes were not provided for patients with symptomatic and asymptomatic carotid stenosis separately, and by the inclusion of patients at different risk for surgical complications. The German Prospective Registry of CAS (Pro-CAS) reported outcomes from 2921 procedures in symptomatic and 2412 procedures in asymptomatic patients in 2008 [11]. No restrictions on the indications for CAS, stent types, or use of protection devices were in place. The combined risks of in-hospital stroke or death were 4.3% among patients with symptomatic and 2.7% among patients with asymptomatic stenosis. Importantly however, follow-up of patients was not done by independent neurologists and did not include the full 30-day peri-procedural period. Therefore, these figures are difficult to compare with short-term outcomes of CEA in the large clinical trials.
The question whether endovascular treatment is a safe and effective alternative to CEA could only be answered by randomised trials. Most of the randomised evidence is available from investigator-initiated trials comparing endovascular treatment with CEA in patients with symptomatic carotid stenosis, who were considered equally suitable for both treatments. The first such trial on a larger scale was the Carotid And Vertebral Artery Transluminal Angioplasty Study (CAVATAS). CAVATAS was a family of three trials, the largest of which randomly assigned 505 patients with mainly symptomatic, severe carotid stenosis to either endovascular treatment or CEA, between 1992 and 1997 [12]. More than 90% of patients had ischaemic symptoms associated with carotid stenosis in the previous 6 months before randomisation. Endovascular treatment consisted of balloon angioplasty without stent insertion in the majority of patients. Stents were only used in 23% of patients in the endovascular arm, usually after unsatisfactory results with angioplasty alone. The peri-procedural risk of stroke causing symptoms for more than 7 days or death was 10.0% in both treatment arms. A later analysis of CAVATAS data showed a non-significant increase in stroke risk in the endovascular arm if minor events lasting less than seven days were included [13].
In 2006, two European randomised multi-centre trials published short-term results of the comparison between CAS and CEA for symptomatic carotid stenosis: The Endarterectomy versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial was stopped early by the data monitoring committee after inclusion of 527 patients, because the 30-day stroke or death rate was significantly higher in the CAS arm (9.6%) than in the CEA arm (3.9%, p = 0.01) [14]. The Stent-Supported Percutaneous Angioplasty of the Carotid Artery versus Endarterectomy (SPACE) trial was stopped after inclusion of 1214 patients for both reasons of futility and lack of funding [15, 16]. Short-term outcomes were comparable in both treatments, with risks of death or stroke between randomisation and 30 days after treatment of 7.4% in the CAS group and 6.6% in the CEA group. However, the trial was unable to prove its primary hypothesis that CAS was not inferior to CEA by a predefined margin.
In 2010, an interim analysis of the International Carotid Stenting Study (ICSS), the largest trial comparing CAS versus CEA for symptomatic carotid stenosis with a total of 1713 patients randomised, was published [17]. Within the first 120 days of randomisation, the primary short-term outcome measure, the combination of stroke, myocardial infarction or death, occurred in 8.5% of patients randomised to CAS and 5.2% of patients randomised to CEA (p = 0.006). In the same year, the North American Carotid Revascularization Endarterectomy vs Stenting Trial (CREST), which enrolled 1321 patients with symptomatic and 1181 patients with asymptomatic stenosis, published results up to four years after randomisation [18]. The primary composite endpoint of death, stroke or myocardial infarction between randomisation and 30 days after treatment, or ipsilateral stroke during follow-up, occurred in 7.2% of CAS and in 6.8% of CEA patients. Among patients with symptomatic carotid stenosis, peri-procedural rates of death or stroke in the CAS arm (6.0%) were lower than in the European trials, but still almost twice as high as in the CEA arm of CREST (3.2%, p = 0.02).
To date, 5778 patients with symptomatic carotid stenosis considered to be at standard surgical risk have been randomly assigned to endovascular therapy or endarterectomy in eleven randomised controlled clinical trials, including the recent large CAS trials mentioned above as well as earlier single-centre or small multi-centre trials. In the current update of the Cochrane systematic review of these trials, the combined results show a significant increase in the risk of stroke or death between randomisation and 30 days after treatment with CAS (8.2%) compared with CEA (5.0%; random-effects odds ratio [OR] 1.72, 95% confidence interval [CI] 1.29–2.31, p = 0.0003; fig. 1) [19]. Despite differences in centre selection and treatment techniques between trials, these results consistently show that on average, CEA was the safer treatment.
Comparison of short-term risks in patient subgroups
Due to limitations in sample size, however, single trials could not reliably answer the question whether CAS might still be a safe alternative to CEA in certain patient subgroups. For this reason, the Investigators of EVA-3S, SPACE, and ICSS pooled baseline and outcome data of all 3433 patients randomised to CAS or CEA in these trials in the Carotid Stenting Trialists’ Collaboration (CSTC) [20]. The risk of any stroke or death within the first 120 days of randomisation (the primary short-term outcome event) was 8.9% in the CAS group and 5.8% in the CEA group (risk ratio [RR] 1.53, 95% CI 1.20–1.95, p = 0.0006). Among 16 predefined baseline variables, only age significantly influenced the balance of treatment risks between CAS and CEA: the primary outcome event rate was 12.0% in the CAS group and 5.9% in the CEA group among patients 70 years or older (RR 2.04, 95% CI 1.48–2.82). In contrast, in the younger age group, event rates were nearly identical in patients receiving CAS (5.8%) and those undergoing surgery (5.7%; RR 1.00, 95% CI 0.68–1.47; interaction p-value 0.0053). Figure 2 shows the observed risk ratios for short-term stroke or death with CAS versus CEA in 6 age strata in the CSTC analysis, which increase in a nearly linear fashion with advancing age. This argues for a biologic mechanism mediating the association between age and stroke risk in CAS. Potential mechanisms include vascular anatomy less favourable to CAS, and higher risk of dislodging emboli during catheterisation due to increased burden of atherosclerosis or decreased plaque stability with increasing age. The CSTC analysis showed furthermore that CAS appeared to be as safe as CEA among patients in whom the stenosis initially became symptomatic with a retinal ischaemic event (amaurosis fugax or retinal stroke; primary outcome event 4.8% vs 4.7%, RR 1.03, 95% CI 0.50–2.09), and patients who had a severe stenosis or occlusion of the contralateral carotid artery (primary outcome event 7.7% vs 7.2%; RR 1.06, 95% CI 0.56–2.01). However, these patient subgroups were small and there was no significant interaction between qualifying event type or contralateral carotid disease with the treatment risk ratio.
In the pooled CSTC analysis, there was no significant difference in the occurrence of disabling stroke or death within the first 120 days (CAS: 4.8%, CEA: 3.7%, RR 1.27, 95% CI 0.92–1.74) which highlights the fact that the difference in peri-procedural risks between treatments was mainly driven by a higher occurrence of minor strokes in the CAS group, which did not lead to disability. This observation had also been made in single trials and initially led to concerns that the reported differences in peri-procedural stroke rates might have been subject to ascertainment bias: patients were usually referred to neurology wards after treatment by CAS, where minor stroke symptoms might have been more readily detected than on surgical wards or high-dependency units, where patients were sent to after CEA.
Silent cerebral ischaemia
In carotid revascularisation, cerebral ischaemia may occur without overt symptoms of stroke or TIA. Transcranial Doppler sonography and diffusion-weighted brain imaging (DWI), a magnetic resonance sequence which is highly specific and sensitive to acute cerebral ischaemia, have been used to detect subclinical embolic events in CAS and CEA. A systematic review of non-randomised studies using DWI before and after treatment showed that an average of 37% of patients had new ischaemic lesions after CAS, compared with 10% of patients after CEA [21]. Within ICSS, a multi-centre prospective substudy investigated 124 patients randomised to CAS and 107 patients randomised to CEA with DWI before and after treatment [22]. 50% of CAS patients and 17% of CEA patients had new ischaemic lesions on DWI after treatment (OR 5.21, 95% CI 2.78–9.79, p <0.0001; fig. 3). Since assessment of MRI could be done without knowledge of the allocated treatment, these results argued against the presence of bias explaining the observed differences in rates of clinically manifest strokes. Furthermore, the use of DWI allowed detecting differences between treatments which were consistent with the clinical outcomes of the main trial in only a fraction of the study population. Therefore, DWI may potentially serve as a surrogate outcome measure in future pilot studies evaluating new treatment strategies or investigating disease mechanisms [21].
Myocardial infarction
In the recent randomised CAS trials, stroke was uniformly defined as a focal neurological deficit of vascular cause lasting for at least 24 hours. In contrast, there were important differences in the definition and assessment of myocardial infarction (MI) between trials. In ICSS and EVA-3S, the WHO definition of MI was used which required two out of the following three criteria: prolonged typical chest pain, elevation of specific cardiac enzymes more than twice the upper limit of normal, and specific ECG abnormalities. As patients were not systematically screened with ECG and enzyme measurement before and after treatment, most MI events recorded in those trials can be expected to have been clinically symptomatic.
In contrast, the CREST trial defined MI by a rise in heart enzymes (serum creatinine kinase MB fraction or troponin levels twice the upper limit of normal), in conjunction with either typical chest pain or ECG changes consistent with myocardial ischaemia [18]. Patients were routinely screened with enzyme measurement and ECG before and after treatment. The risks of peri-procedural MI according to the CREST definition were 2.3% in the CAS arm and 1.0% in the CEA arm, among patients with symptomatic carotid stenosis. 15 of 42 peri-procedural MIs (36%) occurring in the CREST study population as a whole were asymptomatic, but no break-down of symptomatic and asymptomatic MIs according to treatment group was provided [23]. This difference in MI risk was not significant but it counterbalanced the significant difference in peri-procedural stroke or death, which was in favour of CEA. Hence, there was no difference in the primary composite outcome in CREST, which included MI. On one hand, MI had less impact on patients’ quality of life than stroke in CREST [18]. On the other hand, the CREST investigators recently reported that mortality during follow-up was increased even among patients with heart enzyme elevation alone [23]. Nevertheless, the inclusion of potentially asymptomatic myocardial ischaemia (favouring CAS) in the composite primary outcome measure of a clinical trial such as CREST should be seen in the context of the large difference in asymptomatic cerebral ischaemia observed in the MRI substudy of ICSS, which favoured CEA [22].
Pooling the results of patients with symptomatic carotid stenosis from eight randomised trials including CREST, the risk of fatal or non-fatal MI up to 30 days after treatment, according to whatever definition was used in the trials, was lower in patients treated endovascularly (0.4%) than among those undergoing CEA (1.0%; random-effect OR 0.44, 95% CI 0.23–0.87, p = 0.02; fig. 4) [19].
Cranial nerve palsy and access site haematoma
Perhaps the most consistent difference in short-term outcomes across randomised trials comparing endovascular treatment with CEA is the higher rate of cranial nerve palsy (CNP) among patients treated surgically. In the updated Cochrane systematic review, CNP was significantly reduced among patients treated endovascularly (0.3%) compared with patients undergoing CEA (5.5%) in 11 trials which were pooled irrespective of symptom status and surgical risk (random-effect OR 0.08, 95% CI 0.05–0.14, p <0.00001; fig. 5) [19]. It is perhaps surprising that CNP may occur with endovascular treatment, at all; reported reasons include iatrogenic carotid artery dissection, or that the randomly assigned endovascular procedure was abandoned and the patient subsequently referred to CEA, which then caused CNP.
Groin haematoma at the site of catheter insertion in endovascular treatment was also less common than neck haematoma in CEA, across nine trials of patients with symptomatic or asymptomatic carotid stenosis (0.9% vs 2.7%, random-effect OR 0.37, 95% CI 0.18–0.77, p = 0.008) [19].
Cerebral protection devices
Cerebral protection devices (CPD) have been introduced in an attempt to prevent distal embolisation of plaque debris or thrombotic material during endovascular treatment of carotid stenosis. This concept was first described in 1996 in a series by Theron et al. [24]. In a systematic review of non-randomised studies including patients with symptomatic or asymptomatic stenosis, 30-day stroke or death rates were 1.8% in patients stented with and 5.5% in patients stented without CPD (p <0.001) [25]. This was mainly due to a difference in stroke occurrence (0.8% vs 4.8%) whereas mortality did not differ. Another systematic review reported a higher proportion of patients having (mostly) silent ischaemic brain lesions on DWI after unprotected CAS than after protected CAS (45% vs 33%; p <0.01) [21]. However, many of the contributing studies compared historical control groups of patients undergoing unprotected CAS against more recent series of protected stenting cases in the same institutions (after routine use of CPD was introduced). Therefore, these comparisons might have been biased by a learning curve effect. In addition, selection of patients with less favourable vascular anatomy for unprotected CAS might have caused further bias.
There have been no randomised clinical trials comparing unprotected versus protected stenting. In EVA-3S, the use of CPDs in the CAS arm was made mandatory after 73 patients had undergone CAS, because an interim analysis showed that strokes occurred four times as often in unprotected procedures than with protection [26]. In the final analysis stroke rates were 25% and 7.9%, respectively [14]. In contrast, CPD use was optional throughout the SPACE trial, and recommended wherever feasible but not mandatory in ICSS. In these trials, there was no difference between patients stented with and those stented without CPD [15, 27]. In CREST finally, a single stent and – wherever feasible – a single filter CPD, which both were provided for the trial by the same manufacturer, were specified to be used in all CAS procedures [18].
The only randomised evidence on CPD effect stems from two small trials using outcome assessment on DWI [28, 29]. In both studies, more patients undergoing protected stenting than patients undergoing unprotected stenting had ischaemic lesions on DWI after treatment. This finding was confirmed in the ICSS-MRI substudy, where the effect of CAS versus CEA on DWI lesion rates was compared between centres having a policy of unprotected stenting and those having a policy of routine CPD use; the odds ratio for having new ischaemic lesions on DWI after CAS versus CEA was 2.70 (1.16–6.24) among the former and 12.20 (4.53–32.84) among the latter centres [22].
The most commonly used CPD types in these studies were distal filter devices. Although these filters might capture some emboli, crossing the stenosis with the device before the filter is deployed might cause additional risk of dislodging plaque or thrombus material. A different approach towards cerebral protection has been taken with proximal occlusion devices which exert a reversal of blood flow across the stenosis during the time of intervention, which in theory should lower the risk of distal embolism. Indeed, the risk for clinically manifest stroke and cerebral ischaemia on DWI in non-randomised studies compared favourably with reported event rates using distal filter devices [30–35]. However, not all patients tolerate flow reversal in the carotid artery. The current evidence provides no final answer as to whether CPD are effective in preventing peri-procedural strokes and to which type of CPD is best.
The role of experience
Another controversial issue is the role operator experience had on the outcomes of CAS in randomised trials. The investigators of the SPACE trial observed an inverse relationship between the total number of patients enrolled at a given centre and the risk of peri-procedural ipsilateral stroke or death in the CAS arm at that centre, suggesting a positive effect of experience [36]. No such relationship was found in the CEA arm. In contrast, those radiologists who had performed the highest numbers of CAS procedures before joining the trial had the most complications in EVA-3S [37]. This seemingly counterintuitive finding, which to a lesser degree was also present in a large CAS registry [38], was perhaps explained by the fact that experienced interventionalists accepted patients with more difficult vessel anatomy in the trial than their less experienced peers. In CREST, which had a rigorous credentialing procedure before admitting a centre into the trial, peri-procedural stroke or death rates in the CAS arm were lower than in the CSTC pooled analysis of European trials, but so was the complication rate with CEA. Including outcome events up to 30 days after treatment, the hazard ratio for peri-procedural stroke or death among symptomatic patients in the CREST trial (1.89) was almost identical to the risk ratio observed in the per-protocol CSTC analysis (1.74) [18, 20]. The CSTC also evaluated outcomes according to centre enrolment; among the larger centres which enrolled more than 50 patients in total into the trials, risk ratios of CAS versus CEA were somewhat lower than among the smaller centres (1.28, 95% CI 0.91–1.79 vs 1.84, 95% CI 1.29–2.61), but still favoured CEA [20].
Thus, while common sense suggests that higher experience at CAS would lower the risk of complications at the interventionalist level (as it has been demonstrated for many surgical procedures), the difference in complication rates between CAS and CEA observed in clinical trials is unlikely to have been entirely explained by lack of experience. The strong effect of patient age, for example, on CAS risk suggests that patient-related factors probably explain most of the peri-procedural risk associated with CAS.
Long-term efficacy of carotid stenting: stroke prevention and recurrent stenosis
The main reason for revascularisation of symptomatic or asymptomatic carotid stenosis is the prevention of stroke in the long term. CEA has been shown to be effective at preventing ipsilateral stroke in symptomatic or asymptomatic carotid stenosis over long-term follow-up periods of 10 years or longer [4, 8]. The only trial on endovascular treatment which has prospectively followed-up patients for a similar period is CAVATAS, with a median duration of 5 years and a maximum of 11 years follow-up [13]: the cumulative 8-year incidence of ipsilateral stroke was 11.3% in the endovascular arm and 8.6% in the surgery arm. Among the newer trials evaluating primary carotid stenting, there were no differences in the medium-term recurrences of ipsilateral stroke between CAS and CEA in EVA-3S (median 3.5, maximum 4 years follow-up), SPACE (2 years follow-up) and CREST (median 2.5, maximum 4 years follow-up), but data beyond four years after treatment are lacking [16, 18, 39]. ICSS has completed long-term follow-up at the end of 2011, 10 years after the first patient had been randomised (median 5 years follow-up), and the results are being analysed at the time of writing of this review.
A particular concern about endovascular treatment has been that residual or recurrent stenosis of the treated carotid artery (here termed “restenosis”) would limit the efficacy of the procedure in preventing strokes. Therefore, all the large multi-centre trials have included duplex ultrasound of the carotid artery at regular intervals after treatment. In the long-term analysis of the CAVATAS trial, the cumulative incidence of severe (≥70%) restenosis 5 years after treatment was 30.7% in the endovascular group compared with 10.5% in the surgery group (p <0.0001) [40]. However, the increase in restenosis occurred largely among patients treated with balloon angioplasty alone (without stent insertion). The subgroup of patients receiving stents had a significantly lower restenosis rate than those treated by balloon angioplasty alone, which was in the range of the surgical arm. Among the newer trials of primary stenting, information on restenosis is only available from shorter observation periods. In the EVA-3S trial, moderate (cumulative 3-year rate 12.5% vs 5.0%, p = 0.02), but not severe restenosis (3.3% vs 2.8%) occurred significantly more often among patients treated by CAS than CEA (mean follow-up 2.1 years) [41]. In SPACE, 10.7% of patients in the CAS arm and 4.6% in the CEA arm had severe restenosis at 2 years (p = 0.0009) [16].
Even though there might be a small excess of severe restenosis following stent treatment, it remains unclear whether restenosis causes stroke. Among patients with severe residual or recurrent stenosis of the treated carotid in the first year, the later risk of ipsilateral stroke or TIA was higher than among patients without restenosis in the CAVATAS trial, but the risk of stroke alone was not significantly elevated [40]. Restenosis in the carotid artery typically occurs in the first 1–2 years after CAS or CEA and is most often caused by neo-intimal hyperplasia (rather than recurrent atherosclerosis) which appears to be less prone to cause arterio-arterial embolism.
Carotid stenting versus endarterectomy for asymptomatic carotid stenosis
Compared with the wealth of data from randomised trials comparing CAS with CEA for symptomatic carotid disease, evidence on endovascular treatment for asymptomatic carotid stenosis is sparse. The largest amount of data stems from the CREST trial, in which 1181 patients with asymptomatic carotid stenosis were randomised between CAS and CEA [18]. The risk of peri-procedural stroke or death did not differ significantly between treatments (CAS: 2.5%, CEA: 1.4%), but the hazard ratio for this outcome, 1.88 (95% CI 0.79–4.42), was almost identical to the hazard ratio among symptomatic patients in the same trial (1.89, 1.11–3.21). Combining peri-procedural events with ipsilateral strokes during follow-up of up to 4 years after treatment, risks were 4.5% versus 2.7% in patients with asymptomatic stenosis (HR 1.86, 0.95–3.66).
Carotid stenting versus endarterectomy in patients at high surgical risk
Several registries have been initiated by companies seeking approval by the US Food and Drug Administration (FDA) for use of stents in patients who are perceived to be at increased risk for complications with surgery. The largest industry-led registry, CAPTURE (Carotid ACCULINK/ACCUNET Post Approval Trial to Uncover Unanticipated or Rare Events),is a post-market surveillance registry in the U.S.A. which enrolled 3500 surgical high-risk patients up to 2007 (482 with symptomatic stenosis ≥50%, and 3018 with asymptomatic carotid stenosis ≥80%), who underwent treatment with the Carotid RX Acculink® stent and the Accunet® filter-type CPD [38]. The risk of peri-procedural stroke or death was 10.6% in patients with symptomatic and 4.9% in patients with asymptomatic stenosis [42].
The first large-scale randomised trial comparing CAS versus CEA, Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE), also included patients at increased surgical risk [43]. Eligibility criteria included significant cardiac or severe pulmonary disease, contralateral carotid occlusion, and stenosis occurring after neck irradiation or previous CEA. 334 patients were included. The trial demonstrated non-inferiority of CAS compared with CEA in the occurrence of the primary composite outcome of death, stroke, or myocardial infarction within 30 days after the intervention or death or ipsilateral stroke between 31 days and 1 year (CAS: 12.2%, CEA: 20.1%, p-value for non-inferiority = 0.004, p-value for superiority = 0.053). However, most of the difference in the primary outcome event rate was attributable to a higher incidence of myocardial infarction in the CEA arm compared with the CAS arm (7.5% vs 3.0%). Of note, the diagnosis of MI merely required a creatinine kinase elevation of more than twice the upper limit of normal with a positive MB fraction.
The results of SAPPHIRE and registry data have been used as a basis to consider CAS as an alternative to CEA in patients with co-morbidities which are known or thought to increase the risk of complications with surgery. However, an important limitation to the applicability of findings from registries such as CAPTURE and the SAPPHIRE trial is the fact that the majority of included patients had asymptomatic carotid stenosis. The benefit of revascularisation in patients with asymptomatic carotid stenosis and concomitant diseases which increase peri-procedural risk or limit life-expectancy is uncertain at best; many such patients might better be managed with optimal medical care alone.
Conclusion and personal recommendations
Randomised controlled trials have consistently shown a higher rate of peri-procedural stroke or death with endovascular treatment (including balloon angioplasty in the early years, and more recently, primary stenting) than with CEA, for treatment of symptomatic carotid stenosis. However, the excess risk with CAS is largely confined to elderly patients; stenting appears to be as safe as CEA in patients younger than about 70 years. CAS reduces the risk of myocardial ischaemia, but this effect was primarily evident in trials where patients were screened with heart enzyme measurement or ECG before and after treatment, which led to the inclusion of asymptomatic events. Other advantages of endovascular treatment include reductions in the risk of cranial nerve palsy and access site haematoma. Primary stenting may be associated with a small increase in restenosis rates, but this has not resulted in a measurable increase in ipsilateral stroke recurrence during medium-term follow-up. Nevertheless, longer-term follow-up data from existing randomised trials are needed.
Stenting may therefore be considered as a safe and (probably) effective alternative to CEA to treat symptomatic carotid stenosis of ≥50% (according to the NASCET measurement of degree) among patients who are younger than about 70 years. In addition, CAS may be considered in patients at increased risk for complications with surgery, patients in whom the stenosis occurred after previous CEA or neck irradiation, and patients with stenoses at surgically inaccessible sites, so long as these patients are considered to benefit from revascularisation. In asymptomatic carotid stenosis, it would be premature to recommend stenting as an alternative to CEA in all or subgroups of patients. Instead, enrolment of patients into ongoing randomised trials which compare CAS versus CEA (such ACST-2 or SPACE-2) should be encouraged [44, 45].
References
1 de Weerd M, Greving JP, Hedblad B, Lorenz MW, Mathiesen EB, O’Leary DH, et al. Prevalence of asymptomatic carotid artery stenosis in the general population: an individual participant data meta-analysis. Stroke. 2010;41(6):1294–7.
2 Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1991;325(7):445–53.
3 Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339(20):1415–25.
4 Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet. 1998;351(9113):1379–87.
5 North American Symptomatic Carotid Endarterectomy Trial. Methods, patient characteristics, and progress. Stroke. 1991;22(6):711–20.
6 Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003;361(9352):107–16.
7 Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273(18):1421–8.
8 Halliday A, Harrison M, Hayter E, Kong X, Mansfield A, Marro J, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet. 2010;376(9746):1074–84.
9 Diethrich EB, Ndiaye M, Reid DB. Stenting in the carotid artery: initial experience in 110 patients. J Endovasc Surg. 1996;3(1):42–62.
10 Roubin GS, New G, Iyer SS, Vitek JJ, Al-Mubarak N, Liu MW, et al. Immediate and late clinical outcomes of carotid artery stenting in patients with symptomatic and asymptomatic carotid artery stenosis: a 5-year prospective analysis. Circulation. 2001;103(4):532–7.
11 Theiss W, Hermanek P, Mathias K, Bruckmann H, Dembski J, Hoffmann FJ, et al. Predictors of death and stroke after carotid angioplasty and stenting: a subgroup analysis of the Pro-CAS data. Stroke. 2008;39(8):2325–30.
12 Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet. 2001;357(9270):1729–37.
13 Ederle J, Bonati LH, Dobson J, Featherstone RL, Gaines PA, Beard JD, et al. Endovascular treatment with angioplasty or stenting versus endarterectomy in patients with carotid artery stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): long-term follow-up of a randomised trial. Lancet Neurol. 2009;8(10):898–907.
14 Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355(16):1660–71.
15 Ringleb PA, Allenberg J, Bruckmann H, Eckstein HH, Fraedrich G, Hartmann M, et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368(9543):1239–47.
16 Eckstein HH, Ringleb P, Allenberg JR, Berger J, Fraedrich G, Hacke W, et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol. 2008;7(10):893–902.
17 Ederle J, Dobson J, Featherstone RL, Bonati LH, van der Worp HB, de Borst GJ, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet. 2010;375(9719):985–97.
18 Brott TG, Hobson RW, Howard G, Roubin GS, Clark WM, Brooks W, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363(1):11–23.
19 Bonati LH, Lyrer P, Ederle J, Featherstone RL, Brown MM. Percutaneous transluminal angioplasty and stenting for carotid artery stenosis. Cochrane Database of Systematic Reviews, update in progress 2012.
20 Bonati LH, Dobson J, Algra A, Branchereau A, Chatellier G, Fraedrich G, et al. Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet. 2010;376(9746):1062–73.
21 Schnaudigel S, Groschel K, Pilgram SM, Kastrup A. New brain lesions after carotid stenting versus carotid endarterectomy: a systematic review of the literature. Stroke. 2008;39(6):1911–9.
22 Bonati LH, Jongen LM, Haller S, Flach HZ, Dobson J, Nederkoorn PJ, et al. New ischaemic brain lesions on MRI after stenting or endarterectomy for symptomatic carotid stenosis: a substudy of the International Carotid Stenting Study (ICSS). Lancet Neurol. 2010;9(4):353–62.
23 Blackshear JL, Cutlip DE, Roubin GS, Hill MD, Leimgruber PP, Begg RJ, et al. Myocardial infarction after carotid stenting and endarterectomy: results from the carotid revascularization endarterectomy versus stenting trial. Circulation. 2011;123(22):2571–8.
24 Theron JG, Payelle GG, Coskun O, Huet HF, Guimaraens L. Carotid artery stenosis: treatment with protected balloon angioplasty and stent placement. Radiology. 1996;201(3):627–36.
25 Kastrup A, Groschel K, Krapf H, Brehm BR, Dichgans J, Schulz JB. Early outcome of carotid angioplasty and stenting with and without cerebral protection devices: a systematic review of the literature. Stroke. 2003;34(3):813–9.
26 Mas JL, Chatellier G, Beyssen B. Carotid angioplasty and stenting with and without cerebral protection: clinical alert from the Endarterectomy Versus Angioplasty in Patients With Symptomatic Severe Carotid Stenosis (EVA-3S) trial. Stroke. 2004;35(1):e18–e20.
27 Doig DD, Featherstone RL, Brown MM. Cerebral protection devices do not protect against peri-procedural stroke: data from the International Carotid Stenting Study. Cerebrovasc Dis. 2010;29(Suppl 2):4–5.
28 Barbato JE, Dillavou E, Horowitz MB, Jovin TG, Kanal E, David S, et al. A randomized trial of carotid artery stenting with and without cerebral protection. J Vasc Surg. 2008;47(4):760–5.
29 MacDonald S, Evans DH, Griffiths PD, McKevitt FM, Venables GS, Cleveland TJ, et al. Filter-protected versus unprotected carotid artery stenting: a randomised trial. Cerebrovasc Dis. 2010;29(3):282–9.
30 Parodi JC, Schonholz C, Parodi FE, Sicard G, Ferreira LM. Initial 200 cases of carotid artery stenting using a reversal-of-flow cerebral protection device. J Cardiovasc Surg. (Torino) 2007;48(2):117–24.
31 Asakura F, Kawaguchi K, Sakaida H, Toma N, Matsushima S, Kuraishi K, et al. Diffusion-weighted MR imaging in carotid angioplasty and stenting with protection by the reversed carotid arterial flow. AJNR Am J Neuroradiol. 2006;27(4):753–8.
32 El-Koussy M, Schroth G, Do DD, Gralla J, Nedeltchev K, von BF, et al. Periprocedural embolic events related to carotid artery stenting detected by diffusion-weighted MRI: comparison between proximal and distal embolus protection devices. J Endovasc Ther. 2007;14(3):293–303.
33 Faraglia V, Palombo G, Stella N, Rizzo L, Taurino M, Bozzao A. Cerebral embolization during transcervical carotid stenting with flow reversal: a diffusion-weighted magnetic resonance study. Ann Vasc Surg. 2009;23(4):429–35.
34 Leal JI, Orgaz A, Fontcuberta J, Flores A, Doblas M, Garcia-Benassi JM, et al. A prospective evaluation of cerebral infarction following transcervical carotid stenting with carotid flow reversal. Eur J Vasc Endovasc Surg. 2010;39(6):661–6.
35 Pinter L, Ribo M, Loh C, Lane B, Roberts T, Chou TM, et al. Safety and feasibility of a novel transcervical access neuroprotection system for carotid artery stenting in the PROOF Study. J Vasc Surg. 2011;54(5):1317–23.
36 Fiehler J, Jansen O, Berger J, Eckstein HH, Ringleb PA, Stingele R. Differences in complication rates among the centres in the SPACE study. Neuroradiology. 2008;50(12):1049–53.
37 Forsting M. Shortcomings and promises of recent carotid-stenting trials. Lancet Neurol. 2007;6(2):101–2.
38 Gray WA, Yadav JS, Verta P, Scicli A, Fairman R, Wholey M, et al. The CAPTURE registry: results of carotid stenting with embolic protection in the post approval setting. Catheter Cardiovasc Interv. 2007;69(3):341–8.
39 Mas JL, Trinquart L, Leys D, Albucher JF, Rousseau H, Viguier A, et al. Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol. 2008;7(10):885–92.
40 Bonati LH, Ederle J, McCabe DJ, Dobson J, Featherstone RL, Gaines PA, et al. Long-term risk of carotid restenosis in patients randomly assigned to endovascular treatment or endarterectomy in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): long-term follow-up of a randomised trial. Lancet Neurol. 2009;8(10):908–17.
41 Arquizan C, Trinquart L, Touboul PJ, Long A, Feasson S, Terriat B, et al. Restenosis is more frequent after carotid stenting than after endarterectomy: the EVA-3S study. Stroke. 2011;42(4):1015–20.
42 Gray WA, Yadav JS, Verta P, Scicli A, Fairman R, Wholey M, et al. The CAPTURE registry: predictors of outcomes in carotid artery stenting with embolic protection for high surgical risk patients in the early post-approval setting. Catheter Cardiovasc Interv. 2007;70(7):1025–33.
43 Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351(15):1493–501.
44 Rudarakanchana N, Dialynas M, Halliday A. Asymptomatic Carotid Surgery Trial-2 (ACST-2): rationale for a randomised clinical trial comparing carotid endarterectomy with carotid artery stenting in patients with asymptomatic carotid artery stenosis. Eur J Vasc Endovasc Surg. 2009;38(2):239–42.
45 Reiff T, Stingele R, Eckstein HH, Fraedrich G, Jansen O, Mudra H, et al. Stent-protected angioplasty in asymptomatic carotid artery stenosis vs endarterectomy: SPACE2 – a three-arm randomised-controlled clinical trial. Int J Stroke. 2009;4(4):294–9.
46 Naylor AR, Bolia A, Abbott RJ, Pye IF, Smith J, Lennard N, et al. Randomized study of carotid angioplasty and stenting versus carotid endarterectomy: a stopped trial. J Vasc Surg. 1998;28(2):326–34.
47 Alberts MJ. Results of a multicentre prospective randomized trial of carotid artery stenting vs carotid endarterectomy. Stroke. 2001;32:325.
48 Brooks WH, McClure RR, Jones MR, Coleman TC, Breathitt L. Carotid angioplasty and stenting versus carotid endarterectomy: randomized trial in a community hospital. J Am Coll Cardiol. 2001;38(6):1589–95.
49 Ling F, Jiao LQ. Preliminary report of trial of endarterectomy versus stenting for the treatment of carotid atherosclerotic stenosis in China (TESCAS-C). Chinese Journal of Cerebrovascular Diseases. 2006;3(1):4–8.
50 Hoffmann A, Taschner C, Engelter ST, Lyrer P, Rem J, Raude EW. Carotid artery stenting versus carotid endarterectomy. A prospective, randomised trial with long termfollow up (BACASS). SchweizerArchiv für Neurologie und Psychiatrie. 2006;157:191.
51 Liu CW, Liu B, Ye W, Wu WW, Li YJ, Zheng YH, et al. Carotid endarterectomy versus carotid stenting: a prospective randomized trial. Zhonghua Wai Ke Za Zhi. 2009;47(4):267–70.
52 Steinbauer MG, Pfister K, Greindl M, Schlachetzki F, Borisch I, Schuirer G, Feuerbach S, Kasprzak PM. Alert for increased long-term follow-up after carotid artery stenting: results of a prospective, randomized, single-center trial of carotid artery stenting vs carotid endarterectomy. J Vasc Surg. 2008;48(1):93–8.
53 Brooks WH, McClure RR, Jones MR, Coleman TL, Breathitt L. Carotid angioplasty and stenting versus carotid endarterectomy for treatment of asymptomatic carotid stenosis: a randomized trial in a community hospital. Neurosurgery. 2004;54(2):318–24.




