
Figure 1
The newly designed CareCentre infection control station, designed by the DOME project and manufactured by Bristol MaidTM. © Bristol MaidTM 2011. Reprinted with kind permission.
DOI: https://doi.org/10.4414/smw.2012.13634
In November 2011, approximately 550 professionals attended the international conference “Patient safety – moving forward!” organised by the Swiss Patient Safety Foundation in Basel. The 2½-day programme included a preconference, 11 plenary talks and 24 parallel sessions designed as minicourses (abstracts are available at http://www.patientensicherheit.ch/dms/de/Kongress/Abstract-Band/Abstract-Band_final/Abstract-Band.pdf). Participants had the opportunity to meet internationally acknowledged patient safety experts and to exchange knowledge and experiences with national and international researchers, practitioners, and health care executives. The talks and presentations covered the entire range of patient safety, from distinct clinical safety concerns and potential solutions, methodological approaches and measurement issues, to education in patient safety, legal aspects, public health strategies and political concepts. Besides well-known, but nevertheless urgent, matters such as medication safety and nosocomial infections, several emerging issues were discussed that have attracted increasing attention recently.
Diagnosis is the centrepiece of medical care. If the diagnosis is wrong the treatment will not be right. Diagnostic errors include heuristics and biases in diagnostic reasoning, failure to order, perform, follow-up and interpret tests at the appropriate time, as well as errors in judgment and decision-making, such as choosing and initiating the right treatment. Research suggests that diagnostic errors pose a common and serious threat to patients. In the Dutch adverse event study based on chart review, adverse diagnostic events occurred in 0.4% of hospital admissions and represented 6.4% of all adverse events [1]. Autopsy studies reveal an error rate of 10–15% [2, 3]. In one of the earlier adverse event studies, the Harvard Medical Practice Study II, diagnostic errors accounted for 17% of preventable adverse events [4]. In a retrospective review of closed malpractice claims 59% involved diagnostic errors that harmed patients [5]. In physicians’ self-reports of diagnostic errors, the latter occurred most frequently in the testing phase (failure to order, report, and follow-up laboratory results) (44%), followed by clinician assessment errors (failure to consider and overweighing competing diagnoses) (32%), history taking (10%), physical examination (10%), and referral or consultation errors and delays (3%) [6]. In a recent analysis of diagnostic errors involving internists, cognitive factors contributed to the diagnostic error in 74% of cases [7]. The most common cognitive problems involved faulty synthesis. Despite this evidence, diagnostic errors have lagged behind other concerns since the start of the safety field [8]. A number of causes may help to illuminate this finding: first, diagnostic errors are among the errors most embarrassing and unacceptable to the medical profession and are often perceived as a lack of clinical competence. Second, errors in diagnosis are hard to detect and identify. In outpatient care in particular, it may take months, if ever, until missed diagnoses are identified [9]. Third, a large proportion of diagnostic errors are due to flawed reasoning and overconfidence rather than technical failures, and there are no easy solutions to hand for these types of error [10, 11]. It is thus not surprising that a number of interventions have been suggested but few empirical studies have yet tested interventions to prevent diagnostic errors and their associated harm [12]. Electronic tracking of patients and implementation of computerised notification systems have been shown to reduce failures in follow-up of test results [12]. Nendaz and colleagues report that a case-based clinical reasoning seminar, designed to give students insight into cognitive features of their reasoning, improved aspects of diagnostic competence [13]. However, effects on safety outcomes still need to be confirmed. Use of checklists to reduce diagnostic errors has also been recommended by experts in the field [14]. Clearly, considerable progress needs to be made in the understanding and prevention of diagnostic errors during the next decade.
A fundamental experience in ethnographic observation of care processes is that the clinical environment, be it a hospital, a medical office, or a nursing home, seems as if it has been designed to provoke errors. In many instances, cutting edge clinical care is provided with infrastructure and equipment that has not fundamentally changed in recent decades. Clinicians that are able figuratively to step back from their environment and look at it with fresh eyes are often overwhelmed by the many major and minor obstacles in everyday processes (and sometimes their triviality) that are like sand in the gearbox: a cable of a diagnostic device that is too short to allow for unrestricted movements; look-alike medication boxes; a walk-through room designated for patient handover; plasma bags whose bar code label is crumpled or covered in a thick layer of ice; non-IV equipment that is connectable with IV equipment; chemotherapy patient records that require the administering nurse to switch and scroll between landscape and portrait paper formats. Nurses and physicians share a wealth of experience as to how they struggle with their work environment, infrastructure, equipment and materials, and they frequently develop work-arounds and short-cuts to bypass these deficiencies. Sometimes with serious risks for patient safety.

Figure 1
The newly designed CareCentre infection control station, designed by the DOME project and manufactured by Bristol MaidTM. © Bristol MaidTM 2011. Reprinted with kind permission.
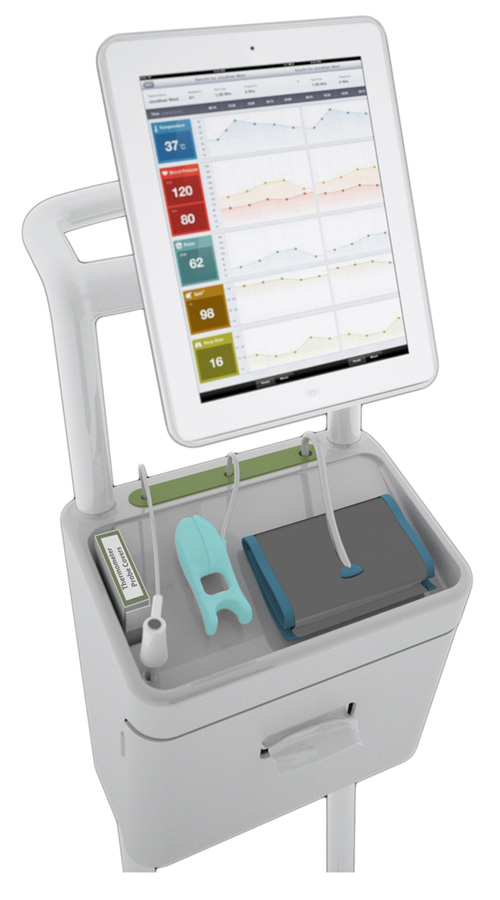
Figure 2
New trolley for bedside monitoring of vital signs with easy-clean design and improved cable management, designed by the DOME project. © The Helen Hamlyn Centre for Design, The Royal College of Art 2011. Reprinted with kind permission.
While the relevance of human factors for the safety of technical devices has been recognised earlier [15], the importance of interior design of rooms, equipment and materials and work flow for patient safety on wards has long been ignored. However, recent research suggests that sometimes simple changes can have substantial effects on safety. For example, Birnbach and colleagues used simulation-based testing in real-size replications of proposed hospital architectural designs [16]. Physicians examined standardised patients in two hospital room replications that differed only by the placement of the alcohol-based hand-rub dispenser. The hand hygiene compliance rate was significantly higher when the dispenser was in clear view of the physicians as they observed the patient (54% sanitised their hands). Only 12% of physicians sanitised their hands when the dispenser was not in their visual field. This is only one example of how thoughtful design features, if properly evaluated and assessed, can make patient safety “easy” for clinicians in their everyday work.
At the Swiss conference, researchers presented results of the DOME project (D esigning Out Medical Error, available at http://www.domeproject.org.uk) [17, 18]. The project aims to increase patient safety on surgical wards by developing equipment and products that fit the requirements of healthcare processes and staff. In prior research five error-prone processes were identified and prioritised for action: hand disinfection, information handover, vital signs monitoring, isolation of infection, and medication delivery. The approach followed by DOME is groundbreaking in several respects: it is truly multidisciplinary and combines expertise from clinicians, safety experts, design specialists, psychologists, and ergonomists. Second, DOME is strongly devoted to research. The team used several methodological approaches to map care processes and understand the work-flow and requirements of health care workers’ tasks. Interventions were designed for each of the error-prone processes and tested in a simulated ward environment in repeated evaluation and improvement cycles. Finally, the research team cooperates with manufacturers to ensure that safe designs are spread and produced en masse. DOME does thus not design nice-looking (but unusable) toys with no added value. Oliver Anderson and his colleagues from Imperial College London presented the outcomes of the DOME project at the Basel patient safety conference to an enthusiastic and excited reception from participants. One of the most powerful products is an all-in-one infection control station for the end of a patient’s bed (fig. 1). The CareCentre is equipped with an alcohol gel dispenser, integrated gloves and aprons, a medication locker, a flat surface for writing, a folder holder, and a touch-free, infrared controlled bin. Infection prevention practices improved significantly with the new CareCenter (53% adherence with CareCentre vs. 16% adherence with control; unpublished data presented at the Basel conference). A new, easy-to-clean trolley was designed for measurement of vital signs at the bedside (fig. 2). Some of the new interventions are currently under investigation in clinical trials, such as a respiratory rate recorder for reliable and accurate manual measurement of vital signs at the bedside. DOME is a promising and innovative project, regarding both the approach to improvements in safety as well as the designed products.
In recent years, methodologically sound studies have shown that improvements in patient safety are feasible and realistic.
Peter Pronovost and colleagues have developed and implemented an evidence-based multifaceted intervention to reduce the incidence of catheter-related bloodstream infections in intensive care units (ICUs) [19]. The programme consists of three core elements: translating evidence into practice at the bedside, improving culture and teamwork, and having a data collection system to monitor central catheter-associated bloodstream infections and other relevant measures [20]. In the initial study more than 100 ICUs in the US implemented specific procedures (hand washing, full-barrier precautions during the insertion of central venous catheters, cleaning the skin with chlorhexidine, avoiding the femoral site, and removing unnecessary catheters) and monitored the effects on infection rates. The median rate of catheter-related bloodstream infection decreased from 2.7 (mean 7.7) infections per 1000 catheter-days at baseline to 0 (mean 2.3) at 0 to 3 months after implementation of the study intervention and was sustained at 0 (mean 1.4) during 18 months’ follow-up. The incidence-rate ratios decreased continuously from 0.62 (at 0 to 3 months after implementation) to 0.34 (at 16 to 18 months). In other words, patients’ risk of acquiring catheter-related bloodstream infection decreased substantially by two thirds. In a retrospective comparative study the project also achieved significant decreases in inpatient mortality in the state of Michigan compared with the surrounding area [21].
In a multinational study Haynes and colleagues investigated the effects of the WHO surgical safety checklist [22]. This 19-item checklist is used at three stages in surgical care (before induction of anaesthesia, immediately before incision, and before the patient leaves the operating room). The checklist is completed by the surgical team and requires oral confirmation of the completion of essential steps in care provision, such as antibiotic prophylaxis, surgical site marking, and team time-out. Considerable improvements in mortality and morbidity were recorded in both low and high income countries, and in emergency operations in particular [23]. Comparable results have been obtained with similar surgical checklists such as the SURPASS tool [24]. A systematic review and meta-analysis estimated that with the use of WHO or SURPASS checklists the relative risk is 0.57 for surgical mortality and 0.63 for any inpatient complication [25]. A recent study confirmed the positive effects of the checklist on patient safety, but only in patients for whom the checklist was fully completed [26]. Patients did not benefit from partial completion or complete noncompliance with the checklist. At the Basel conference, several researchers and practitioners reported local experiences with the checklist.
Other examples of effective patient safety interventions include the large scale introduction of surgical team training to lower surgical mortality [27], a nurse-led programme which reduces medication error rates by implementation of six medication administration safety processes [28, 29], pharmacist-led medication reconciliation [30, 31], and use of information technology [32–34] (table 2). These success stories confirm that interventions exist, can be implemented and can have sustainable, measurable positive effects and make a difference in patient safety. But what have we achieved on the systems level since the US Institute of Medicine published their report “To Err is Human” in 2000 [35]? Has healthcare become safer? Have risks for the “average patient” seeking care significantly declined in recent years? Is it ultimately time to sit back, relax and bring in the harvest?
The evidence on this question is mixed and not as unambiguous and positive as one would hope after reviewing the evidence on successful interventions. On the organisational and systems level, improvements in safety are not easily achieved, and not even easily measured. For example, Landrigan et al. investigated temporal trends in rates of patient harm caused by medical care in North Carolina, a state with above average engagement in safety campaigns [36]. The researchers applied the Institute for Healthcare Improvement’s Global Trigger Tool for Measuring Adverse Events to patient records of patients discharged from 10 randomly selected hospitals between 2002 and 2007. They found harm to be persistently common and no significant decreases in the rate of harms over time. Rates of preventable harms and harms of greater severity also remained stable during the 6-year period. At the Swiss conference Maike Langelaan and colleagues reported the results of two adverse events studies based on chart review methodology conducted in hospitals in the Netherlands [37]. The first Dutch adverse event study conducted in 2004 [38] was followed by several patient safety campaigns and a second adverse event study in 2008. There was no significant decrease in preventable adverse events between the study periods. Charles Vincent presented similar data from France in his plenary talk [39].
Results from the UK “Safer Patients Initiative” (SPI1 and SPI2), a large scale safety improvement programme, are complex and ambivalent [40, 41]. Improvements in safety climate, practices and outcomes have been achieved in both control hospitals and hospitals that participated in SPI. However, only a few selective incremental effects of the initiative were detectable. In other words, improvements over time were observed on many safety items such as consumption of hand disinfectants and simultaneously decreasing rates of Clostridium difficile and methicillin resistant Staphylococcus aureus, but the difference in differences between control and SPI hospitals was not significant. Of 57 quantitative outcome measures, 24 favoured the SPI hospitals, 22 favoured the control hospitals and in 11 outcomes there was no difference at all [41]. One of the major explanations for the counterintuitive results of SPI2 was the spread of contemporaneous policy and professional forces in the control environment. During the study period of SPI other safety campaigns were underway and improvement activities accelerated in many hospitals. Rather than testing the effect of “treatment”, SPI was thus more like evaluating “dose” and it can be concluded that the “dose” given in SPI was too small to achieve incremental effects compared to the control dose.
A number of lessons can be learned from the SPI experience: First, the programme directors and commissioners are to be congratulated on the efforts and resources expended on evaluation and the use of concurrent controls in particular. Without these controls the improvements over time observed in SPI hospitals would have been causally linked to SPI, and may have masked how improvements were achieved, making it even harder to generalise and transfer the approach to other settings. Second, improvement programmes should be designed and interventions should be selected on the basis of baseline data, i.e., actual safety targets. One problem with SPI was that hospitals were already performing to high standards and improvements are then hard to achieve and measure. Third, measurement and data quality control are crucial for any patient safety improvement project and have long been ignored as a key – and resource-intensive – force [42]. Fourth, the SP initiative may have been too diffuse and not well-grounded in theories of organisational change [43]. Interventions and measures need to be evidence-based, pilot-tested and accepted by clinicians. Different areas of patient safety probably need different theories of change, methods, interventions, and measures, but all should be based on “health care delivery” science [44]. The call for more science is justified in particular for large-scale improvement programmes for which we do not yet fully understand what works and why. Ex-post theorisation seems a valuable approach to increasing improvement programmes' generalisability and transferability to other settings [45].
| Table 1: Conference participants’ backgrounds, results from the evaluation survey (n = 327). | |||
| Background characteristic | n | (%) | |
| Female gender | 184 | 57% | |
| Educational background | |||
| Medicine | 93 | 28% | |
| Nursing | 114 | 35% | |
| Pharmacy | 20 | 6% | |
| Quality/risk management | 105 | 32% | |
| Administration, economics, law | 50 | 15% | |
| Other | 44 | 13% | |
| Current workplace | |||
| Hospital | 229 | 70% | |
| Nursing home | 8 | 2% | |
| Outpatient care | 12 | 4% | |
| Public administration | 15 | 5% | |
| Insurance business | 4 | 1% | |
| Consultancy | 12 | 4% | |
| Educational institute | 23 | 7% | |
| Other | 38 | 12% | |
| Table 2: Examples of effective patient safety interventions. | |
| Targeted patient safety problem | Intervention |
| Catheter-related bloodstream infections | Implementation of specific evidence-based procedures, improving culture and teamwork, and infection incidence monitoring [19] |
| Surgical mortality and morbidity | Surgical safety checklists [22, 24] |
| Surgical mortality | Team training [27] |
| Medication errors | Implementation of six medication administration safety processes [28, 29] |
| Adverse drug events | Pharmacist-led medication reconciliation [30, 31] |
| Medication errors / adverse drug events | Information technology, e.g. electronic prescribing [32–34] |
One reason for the observation that system-wide progress in patient safety is slow is that compliance even with simple and inexpensive interventions such as hand disinfection remains low [36] and the penetration of evidence-based safety practices has been quite modest and often needs years of change [46]. It is surprising that this slow and fractious progress seems to be to some extent accepted by health policy leaders and clinicians, given the safety epidemic and the widespread perception of medical error in the general public. For example, in a recent citizen survey conducted in eleven high-income countries, one out of ten citizens reported medical or medication error [47]. However, this rate varied widely between countries (range: 5% in the UK, 11% in Switzerland, 13% in the US, 16% in Norway).
In many countries adoption of safe medication practices has been particularly slow and surveillance data of adverse drug events are generally lacking [46, 48]. Longo at al. report on a survey among all acute care hospitals in Missouri and Utah in 2002 and 2004 which assessed the development, implementation and spread of patient safety systems such as computerised test results, drug storage, administration and safety procedures, or handling of adverse event/error reporting. The results show that improvements have been achieved in many items over time, but are only modest at best. It is striking that in 2002 only 28% of hospitals had a patient safety programme budget. This figure rose to 39% in 2004 but is still disappointing. Clearly, great care must be taken to ensure that the public does not lose confidence in, and patience with, the health care system, and that the safety movement does not end up in circularities.
There are instances in which individual clinicians, providers, executives and directors, health care leaders, and health care system administrations are to be held accountable: for not investing in safety programmes, for not providing their staff with the necessary support and resources, for not teaching and instructing students and residents in the cornerstones of safety, for not implementing evidence-based safety standards, for not using hard edges, and, when all is said and done, for not disinfecting their hands. As Robert Wachter argues, (no) blame needs to be sensitively balanced with accountability [49].
At the Swiss conference, accountability, and sometimes lack thereof, was noticeable. It was noticeable and encouraging in each and every report of clinicians and other professionals that devote their time and efforts to promoting change in patient safety.
However, it was also noticeable in what was not present.
Our subjective impressions at the conference are confirmed by participant evaluation. Table 1 displays background characteristics of conference participants that responded to the electronic evaluation survey (60% response rate). One number in the table is eye-catching, and disturbing: the relatively low fraction of physicians among participants. The limited participation by physicians is not a matter of chance but seems to some extent symptomatic. One common concern of quality and risk managers when discussing opportunities for participation in safety projects or implementation of safety measures is: what can I do to get clinical staff involved? In fact, a major obstacle for any patient safety initiative is the motivation of physicians, and, to a lesser extent, nurses. Hours over hours are spent considering how evidence-based patient safety measures, like the surgical safety checklist or hand hygiene, can be made “tasteful”, what clinicians need to adapt the recommended behaviours, and what incentives hospitals can implement to increase staff compliance. Indeed, sometimes it feels like “pushing an elephant up the stairs”.
Why is this?
The vast majority of clinicians are involved in safety every day. Preventing harm to individual patients is central to most clinicians and belongs to the intrinsic motivation of health professionals. Ironically, the same clinicians are often reluctant to engage in system-wide safety initiatives. To many physicians, saving additional lives (through advanced therapy) seems psychologically more attractive than avoiding deaths through prevention of harm. It is much easier to enthuse surgeons about a new microinvasive device, or innovations such as fast-track surgery, than about the surgical safety checklist, team training, or structured handovers. Asked why he did not attend the Swiss Patient Conference, a senior consultant argued: Yes, it’s certainly interesting. But I do not have the time. Inspiration about patient safety would be a nice-to-have but keeping up with developments in my clinical area is more directly relevant because that is what is expected from me. This statement reveals a fundamental misconception: that patient safety is something “additional” to clinical care provision. In the same way as the safety movement has long failed to embrace science, clinical medicine is still too often failing to embrace the patient safety agenda. The unavailability of safety data at the local level has been identified as a major barrier to clinician engagement in safety [48]. It is simply unacceptable that in many Swiss hospitals, clinicians do not have monitoring data about hand disinfection compliance rates at their department regularly available. Similarly, at most hospitals no data is available regarding medication safety, including safe administration practices, or diagnostic performance. It is hardly possible to engage clinicians in safety if you cannot even document the target. In addition to the data availability problem, clinicians often underestimate the impact of human factors, system and process design, and communication on safety. The best surgeon in the theatre and the best physician on ward is no guarantee of safety if the handover between and across professions is unstructured, incomplete or erroneous. Put simply, safety is more than the sum of dedicated and safe-acting individuals.
From the various reports presented at the Swiss Patient Safety Conference it can be concluded that there is still a long way to go on the safety journey, but considerable improvements are achievable. Collaboration, expertise and engagement from clinicians, safety experts, health services researchers and psychologists is needed – the recent meeting in Basel proved that this community exists – and is growing.
1 Zwaan L, de Bruijne M, Wagner C, Thijs A, Smits M, van der Wal G, et al. Patient record review of the incidence, consequences, and causes of diagnostic adverse events. Arch Intern Med. 2010;170(12):1015–21.
2 Shojania KG, Burton EC, McDonald KM, Goldman L. Changes in rates of autopsy-detected diagnostic errors over time. JAMA: The Journal of the American Medical Association 2003;289(21):2849–56.
3 Sonderegger-Iseli K, Burger S, Muntwyler J, Salomon F. Diagnostic errors in three medical eras: a necropsy study. Lancet. 2000;355(9220):2027–31.
4 Leape LL, Brennan TA, Laird N, Lawthers AG, Localio AR, Barnes BA, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324(6):377–84.
5 Gandhi TK, Kachalia A, Thomas EJ, Puopolo AL, Yoon C, Brennan TA, et al. Missed and delayed diagnoses in the ambulatory setting: a study of closed malpractice claims. Ann Intern Med. 2006;145(7):488–96.
6 Schiff GD, Hasan O, Kim S, Abrams R, Cosby K, Lambert BL, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169(20):1881–7.
7 Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493–9.
8 Wachter RM. Why diagnostic errors don’t get any respect – and what can be done about them. Health Aff. 2010;29(9):1605–10.
9 Gandhi TK, Lee TH. Patient Safety beyond the Hospital. N Engl J Med. 2010;363(11):1001–3.
10 Zwaan L, Thijs A, Wagner C, van der Wal G, Timmermans DRM. Relating faults in diagnostic reasoning with diagnostic errors and patient harm. Acad Med. 2012;87(2).
11 Berner ES, Graber ML. Overconfidence as a cause of diagnostic error in medicine. The American Journal of Medicine 2008;121(5, Supplement):S2-S23.
12 Singh H, Graber ML, Kissam SM, Sorensen AV, Lenfestey NF, Tant EM, et al. System-related interventions to reduce diagnostic errors: a narrative review. BMJ Quality & Safety 2011;doi: 10.1136/bmjqs-2011-000150.
13 Nendaz MR, Gut AM, Louis-Simonet M, Perrier A, Vu NV. Bringing explicit insight into cognitive psychology features during clinical reasoning seminars: a prospective, controlled study. Education for health (Abingdon, England) 2011;24(1):496.
14 Ely JW, Graber ML, Croskerry P. Checklists to Reduce Diagnostic Errors. Acad Med. 2011;86(3):307–13.
15 Sawyer D. Do it by Design. An introduction to human factors in medical devices. Rockville: US Department of Health and Human Services; 1996.
16 Birnbach DJ, Nevo I, Scheinman SR, Fitzpatrick M, Shekhter I, Lombard JL. Patient safety begins with proper planning: a quantitative method to improve hospital design. Qual Saf Health Care. 2010;19(5):462–5.
17 Boyce N. War on error. Lancet. 2012;379(9816):605.
18 Anderson O, Davey G, West J. Make it better. Designing out medical error. London: Helen Hamlyn Centre, Royal College of Art; 2011.
19 Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, et al. An Intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725–32.
20 Sawyer M, Weeks K, Goeschel CA, Thompson DA, Berenholtz SM, Marsteller JA, et al. Using evidence, rigorous measurement, and collaboration to eliminate central catheter-associated bloodstream infections. Crit Care Med. 2010;38(S8):S292–S298.
21 Lipitz-Snyderman A, Steinwachs D, Needham DM, Colantuoni E, Morlock LL, Pronovost PJ. Impact of a statewide intensive care unit quality improvement initiative on hospital mortality and length of stay: retrospective comparative analysis. BMJ. 2011;342:doi: 10.1136/bmj.d219.
22 Haynes AB, Weiser TG, Berry WR, Lipsitz SR, Breizat AH, Dellinger EP, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360(5):491–9.
23 Weiser TG, Haynes AB, Dziekan G, Berry WR, Lipsitz SR, Gawande AA, et al. Effect of a 19-item surgical safety checklist during urgent operations in A Global Patient Population. Ann Surg. 2010;251(5).
24 de Vries EN, Prins HA, Crolla RMPH, den Outer AJ, van Andel G, van Helden SH, et al. Effect of a comprehensive surgical safety system on patient outcomes. N Engl J Med. 2010;363(20):1928–37.
25 Borchard A, Schwappach DLB, Barbir A, Bezzola P. A Systematic review of the effectiveness, compliance, and critical factors for implementation of safety checklists in surgery. Ann Surg. 2012; in press.
26 van Klei WA, Hoff RG, van Aarnhem EEHL, Simmermacher RKJ, Regli LPE, Kappen TH, et al. Effects of the Introduction of the WHO “surgical safety checklist” on in-hospital mortality: a cohort study. Ann Surg. 2012;255(1):44–9.
27 Neily J, Mills PD, Young-Xu Y, Carney BT, West P, Berger DH, et al. Association between implementation of a medical team training program and surgical mortality. JAMA. 2010;304(15):1693–700.
28 Kliger J, Blegen MA, Gootee D, O’Neil E. Empowering frontline nurses: a structured intervention enables nurses to improve medication administration accuracy. Jt Comm J Quality Safety. 2009;35(12):604–12.
29 Kliger J, Singer S, Hoffman F, O’Neil E. Spreading a medication administration intervention organizationwide in six hospitals. Jt Comm J Quality Safety. 2012;38(2):51–60.
30 Kwan Y. Pharmacist medication assessments in a surgical preadmission clinic. Arch Intern Med. 2007;167(10):1034–40.
31 Kaboli PJ HAMBSJ. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;166(9):955–64.
32 Hug B, Witkowski D, Sox C, Keohane C, Seger D, Yoon C, et al. Adverse drug event rates in six community hospitals and the potential impact of computerized physician order entry for prevention. J Gen Intern Med. 2010;25(1):31–8.
33 van Rosse F, Maat B, Rademaker CMA, van Vught AJ, Egberts ACG, Bollen CW. The effect of computerized physician order entry on medication prescription errors and clinical outcome in pediatric and intensive care: a systematic review. Pediatrics. 2009;123(4):1184–90.
34 Westbrook JI, Reckmann M, Li L, Runciman WB, Burke R, Lo C, et al. Effects of two commercial electronic prescribing systems on prescribing error rates in hospital in-patients: a before and after study. PLoS Med. 2012;9(1):e1001164.
35 Institute of Medicine. To err is human. Building a safer health system. Washington, DC: National Academy Press; 2000.
36 Landrigan CP, Parry GJ, Bones CB, Hackbarth AD, Goldmann DA, Sharek PJ. Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363(22):2124–34.
37 Langelaan M, Baines R, de Bruijne M, Broekens M, van de Steeg L, Siemerink K, et al. Monitoring adverse events in hospitals. Paper presented at the Swiss Patient Safety Conference, Basel November 29th 2011. 2011.
38 Zegers M, de Bruijne MC, Wagner C, Hoonhout LHF, Waaijman R, Smits M, et al. Adverse events and potentially preventable deaths in Dutch hospitals: results of a retrospective patient record review study. Qual Saf Health Care. 2009;18(4):297–302.
39 Vincent C. Patient safety past and future. Paper presented at the Swiss Patient Safety Conference, Basel November 29th 2011. 2011.
40 Benning A, Ghaleb M, Suokas A, Dixon-Woods M, Dawson J, Barber N, et al. Large scale organisational intervention to improve patient safety in four UK hospitals: mixed method evaluation. BMJ. 2011;342:doi:10.1136/bmj.d195.
41 Benning A, Dixon-Woods M, Nwulu U, Ghaleb M, Dawson J, Barber N, et al. Multiple component patient safety intervention in English hospitals: controlled evaluation of second phase. BMJ. 2011;342:doi:10.1136/bmj.d199.
42 Needham DM, Sinopoli DJ, Dinglas VD, Berenholtz SM, Korupolu R, Watson SR, et al. Improving data quality control in quality improvement projects. Int J Qual Health Care. 2009;21(2):145–50.
43 Pronovost PJ, Berenholtz SM, Morlock L. Is quality of care improving in the UK? BMJ. 2011;342:doi:10.1136/bmj.c6646.
44 Pronovost PJ, Goeschel CA. Viewing health care delivery as science: challenges, benefits, and policy implications. Health Serv Res. 2010;45(5p2):1508–22.
45 Dixon-Woods M, Bosk CL, Aveling EL, Goeschel CA, Pronovost PJ. Explaining Michigan: ceveloping an ex post theory of a quality improvement ürogram. Milbank Q. 2011;89(2):167–205.
46 Longo DR, Hewett JE, Ge B, Schubert S. The long road to patient safety. JAMA: The Journal of the American Medical Association. 2005;294(22):2858–65.
47 Schwappach DLB. Risk factors for patient-reported medical errors in eleven countries. Health Expect. 2012;doi:10.1111/j.1369-7625.2011.00755.x.
48 Vincent C, Aylin P, Franklin BD, Holmes A, Iskander S, Jacklin A, et al. Is health care getting safer? BMJ. 2008;337:doi:10.1136/bmj.a2426.
49 Wachter RM, Pronovost PJ. Balancing “no blame” with accountability in patient safety. N Engl J Med. 2009;361(14):1401–6.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.