
Figure 1
Study design and patient recruitment. AMI: acute myocardial infarction. PCI: percutaneous coronary intervention. i.c.: intracoronary. BM-MNC: autologous bone marrow derived mononucleated cells. FU: follow-up.
DOI: https://doi.org/10.4414/smw.2012.13632
A 5-year follow-up from the Stem Cell Transplantation in Ischaemic Myocardium Study
Despite major advances in management of acute myocardial infarction (AMI), maladaptive ventricular remodelling process occurs after AMI, leading to congestive heart failure. Recently, reparative therapy by either intramyocardial or intracoronary injection of autologous bone marrow-derived mononucleated cells (BM-MNC) has been proved to be safe and effective when administered after AMI. First-in-man studies conducted between 2002 and 2004 demonstrated significant improvement in left ventricular (LV) function in patients treated in the early phase after AMI [1–4]. Several double-blind placebo controlled randomised clinical trials have however shown less consistent results in terms of improvement of left ventricular (LV) function [5–12] (table 1).
Little is known about the long-term durability of the potential therapeutic effect of regenerative BM-MNC therapy. Assmus et al. reported the 2-year results of the Repair-AMI trial [13] where a sustained improvement in LV function in the BM-MNC treated group could have been shown for a subgroup of the patients undergoing cardiac magnetic resonance imaging (CMR). Likewise, in a larger, non-randomised cohort with chronic ischaemic cardiomyopathy, Strauer et al. [14] showed that intracoronary BM-MNC treatment had a significant effect on quality of life, improvement in LV function and in survival 5 years after initial treatment. In contrast, Meyer et al. recently reported 5-year results of one randomised trial showing that LV function assessed by CMR decreased by about 3 absolute points in both controls and a BM-MNC treated group [15].
We aimed to evaluate long-term (5 years) efficacy results of Stem Cell Transplantation in Ischaemic Myocardium trial (STIM), a single centre trial including patients with recent AMI treated by intracoronary BM-MNC administration.
| Table 1: Randomised clinical trials with intracoronary BM-MNC treatment in the acute phase of myocardial infarction. | |||||
| Study | n | Cell type | Modality | 1° Endpoint | Results |
| BOOST | 60 | BM-MNC | i.c., randomised | Improvement in LVEF (CMR) at 6 months | No safety concern |
| Wollert et al. Lancet 2004 (6 months ) | Significant improvement in LVEF | ||||
| Meyer et al. Circ 2006 (18 months ) | ns (improvement of control group) | ||||
| Meyer et al. EHJ 2009 (5 years) | Decrease of LVEF in control and BM-MNC group | ||||
| Janssens et al. | 67 | BM-MNC | i.c., randomised, double blind | Improvement in LVEF (CMR) at 4 months | No safety concern |
| Lancet 2006 | Placebo controlled | No improvement in LVEF | |||
| Reduction of scar size (DE-CMR) | |||||
| REPAIR AMI | 201 | BM-MNC | i.c., randomised, double blind | Improvement in LVEF (LV-Angio) at 4 months | No safety concern |
| Schächinger et al. NEJM 2006 (4 months ) | Placebo controlled | Significant improvement in LVEF | |||
| Assmus et al. Circ Heart Fail 2010 (2 years) | Sustained improvement in LVEF | ||||
| ASTAMI | 100 | BM-MNC | I.c, randomised | Improvement in EF (CMR) at 6 months | No safety concern |
| Lunde et al. NEJM 2006 | No improvement in LVEF | ||||
| FINCELL study | 80 | BM-MNC after thrombolysis | i.c., randomised | Improvement in LVEF (LV-Angio/echo) at 6 months | No safety concern |
| Huikuri et al. EHJ 2008 | Significant improvement in LVEF | ||||
| Plewka et al. | 60 | BM-MNC | i.c., randomised | Improvement in LVEF (echo) at 6 months | No safety concern |
| Am J Cardiol. 2009 | Significant improvement in LVEF | ||||
| HEBE-Trial | 200 | BM-MNC / EPC | i.c., randomised | Improvement in LVEF (CMR) at 6 months | No safety concern |
| Hirsch et al. EHJ 2010 | No improvement in LVEF | ||||
| REGENT Trial | 200 | BM-MNC / CD 34+ / CXCR4+ | i.c., randomised | Improvement in LVEF (CMR) at 6 months | No safety concern |
| Tendera et al. Eur Heart J 2009 | No improvement in LVEF | ||||
| BONAMI Trial | 101 | BM-MNC | i.c., randomised | Improvement in viability (SPECT) | Positive in multivariate analysis |
| Roncalli et al. EHJ 2010 | Negative in univariate analysis | ||||
| Topcare AMI (5 years results) | 55 | BM-MNC / EPC | i.c., open-labelled | Assessment of LVEF (CMR) at 5 years | No safety concern |
| Leistner DM et al. Clin Res Cardiol 2011 | Non-controlled | Stable LVEF | |||
| Late Time | 87 | BM-MNC | i.c., randomised; 2-3 weeks vs. placebo | Improvement in LVEF (CMR) at 6 months | No safety concern |
| Traverse JH et al. JAMA 2011 | No improvement in LVEF | ||||
| BM-MNC = autologous bone marrow derived mononucleated cells; i.c. = intracoronary; EPC = Endothelial progenitor cells; CD 34+/ CXCR4+ = selected mononucleated cells co-expressing the CD 133 and the CXCR4 receptor; LVEF = left ventricular ejection fraction; CMR = cardiac magnetic resonance imaging; DE = delayed enhancement. | |||||
60 consecutive patients with acute anterior ST-elevation myocardial infarction (STEMI), successfully treated by primary percutaneous coronary intervention, and initial left ventricular ejection fraction (LVEF) <50% , were screened to participate in a prospective single-centre evaluation between 2004 and 2006. Of these patients, 23 (38%) agreed to undergo intracoronary autologous BM-MNC administration (treatment group) whereas 37 patients refused, or had contraindications against BM-MNC therapy. Of those, 19 consented to clinical and echocardiographic data collection, serving as control group (fig. 1). Inclusion and exclusion criteria are summarised in table 2. All the patients received optimal drug therapy including ACE-inhibitors or angiotensin-receptor II blockers, betablockers, statin, aspirin and clopidogrel. They underwent clinical and echocardiographic follow-up at 21 days, 4 months, 12 months, and thereafter every 12 months. All the patients gave oral and written consent to the study. The study protocol was approved by local IRB and by the competent Swiss Federal authority (“Bundesamt für Gesundheitswesen”).

Figure 1
Study design and patient recruitment. AMI: acute myocardial infarction. PCI: percutaneous coronary intervention. i.c.: intracoronary. BM-MNC: autologous bone marrow derived mononucleated cells. FU: follow-up.
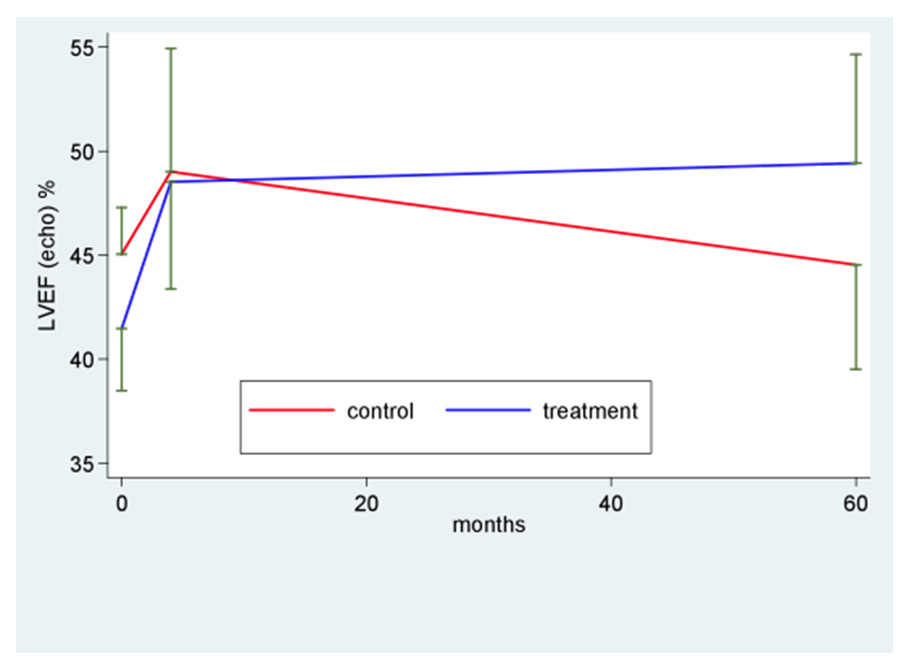
Figure 2
Left ventricular function directly after myocardial infarction (baseline), after 4 and 60 months. LVEF: left ventricular ejection fraction.
Bone marrow (BM) aspiration from the iliac crest was performed under local anaesthesia and the aspirate transferred to an in-house cell processing facility (Cell Therapy Unit of the Cardiocentro Ticino / Molecular Diagnostic Laboratory, Lugano, CH). The mononucleated cell fraction was isolated according to a standard protocol. Briefly, the bone marrow was pre-filtered and then subjected to density gradient centrifugation (ρ = 1.077 g/ml); after washing, the cells were resuspended in 10 ml 5% v/v human albumin and transferred to a 10-ml syringe. An aliquot of the cell product was collected for cell count and viability by Neubauer chamber.
BM-MNC were reinfused at least 3 hours after BM aspiration via an over-the-wire balloon catheter inserted into the infarct related coronary artery, inflated at low pressure (2–4 bar) to completely block blood flow. This manoeuvre was repeated three times for a total administration of 10 ml progenitor cell suspension; between each injection, 5 minutes’ reflow was permitted by deflating the balloon, thus minimising extensive ischaemia. After completion of intracoronary cell reinfusion, coronary angiography was repeated to ascertain vessel patency, absence of embolisation and unimpeded flow of contrast material.
Periprocedural safety of the BM-MNC infusion was monitored by assessment of serum cardiac enzymes and cardiac troponin.
Rest gated myocardial perfusion scintigraphy (G-SPECT) was performed the day before cell delivery and 4 months later. Myocardial G-SPECT acquisition was performed 1 h after IV injection of 800–925 MBq of Tc-99m-sestamibiI using a two-head gamma camera (ECAM Siemens, Erlangen, Germany). Sixty-four images were obtained on a 64x64 matrix for 30 seconds, throughout a 180° arc, beginning from the 45° right anterior oblique projection and ending at the 45° left posterior oblique projection. Transaxial sections were used to generate oblique sections, reoriented along the horizontal and vertical long axis and short axis of the left ventricle. LVEF as well as LV enddiastolic (LVEDV) and endsystolic (LVESV) volumes were determined using the Quantitative Gated SPECT (QGS; Cedars-Sinai Medical Centre) program. We performed a comparison between SPECT and echocardiography as for assessment of LVEF by doing Lin concordance correlation.
Continuous data were described either as median and interquartile range (IQR), or as mean and standard deviation (SD). Categorical data were expressed as counts and percent. They were compared with the Student t test and the Fisher exact test respectively. To evaluate changes over time in LV function and compare them between groups, a general linear regression model was fitted including time, LV function and their interaction. Model of outcome was the value of the LV-function measure at each time. Huber White roust standard errors were computed to account for intra-patient correlation. The Lin concordance correlation coefficient was computed to assess the agreement between SPECT and echo measurements of LVEF. All tests were 2-sided. A p-value <0.05 was considered statistically significant. Raw p-values are presented. Bonferroni correction for post-hoc comparisons should be applied when interpreting results. Stata 11 (StataCorp, College Station, TX, USA) was used for computation.
| Table 2: Inclusion/exclusion criteria of STIM. |
| Inclusion criteria |
| STEMI with visual LVEF at angiogram or echocardiography <50% within 24 h after PCI of the IRA. |
| Treatment by primary PCI within 12 h of chest pain onset or initial treatment with thrombolysis within 12 h followed by PCI within 24 h of chest pain onset. |
| Significant regional LV wall motion dysfunction in the infarct related territory. |
| Age >18 years. |
| Exclusion criteria |
| Abnormal regional wall motion outside the infarct region. |
| Known previous myocardial infarction in the same target vessel. |
| Known preexisting left ventricular dysfunction (EF <50% prior to admission). |
| Need for revascularisation in the non infarct-related coronary within 4 months. |
| Preexisting symptoms of heart failure or known cardiomyopathy. |
| Known active infection or chronic infection with HIV, HBV or HCV.. |
| Chronic inflammatory disease. |
| Serious concomitant disease with a life expectancy of less than one year. |
| Follow-up unlikely (no fixed abode, etc). |
| Severe renal failure (creatinine >250 mmol/l). |
| Relevant liver disease (GOT >2x norm or spontaneous INR >1.5). |
| Anaemia (Hb <8.5 mg/dl), thrombocytopenia (<100.000/µl). |
| Pregnancy. |
Table 3 summarises demographic, clinical, laboratory and echocardiographic characteristics of all patients included. Among the baseline characteristics there was no major difference in patient profile between the 2 groups apart from a trend in favour of higher age and a higher frequency of multivessel disease and more frequent use of drug eluting stents in the control group. Likewise the treatment group showed a trend towards a lower LVEF and significantly higher volumes. The active group was treated with a mean of 158 (80) million mononuclear cells, which were reinfused within a median of 3 days (IQR 2) after AMI, with a mean cell viability of 95% (2%). No bleeding complications or haematoma formation at the iliac crest or in the groin have been observed. TIMI flow of the former infarct related artery was not impeded after injection of BM-MNC, and no rise in cardiac troponin was detected. No malignant ventricular arrhythmias were observed during the in-hospital stay. Mean follow-up time was 70 months in the BM-MNC treated group and 63 months in the untreated control group. At 5 years follow-up (fig. 1) 21 patients were evaluated in the active group (2 patients died) and 11 patients in the control group (7 patients were lost to follow-up or unwilling to present, 1 died 3 years after myocardial infarction).
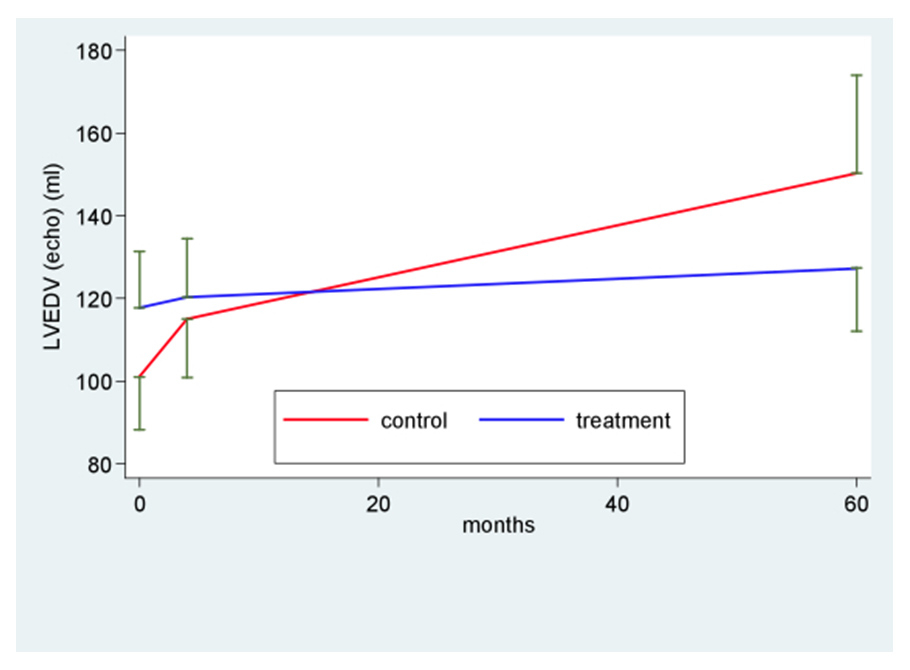
Figure 3
Left ventricular remodelling directly after myocardial infarction (baseline), after 4 and 60 months. LVEDV: left ventricular enddiastolic volume.
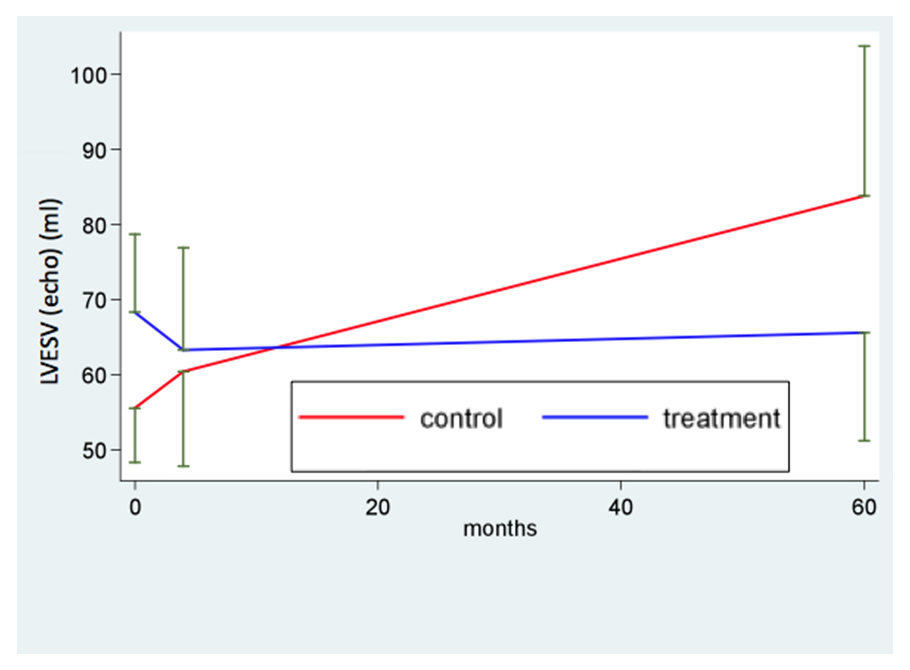
Figure 4
Left ventricular remodelling directly after myocardial infarction (baseline), after 4 and 60 months. LVESV: left ventricular endsystolic volume.
Among the treatment group one patient died 5 months after BM-MNC treatment for end-stage heart failure, listed for heart transplantation. The second patient refused prophylactic ICD implantation and succumbed to sudden cardiac death 24 months after AMI.
A fair agreement between LVEF by G-SPECT analysis and 2D-transthoracic echocardiography was found at baseline and after 4 months (Lin concordance correlation R = 85%, 95%CI 75–95%) and for long term follow up only echocardiography was performed (presented data derives entirely from 2D-echocardiography; detailed data from SPECT can be shown on request).
Baseline LV ejection fraction in both the BM-MNC treated group and in those who refused cell treatment was moderately reduced (fig. 2). At 4 months’ follow-up [16], LVEF at echocardiography increased from 41% to 49% in the treatment group (p <0.001) and from 45% to 49% in the control group (p = 0.31) (fig. 2). At this time point the difference between the treatment and the control group was not statistically significant (p = 0.27).
Long term follow-up (fig. 2; table 4) revealed stable LV function in the BM-MNC treated group (LVEF = 49% at 60 months) whereas LV function in the untreated group decreased to LVEF of 45%. Comparison of time profiles between groups favours BM-MNC treatment (+8.5; 95%CI 2.1; 14.8; p value of interaction = 0.035). Likewise, negative ventricular remodelling was chiefly observed in the control group with a significant increase in LVEDV and LVESV, whereas in the BM-MNC treatment group both LV enddiastolic and endsystolic volume remained stable. Comparison of time profiles between groups favours BM-MNC treatment with a difference of 39.7 ml for LVEDV (p = 0.018) and 31.0 ml for LVESV (p = 0.01) (fig. 3 and 4 respectively, table 4).
| Table 3: Baseline characteristics of the included patients and concomitant therapy. | |||
| Characteristic | BM-MNC (N = 23) | Control ( N = 19) | P value |
| Risk factor | |||
| Age (years) | |||
| Mean | 55 (10.5) | 60.4 (10.9) | 0.13 |
| Median | 56 | 60.5 | |
| Male sex – no. (%) | 18 (78) | 15 (79) | 1.00 |
| Hypertension – no. (%) | 11 (48) | 7 (36) | 0.54 |
| Hyperlipidaemia – no. (%) | 21 (91) | 17 (89) | 1.00 |
| Diabetes – no. (%) | 3 (13) | 2 (11) | 1.00 |
| Smoking (current or former) – no. (%) | 15 (65) | 11 (58) | 0.75 |
| Family history of CAD – no. (%) | 6 (26) | 5 (26) | 1 |
| Coronary artery disease – no. (%) | |||
| 1-vessel disease | 15 (65) | 8 (42) | 0.25 |
| 2-vessel disease | 7 (30) | 8 (42) | |
| 3-vessel disease | 1 (4) | 3 (16) | |
| Infarct treatment | |||
| Primary PCI | 20 (87) | 18 (95) | 0.61 |
| Thrombolysis followed by accelerated PCI of the IRA | 3 (13) | 1 (5) | |
| Infarct related vessel – no. (%) | |||
| LAD | 23 (100) | 19 (100) | 1.00 |
| LCX | |||
| RCA | |||
| PCI for additional stenoses in the non IRA – no. (%) | 8 (35) | 5 (26) | 0.74 |
| Time from symptom onset to first reperfusion therapy – hr | |||
| Mean | 5.9 (3.9) | 4.7 (2.5) | 0.30 |
| Median | 4 | 4 | |
| TIMI flow before PCI | |||
| Mean | 0.3 (0.5) | 0.1 (0.5) | 0.15 |
| Median | |||
| DES – no. (%) | 12 (52) | 15 (79) | 0.04 |
| GP IIb/IIIa inhibitor during primary PCI – no. (%) | 11 (48) | 10 (53) | 1.00 |
| Intravenous catecholamines – no. (%) | 5 (22) | 1 (5) | 0.20 |
| Maximal CK - U/l | |||
| Mean | 3,373 (2,029) | 3,205 (2,047) | 0.79 |
| Median | |||
| Cell therapy | |||
| TIMI flow before study therapy | |||
| Mean | 3 (0) | – | – |
| Median | 3 | – | – |
| TIMI flow after study therapy | |||
| Mean | 3 (0) | – | – |
| Median | 3 | – | – |
| No. of injected cells – x10–6 | |||
| Mean | 158 (80) | – | – |
| Median | 140 | – | – |
| Viability of cells – % | |||
| Mean | 95 (2) | – | – |
| Baseline echocardiograpy | |||
| LVEF – % | |||
| Mean | 41 (7) | 45 (5) | 0.11 |
| Median | 43 | 46 | |
| Enddiastolic volume – ml | |||
| Mean | 118 (31) | 101 (25) | 0.06 |
| Endsystolic volume – ml | |||
| Mean | 68 (24) | 56 (14) | 0.04 |
| Medication at discharge | |||
| Aspirin | 23 (100) | 19 (100) | 1.00 |
| Clopidogrel | 23 (100) | 19 (100) | 1.00 |
| ACE inhibitor or AT II blocker | 17 (74) | 16 (84) | 0.48 |
| Betablocker | 16 (70) | 15 (79) | 0.73 |
| Aldosterone antagonist | 2 (9) | 0.49 | |
| Statin | 21 (91) | 18 (95) | 1.00 |
| Medication at 5 years FU | |||
| Aspirin and/or clopidogrel | 21 (100) | 11 (100) | 1.00 |
| ACE inhibitor or AT II blocker | 17 (81) | 8 (73) | 0.92 |
| Betablocker | 13 (62) | 6 (55) | 0.87 |
| Aldosterone antagonist | 1 (5) | 1(9) | 0.49 |
| Statin | 18 (86) | 8 (73) | 0.90 |
| Table 4: Changes over time in left ventricular function according to BM-MNC treatment. Comparison of changes over time is adjusted for baseline values. | |||||
| LV function (mean, SD) | Control group | Within group difference(95% CI); p-value | BM-MNC group | Within group difference (95% CI); p-value | Comparison of time profiles between groups (95% CI); p-value of interaction |
| LVEF – % Baseline 4 months 60 months | 45 (5) 49 (12) 45 (8) | * – 0.5 (–5.4; 4.4); p = 0.31 ^ 3.9 (-–2.1; 10); p = 0.19 ‡ –4.5 (–10.7; 1.7) p = 0.15 | 41 (7) 49 (12) 49 (11) | * 7.95 (3.5; 12.4); p <0.001 ^ 7.0 (3.6; 10.4); p <0.0001 ‡ 0.9 (–3.8; 5.6); p = 0.69 | * 8.5 (2.1; 14.8); p = 0.035 ^ 2.8 (–3.4; 9.0); p = 0.27 ‡ 7.9 (1.6; 14.3); p = 0.015 |
| LVEDV – ml Baseline 4 months 60 months | 101 (25) 115 (27) 150 (35) | * 49.3 (28.6; 69.9); p <0.001 ^ 14.1 (2.4; 26.8); p = 0.03 ‡ 35.2 (14.2; 56.2); p = 0.003 | 118 (31) 120 (33) 127 (33) | * 9.6 (–8.1; 27.2); p = 0.50 ^ 2.5 (–9; 13.9); p = 0.66 ‡ 7.1 (–5.8; 20); p = 0.27 | * 39.7 (12.8; 66.6); p = 0.018 ^ 12.2 (–4.4; 28.8); p = 0.19 ‡ 24.6 (0.6; 48.6); p = 0.035 |
| LVESV – ml Baseline 4 months 60 months | 56 (14) 60 (23) 84 (30) | * 28.3 (10.4; 46.2); p = 0.003 ^ 4.8 (–7.8; 17.6); p = 0.43 ‡ 23.4 (6.5; 40.3); p = 0.01 | 68 (24) 63 (32) 66 (32) | * –2.6 (–17.8; 12.5); p = 0.72 ^ -5.1 (–12.7; 2.7); p = 0.19 ‡ 2.3 (–10.1; 14.7); p = 0.70 | * 31.0 (7.8; 54.1); p = 0.010 ^ 11.1 (–2.2; 24.4); p = 0.04 ‡ 21.3 (0.5; 42.1); p = 0.05 |
| * Overall (baseline vs. 60 months); ^ baseline vs. 4 months; ‡ 4 months vs. 60 months. | |||||
Little is known about the long-term therapeutic effect of intracoronary BM-MNC injection after acute myocardial infarction. Leistner et al. [17] and Meyer et al. [15] recently presented the 5 years’ follow-up data of the TOPCARE AMI and BOOST trials respectively. Whereas in the first report, in the absence of a control group, a persistent improvement in LVEF was shown for the patients treated with BM-MNC, in the second report a decrease in LV function either in the control or the BM-MNC group was demonstrated by CMR.
Our data 4 months after AMI show recovery of LVEF by 8 absolute points in the BM-MNC treatment group and by 4 absolute points in the control group. The effect was maintained over time exclusively in the BM-MNC treated group, whereas LVEF reduction occurred again in nearly all BM-MNC untreated patients, resulting mainly from a constant increase in LV diastolic volumes. While the difference between control and treatment group at 4 months was not significant, at 60 months the absolute gain in LVEF is significantly higher in the treated population as compared with control.
The mechanism for improved myocardial function and significant volume recovery after acute myocardial infarction in patients treated by intracoronary injection of BM-MNC has not been fully elucidated; it is however likely to be chiefly related to paracrine effects leading to neoangiogenesis in the border zone of the myocardial scar. This mechanism has been reported to play a key role in preserving myocardial function in the preclinical setting, as shown by Olivetti et al. [18] and more recently by Yoon et al. [19] reporting a direct correlation between myocardial recovery from acute infarction and capillary density in the infarct border zone. From a clinical point of view this may result in improved microvascular function as described in a subgroup of the Repair AMI trial [20], possibly resulting in improved regional and global LV function in the short term as shown by some [5, 6, 8, 10, 11] but not all studies published so far. As for the Repair AMI study the improved global LV-function resulted from an abrogation of negative LV-remodelling shortly after the myocardial infarction [21].
Another open question in cell therapy directly after AMI is definition of the optimal time point for cell delivery. The patients in the study presented were treated between 2004 and 2006, a time when little was known of this argument. The relatively early time-point chosen (a median of 3 days after AMI) was, at that time, purely empirical. Later on [22], on the basis of expert consensus, the ideal time point for cell delivery was defined as 3–6 days after AMI. More recently Traverse [23] showed that later treatment (2–3 weeks after AMI) did not result in improved left ventricular function compared to the control group. At present two ongoing studies aim to settle the issue of the optimal time point for BM-MNC administration, with results expected for late 2012 respectively: our own group is co-organising a multicentre trial analysing early (5–7 days) versus late (3–4 weeks) treatment [24], whereas another group aims to compare BM-MNC injection either 3 days or 7 days after AMI [25].
The single centre study described presents several limitations, such as small sample size and its open-labelled, non-randomised design. This may have led to a selection bias with a potentially higher compliance in the treatment group, which may have an influence on long-term outcome and may also explain the substantially higher proportion of loss to follow-up in the control group. In addition, although most of the difference lacks statistical significance, the control group is not perfectly matched to the treatment group, especially for a trend to a higher percentage of 3-vessel disease and higher age in the control group. Likewise, LVEDV and LVESV were significantly higher in the treatment group with a trend towards a lower LVEF. Furthermore, ischaemic post-conditioning, due to the technique of BM-MNC injection in the coronary artery, may have had an effect on the patients in the treatment group. However, after a search of the literature it remains unclear whether this may help [26] or harm [27]. Most of the limitations described may to some extent have influenced the long-term results of the control group but to a much lesser degree those of the active treatment group.
In conclusion, our series, representing one of the earliest cell-based therapeutic approaches in Switzerland, may provide additional evidence that BM-MNC therapy limits the extent of ventricular remodelling after acute myocardial infarction, extending current knowledge by showing that the beneficial effect of BM-MNC is sustained by up to 5 years of follow-up. However, given the only moderate improvement in LV function after BM-MNC administration, future research should focus on enhancing the regenerative capacity of bone marrow-derived progenitor cells [28]. Alternatively, given the limitations of autologous cell-based therapy, the future of regenerative medicine may rather be represented by an allogeneic approach, either by using “off the shelf” mesenchymal stem cell products from young and healthy donors [29] or by directly relying on the paracrine effects of progenitor cells [30].
1 Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106(15):1913–8.
2 Fernandez-Aviles F, San Roman JA, Garcia-Frade J, Fernandez ME, Penarrubia MJ, de la Fuente L, et al. Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction. Circ Res. 2004;95(7):7428.
3 Schachinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004;44(8):1690–9.
4 Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation. 2002;106(24):3009–17.
5 Tendera M, Wojakowski W, Ruzyllo W, Chojnowska L, Kepka C, Tracz W, et al. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur Heart J. 2009;30(11):1313–21.
6 Huikuri HV, Kervinen K, Niemela M, Ylitalo K, Saily M, Koistinen P, et al. Effects of intracoronary injection of mononuclear bone marrow cells on left ventricular function, arrhythmia risk profile, and restenosis after thrombolytic therapy of acute myocardial infarction. Eur Heart J. 2008;29(22):2723–32.
7 Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1199–209.
8 Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1210–21.
9 Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367(9505):113–21.
10 Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364(9429):141–8.
11 Roncalli J, Mouquet F, Piot C, Trochu JN, Le Corvoisier P, Neuder Y, et al. Intracoronary autologous mononucleated bone marrow cell infusion for acute myocardial infarction: results of the randomized multicenter BONAMI trial. Eur Heart J. 2010, Dec 2;
12 Hirsch A, Nijveldt R, van der Vleuten PA, Tijssen JG, van der Giessen WJ, Tio RA, et al. Intracoronary infusion of mononuclear cells from bone marrow or peripheral blood compared with standard therapy in patients after acute myocardial infarction treated by primary percutaneous coronary intervention: results of the randomized controlled HEBE trial. Eur Heart J. 2010, Dec 10;
13 Assmus B, Rolf A, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, et al. Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ Heart Fail. 2010;3(1):89–96.
14 Strauer BE, Yousef M, Schannwell CM. The acute and long-term effects of intracoronary stem cell transplantation in 191 patients with chronic heARt failure: the STAR-heart study. Eur J Heart Fail. 2010;12(7):721–9.
15 Meyer GP, Wollert KC, Lotz J, Pirr J, Rager U, Lippolt P, et al. Intracoronary bone marrow cell transfer after myocardial infarction: 5-year follow-up from the randomized-controlled BOOST trial. Eur Heart J. 2009;30(24):2978–84.
16 Torretta M. Stem cells transplantation after acute myocardial infarction: results of a long term follow-up after autologous bone marrow-derived progenitor cells intracoronary infusion. Cardiovascular Medicine. 2008;Volume 11(Supplementum 16, abstract 126):28.
17 Leistner DM, Fischer-Rasokat U, Honold J, Seeger FH, Schächinger V, Lehmann R, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI): final 5-year results suggest long-term safety and efficacy. Clin Res Cardiol. 2011, Jun 3;
18 Olivetti G, Ricci R, Beghi C, Guideri G, Anversa P. Response of the border zone to myocardial infarction in rats. Am J Pathol. 1986;125(3):476–83.
19 Yoon CH, Koyanagi M, Iekushi K, Seeger F, Urbich C, Zeiher AM, Dimmeler S. Mechanism of improved cardiac function after bone marrow mononuclear cell therapy: role of cardiovascular lineage commitment. Circulation. 2010;121(18):2001–11.
20 Erbs S, Linke A, Schächinger V, Assmus B, Thiele H, Diederich KW, et al. Restoration of microvascular function in the infarct-related artery by intracoronary transplantation of bone marrow progenitor cells in patients with acute myocardial infarction: the Doppler Substudy of the Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI) trial. Circulation. 2007;116(4):366–74.
21 Schächinger V, Assmus B, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, et al. Intracoronary infusion of bone marrow-derived mononuclear cells abrogates adverse left ventricular remodelling post-acute myocardial infarction: insights from the reinfusion of enriched progenitor cells and infarct remodelling in acute myocardial infarction (REPAIR-AMI) trial. Eur J Heart Fail. 2009;11(10):973–9.
22 Bartunek J, Wijns W, Heyndrickx GR, Vanderheyden M. Timing of intracoronary bone-marrow-derived stem cell transplantation after ST-elevation myocardial infarction. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl 1):S52–6.
23 Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA. 2011;306(19):2110–9.
24 Sürder D, Schwitter J, Moccetti T, Astori G, Rufibach K, Plein S, et al. Cell-based therapy for myocardial repair in patients with acute myocardial infarction: rationale and study design of the SWiss multicenter Intracoronary Stem cells Study in Acute Myocardial Infarction (SWISS-AMI). Am Heart J. 2010;160(1):58–64.
25 Traverse JH, Henry TD, Vaughan DE, Ellis SG, Pepine CJ, Willerson JT, et al. Rationale and design for TIME: A phase II, randomized, double-blind, placebo-controlled pilot trial evaluating the safety and effect of timing of administration of bone marrow mononuclear cells after acute myocardial infarction. Am Heart J. 2009;158(3):356–63.
26 Garcia S, Henry TD, Wang YL, Chavez IJ, Pedersen WR, Lesser JR, et al. Long-term follow-up of patients undergoing postconditioning during ST-elevation myocardial infarction. J Cardiovasc Transl Res. 2011;4(1):92–8.
27 Freixa X, Bellera N, Ortiz-Pérez JT, Jiménez M, Paré C, Bosch X, et al. Ischaemic postconditioning revisited: lack of effects on infarct size following primary percutaneous coronary intervention. Eur Heart J. 2012;33(1):103–12.
28 Xu Q, Seeger FH, Castillo J, Iekushi K, Boon RA, Farcas R, et al. Micro-RNA-34a contributes to the impaired function of bone marrow-derived mononuclear cells from patients with cardiovascular disease. JACC. 2012;59(23):2107–17.
29 Vassalli G, Moccetti T. Cardiac repair with allogeneic mesenchymal stem cells after myocardial infarction. Swiss Med Wkly. 2011;141w13209.
30 Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103(11):1204–19.
Funding / potential competing interests: Fondazione Cardiocentro Ticino. No other potential conflict of interest relevant to this article was reported.