Figure 1
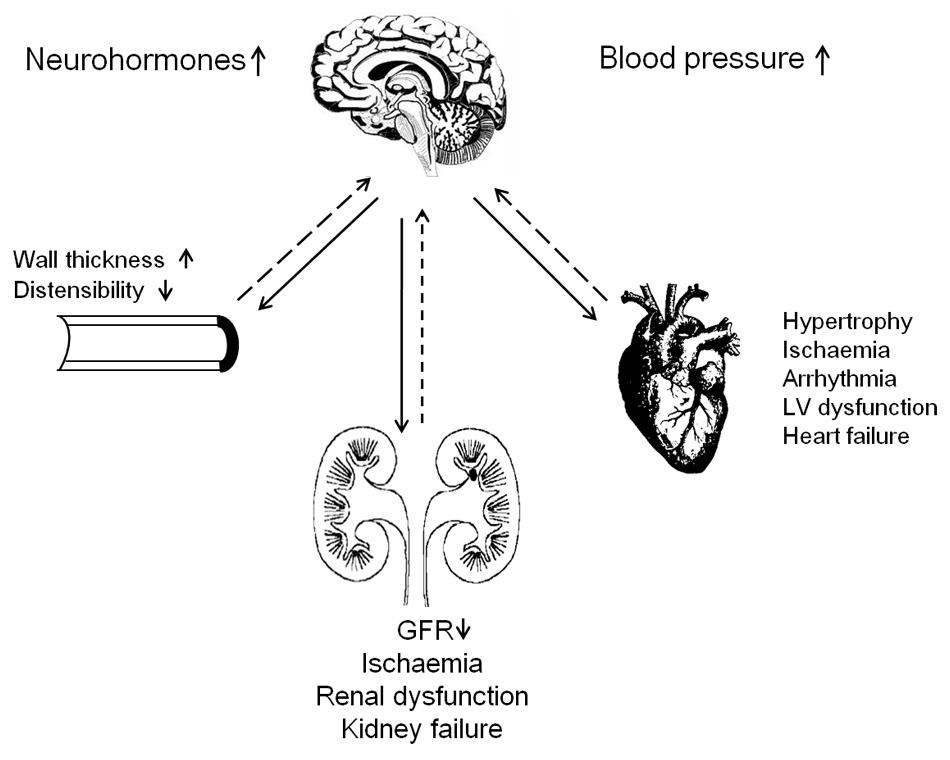
Effect of sympathetic afferent and efferent nerves. Solid and dashed lines represent afferent and efferent nerve traffic, respectively. Abbreviations: LV, left ventricular; GFR, glomerular filtration rate.
DOI: https://doi.org/10.4414/smw.2012.13638
Hypertension affects an estimated 20% to 30% of the world’s adult population [1]. Despite the availability of numerous safe and effective antihypertensive drugs, the percentage of patients achieving adequate blood pressure control meeting guideline targets remains low [1, 2]. Resistant hypertension is a blood pressure that stays above goal in spite of the concomitant use of antihypertensive medications from more than 3 drug classes [3]. Patients who require more than four drug classes to have their blood pressure controlled are also considered to have resistant hypertension. Preferably, the regimen should include a diuretic and all doses should be optimal [3]. Unfortunately, current guidelines for treatment resistant hypertension do not address non-adherence or the white-coat effect as possible underlying causes of so-called treatment resistance.
Renal sympathetic nerves contribute to development and perpetuation of hypertension [4]. The efferent sympathetic nervous outflow to the kidney stimulates renin release, enhances tubular reabsorption of sodium and water, and reduces renal blood flow [4]. Afferent signals from the kidney modulate central sympathetic outflow and thereby contribute to the neurogenic elevation of blood pressure [4]. Excessive activation of the sympathetic nervous system probably contributes to the high blood pressure in treatment-resistant hypertension [5]. Currently, techniques that modulate sympathetic nervous activity via renal denervation [6, 7] possibly offer new avenues for the management of treatment-resistant hypertension. Moreover, other studies, most of them non-randomised [8, 9], suggested that renal denervation cannot only decrease blood pressure in resistant hypertension, but that this procedure might also have indications in the management of glucose intolerance [8], sleep apnoea [8], the polycystic ovary syndrome [10], left ventricular hypertrophy [10], or cardiac diastolic dysfunction [9]. These studies are opening a rapidly expanding area of exciting new research perspectives.
In 2009, Krum and colleagues reported a non-randomised proof-of-concept study (NCT 00483808 and NCT 00664638). The SYMPLICITY HTN-1 study showed that percutaneous radiofrequency catheter-based renal sympathetic denervation was feasible [11]. After the proof-of-concept study [11], the SYMPLICITY HTN-2 investigators published a small randomised clinical trial [12], in which106 (55.8%) patients were randomly allocated to undergo renal denervation plus previous treatment (n= 52) or to maintain previous treatment alone (control group; n= 54) [12]. The primary endpoint was the office blood pressure at 6 months of follow-up. In the renal denervation group, office blood pressure decreased by 32/12 mm Hg (P<0.0001) from the baseline value of 178/96 mm Hg, whereas the corresponding 1/0-mm Hg change from 178/97 to 179/97 mm Hg in the control group was not significant (P ≥0.77) [12].
Subsequently, the SYMPLICITY HTN-1 investigators applied renal sympathetic denervation to 153 patients [13], including the 45 patients from SYMPLICITY HTN-1 study [11]. At 1, 3, 6, 12, 18, and 24 months, the percentage of patients followed up for blood pressure amounted to 90.2, 88.2, 56.2, 41.8, 23.5 and 11.8, respectively [13]. At these time points, the blood pressure reductions averaged 20/10, 24/11, 25/11, 23/11, 26/14, and 32/14 mm Hg [13]. During the first year of follow-up, eGFR remained stable, with a change at 1, 3, 6, and 12 months of +0.1, –1.6, –0.1, and –2.9 mL/min per 1.73 m2, when the percentage of patients remaining in follow-up for renal function was 73.2, 66.7, 56.9, and 41.8 [13]. Only 10 patients (6.5%), had eGFR measured at 2 years. eGFR fell by 16.0 mL/min/1.73 m2 in all patients (table 1) and by 7.8 and 24.2 mL/min/1.73 m2 in patients who did not have (n= 5) or did have (n= 5) a diuretic added to their treatment [13].
| Table 1: Effects of renal denervation on blood pressure and renal function in the Symplicity Registry. | ||||||
| Timeline in months | 1 | 3 | 6 | 12 | 18 | 24 |
| Blood pressure | ||||||
| Number of patients with measurements | 138 (90.2%) | 135 (88.2%) | 86 (56.2%) | 64 (41.8%) | 36 (23.5%) | 18 (11.7%) |
| Mean systolic blood pressure changes (mm Hg) | –20 | –24 | –25 | –23 | –26 | –32 |
| Mean diastolic blood pressure changes (mm Hg) | –10 | –11 | –11 | –11 | –14 | –14 |
| Renal function | ||||||
| Number of patients with measurements | 112 (73.2%) | 102 (66.7%) | 87 (56.9%) | 64 (41.8%) | … | 10 (6.5%) |
| Mean estimated glomerular filtration rate (eGFR) (mL/min/1.73m2) | +0.1 | –1.6 | –0.1 | –2.9 | … | –16.0 |
| Data appear in reference 8. Numbers in parentheses (%) indicate the percentage of patients with follow-up measurements. An ellipsis indicates that the information is unavailable. | ||||||
The SYMPLICITY HTN-1 [11] and SYMPLICITY HTN-2 [12] studies covered only 6 months. The proportion of patients in the SYMPLICITY HTN-1 registry [13] with a follow-up of 1 and 2 years was 41.8% and 11.7% for blood pressure and 41.8% and 6.5% for eGFR (table 1).

Figure 1
Effect of sympathetic afferent and efferent nerves. Solid and dashed lines represent afferent and efferent nerve traffic, respectively. Abbreviations: LV, left ventricular; GFR, glomerular filtration rate.
The definition of treatment-resistant hypertension in the SYMPLICITY reports, although in line with guidelines for the management of hypertension [3, 14, 15] was loose. In SYMPLICITY HTN-1 [11], treatment resistance included intolerance to blood pressure lowering drugs, which often occurs in non-adherent patients. At screening for SYMPLICITY HTN-2 [12], patients recorded the intake of medications for 2 weeks, but the number of patients excluded because of non-adherence was not reported. Moreover, the management of hypertension was suboptimal. The SYMPLICITY researchers did not report how lifestyle measures were reinforced and followed up, for instance by measurement of body mass index or the 24-h urinary sodium excretion [15]. At inclusion, 11% and 5% of the patients enrolled in SYMPLICITY HTN-2 [12] and the SYMPLICITY HTN-1 registry [13] were not taking diuretics, and only 17% and 22% were taking aldosterone antagonists, a drug class strongly recommend in treatment-resistant patients [16], particularly if plasma renin activity is low [17].
In both SYMPLICITY HTN studies [11–12], screening for secondary hypertension was not mandatory and the procedures for a diagnostic workup were not standardised. In SYMPLICITY HTN-1 [11], known secondary hypertension was an exclusion criterion, while secondary hypertension was not even mentioned among the SYMPLICITY HTN-2 eligibility criteria [12].
Poor medication-taking behaviour is a major problem among patients with hypertension, and is one of the main causes of failure to achieve blood pressure control [3]. SYMPLICITY HTN-1 did not address adherence at all [11]. In SYMPLICITY HTN-2 [12], eligible patients had to comply with at least three drugs, including a diuretic. However, this definition was lax, as neither at randomisation nor during follow-up was any attempt made to assess adherence in a systematic manner, for instance by measuring biomarkers of drug intake, drug metabolites or by means of electronic pill boxes [18, 19].
Notwithstanding the overwhelming evidence in favour of the superiority of out-of-the-office blood pressure measurement [20–23], in particular, in treatment-resistant patients [24], in both SYMPLICITY trials [11, 12] and even in the ongoing SYMPLICITY HTN-3 trial (http://clinicaltrials.gov/ct2/show/NCT01418261), the primary endpoint rested on office blood pressure. In SYMPLICITY HTN-1 [11], only 12 of 45 patients (27%) had adequate ambulatory blood-pressure monitoring at baseline and more than 30 days after denervation. The 24-h systolic blood pressure decreased by 11 mm Hg in 9 responders (according to office systolic blood pressure) and by 10 mm Hg in 3 non-responders. In SYMPLICITY HTN-2 [12], all eligible patients received an Omron HEM-705 monitor to record seated blood pressure daily for 2 weeks, 3 times in the morning and 3 times in the evening. The home blood pressure fell by 20/12 mm Hg in 32 patients in the renal denervation group, compared with a rise of 2/0 mm Hg in 40 controls, resulting in a between-group difference of 22/12 mm Hg (P <0.0001) [12]. At 6 months, the 24-h blood pressure decreased by 11/7 mm Hg in 20 patients randomised to renal denervation and did not change (–3/–1 mm Hg) in 25 control patients resulting in a between-group difference of 14/8 mm Hg (P ≤0.02) [12]. The SYMPLICITY HTN-2 investigators did not report the baseline values of the ambulatory or self-measured blood pressures, so that the prevalence of white-coat hypertension cannot be assessed. In summary, in the SYMPLICITY studies [11, 12], out-of-the office blood pressure was not documented at recruitment, white-coat hypertension was not an exclusion criterion, and results based on the 24-h ambulatory blood pressure were available in less than half of the patients.
Animal studies on the safety of the SYMPLICITY™ Catheter System are scarce. Only in 2011, after publication of SYMPLICITY HTN-2 [12] and after the catheter had obtained a EC label in Europe, Rippy and coworkers [25] published results obtained 4 years earlier in 7 swine. In animals sacrificed 6 months after the procedure, the renal arteries showed fibrosis of 10–25% of the total media and underlying adventitia, with mild disruption of the external elastic lamina. In the SYMPLICITY studies [11, 12], imaging of the renal arteries was neither standardised in terms of the technique used at baseline and follow-up nor in terms of the operators, an issue that might be most relevant for duplex imaging.
Future trials of renal denervation in the management of treatment-resistant hypertension should address the following issues:
1 Studies should be randomised with blinded assessment of the primary and secondary endpoints.
2 Renal denervation should be only offered to carefully selected patients. Secondary hypertension and non-adherence should be ruled out. Hypertension should be confirmed by out-of-the office measurement. Treatment should not only include diuretics, but also aldosterone antagonists.
3 Future studies should clarify to what extent changes in the circulating volume, sodium and fluid homeostasis play a role in the blood pressure response to renal denervation and identify the haemodynamic mechanisms underlying the antihypertensive effect, which in most cases requires several months to be fully established.
4 As highlighted by the SYMPLICITY HTN-1 investigators [13], an outstanding question with regard to renal denervation in general and the radio-frequency approach taken in particular is the durability of the blood pressure lowering effect. Efferent nerves can regrow over a period of months to years [6, 26].
5 In view of decline of renal function at 2 years, as reported by the SYMPLICITY HTN-1 investigators [13], long term follow-up of renal function and the integrity of the renal arteries is of major importance.
6 The evidence available from the SYMPLICITY studies [11–13] was obtained with the first-generation 8 French compatible Ardian® catheter, which had a design different from the currently marketed 6 French devices. Newer ablation systems are being tested and will soon be released to the market. Trials comparing different denervation systems should focus on safety and the measurement of the activity of the sympathetic nervous system.
Renal denervation seems to be a procedure promoted by marketers without conclusive supporting evidence from long-term randomised clinical trials. A similar situation occurred with devices used for closure of a patent foramen ovale. Several devices not approved by the Food and Drug Administration were available for the prevention of recurrent stroke [27]. Evidence in support of the use for stroke prevention had only been provided by small and poorly controlled observational studies [28]. The CLOSURE I trial [29] was a large-scale, randomised study comparing device closure with the best medical therapy in patients with a patent foramen ovale, who have sustained a previous stroke or a transient ischaemic attack (TIA). The investigators reported that the 2 treatment groups did not differ in terms of the reduction in the risk of stroke, TIA or death [29]. Moreover, closure of the patent foramen ovale increased the risks of major vascular events and of atrial fibrillation [29].
For now, renal denervation should remain the last resort therapy in adherent and truly resistant patients with severe hypertension, in whom all other efforts to reduce blood pressure have failed. The intervention should only be offered to patients within a context of clinical research in highly skilled tertiary referral centres that participate in international registries constructed independent of the manufacturers. Consensus along these lines is rapidly growing in several European countries [30] as well as elsewhere [23, 31].
1 Staessen JA, Kuznetsova T, Stolarz K. Hypertension prevalence and stroke mortality across populations. JAMA. 2003;289(18):2420–2.
2 Weinehall L, Öhgren B, Persson M, Stegmayr B, Boman K, Hallmans G, et al. High remaining risk in poorly treated hypertension: the “rule of halves” still exist. J Hypertens. 2002;20(10):2081–8.
3 Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117(25):e510–26.
4 DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77(1):75–197.
5 Grassi G. Assessment of sympathetic cardiovascular drive in human hypertension. Hypertension. 2009;54(5):690–7.
6 Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic–nerve ablation for uncontrolled hypertension. N Eng J Med. 2009;361:932–4.
7 Schlaich MP, Sobotka PA, Krum H, Whitbourn R, Walton A, Esler MD. Renal denervation as a therapeutic approach for hypertension. Novel implications for an old concept. Hypertension. 2009;54:1195–201.
8 Witkowski A, Prejbisz A, Florczak E, Kadziela J, Sliwinsky P, Bielen P, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension. 2011;58:559–65.
9 Brandt MC, Mahfoud F, Reda S, Schirmer SH, Erdmann E, Böhm M, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012;59:901–9.
10 Schlaich MP, Straznicky N, Grima M, Ika–Sari C, Dawood T, Mahfoud F, et al. Renal denervation: a potential new treatment modality for polycystic ovary syndrome? J Hypertens. 2011;29:991–6.
11 Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373(9671):1275–81.
12 Symplicity HTN–2 Investigators. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN–2 Trial): a randomised controlled trial. Lancet. 2010;376(9756):1903–9.
13 Symplicity HTN–1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension. Durability of blood pressure reduction out to 24 months. Hypertension. 2011;57(5):911–7.
14 Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 guidelines for the management of arterial hypertension. The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25(6):1105–87.
15 Turner MJ, van Schalkwyk J.M. Is it ethical to perform irreversible renal denervation before a trial low sodium intake for treatment-resistant hypertension? Hypertension. 2011;58(2):1–9.
16 Calhoun DA, White WB. Effectiveness of the selective aldosterone blocker, eplerenone, in patients with resistant hypertension. J Am Soc Hypertens. 2008;2(6):462–8.
17 Eide IK, Torjesen PA, Drolsum A, Babovic A, Lilledahl NP. Low-renin status in therapy-resistant hypertension: a clue to efficient treatment. J Hypertens. 2004;22(11):2217–26.
18 Young LM, Haakenson CM, Lee KK, van Eeckhout JP. Riboflavin use as a drug marker in Veterans Administration cooperative studies. Control Clin Trials. 1984;5(Suppl 4):497–504.
19 Azizi M, Ménard J, Peyrard S, Lièvre M, Marre M, Chatellier G. Assessment of patients’ and physicians’ compliance to an ACE inhibitor treatment based on urinary N-Acetyl Ser-Asp-Lys-Pro determination in the Noninsulin-Dependent Diabetes, Hypertension, Microalbuminuria, Proteinuria, Cardiovascular Events and Ramipril (DIABHYCAR) study. Diabet Care. 2006;29:1331–36.
20 Kikuya M, Hansen TW, Thijs L, Björklund-Bodegård K, Kuznetsova T, Ohkubo T, et al., on behalf of the International Database on ambulatory blood pressure in relation to Cardiovascular Outcome (IDACO) investigators. Diagnostic thresholds for ambulatory blood pressure monitoring based on 10–year cardiovascular risk. Circulation. 2007;115(16):2145–52.
21 Hansen TW, Kikuya M, Thijs L, Björklund-Bodegård K, Kuznetsova T, Ohkubo T, et al., on behalf of the IDACO Investigators. Prognostic superiority of daytime ambulatory over conventional blood pressure in four populatios: a meta-analasis of 7030 individuals. J Hypertens. 2007;25(18):1554–64.
22 Staessen JA, Thijs L, Ohkubo T, Kikuya M, Richart T, Boggia J, et al. Thirty years of research on diagnostic and therapeutic thresholds for the self-measured blood pressure at home. Blood Press Monit. 2008;13(6):352–65.
23 Pickering TG, Houston Miller N, Ogedegbe G, Krakoff LR, Artinian NT, Goff D. Call to action on use and reimbursement for home blood pressure monitoring. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52(1):10–29.
24 Salles GF, Cardoso CR, Muxfeldt ES. Prognostic influence of office and ambulatory blood pressures in resistant hypertension. Arch Intern Med. 2008;168(21):2340–6.
25 Rippy MK, Zarins D, Barman NC, Wu A, Duncan KL, Zarins CK. Catheter-based renal sympathetic denervation: chronic preclinical evidence for renal artery safety. Clin Res Cardiol. 2011;100(12):1095–101.
26 Kaye DM, Esler M, Kingwell B, McPherson G, Esmore D, Jennings G. Functional and neurochemical evidence for partial cardiac sympathetic reinnervation after cardiac transplantation in humans. Circulation. 1993;88:1110–8.
27 Johnston SC. Patent foramen ovale closure – closing the door except for trials. N Engl J Med. 2012;366(11):1048–50.
28 Kitsios GD, Dahabreh IJ, Abu Dabrh AM, Thaler DE, Kent DM. Patent foramen ovale closure and medical treatments for secondary stroke prevention: a systematic review of observational and randomized evidence. Stroke. 2012;43:422–31.
29 Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991–9.
30 Mark C, Mark DB, Trevor C, David C, John D, Huon G, et al. The Joint UK Societies’ consensus statement on renal denervation for resistant hypertension, 2012. Available from: http://www.bhsoc.org/docs/The-Joint-UK-Societies’-Consensus-on-Renal-Denervation-for-resistant-hypertension.pdf.
31 Chobanian AV, Bakris GL, Black BK, Cushman WC, Green LA, Izzo JL, et al., and the National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–52.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.