
Figure 1
Silicone gel filled silicone rubber breast implant, manufactured by the French company Poly Implant Prosthese (PIP).
© Sébastien Nogier / AFP. Reprinted with permission.
DOI: https://doi.org/10.4414/smw.2012.13614
I prepared this article at the request of Swiss Medical Weekly. The topic is timely because of recent public controversy over silicone-based breast implants (fig. 1) manufactured by a now-defunct company, Poly Implant Prosthese (PIP). The purposes of my article are to (1.) provide a general overview of silicone breast implant materials, (2.) describe the general safety of these materials as reported to date, and (3.) summarise current publicly available information about these aspects of the PIP prostheses.

Figure 1
Silicone gel filled silicone rubber breast implant, manufactured by the French company Poly Implant Prosthese (PIP).
© Sébastien Nogier / AFP. Reprinted with permission.
Please note that I am not a clinician and am not qualified to offer clinical opinions as to whether PIP or any other breast implants should be surgically removed. I was trained as a materials scientist and have spent most of my career teaching and doing research related to surgical materials and implants as a member of three different university medical faculties. While I know the technology, I have not designed breast implants, specified their materials, tested their physico-chemical properties or evaluated biologic responses to them.
Two chapters from a 2004 academic text provide valuable, comprehensive overviews of the chemistry and fabrication of medical-grade silicone-based materials [1] and their application in medicine and surgery [2]. However, at the time of writing the two authors were full-time employees of a major producer of such materials, Dow Corning Corporation.
Silicones [3] should not be confused with the chemical element, silicon, which is part of the composition of silicones. Silicones are peculiar. Unlike many other silicon-containing compounds or materials (e.g., SiO2, quartz mineral) silicones do not occur in nature. They are entirely synthetic. They were first synthesised ca. 1900, and the term “silicones” was invented to describe them. Silicones are polymerised siloxanes (a.k.a. polysiloxanes). They are mixed inorganic-organic polymers with the chemical formula (R2SiO)n where R is an organic side group (e.g., methyl, CH3) attached to a siloxane ...-Si-O-Si-O-Si-O-... “backbone” or chain (fig. 2A, B). The specific example shown, polydimethylsiloxane (PDMS), is the most common polysiloxane [4]. Since the chains must be terminated, the complete PDMS formula is CH3[Si(CH3)2O]nSi(CH3)3. PDMS is an oily, sticky liquid with a viscosity that increases as the average chain length (molecular weight) is increased.
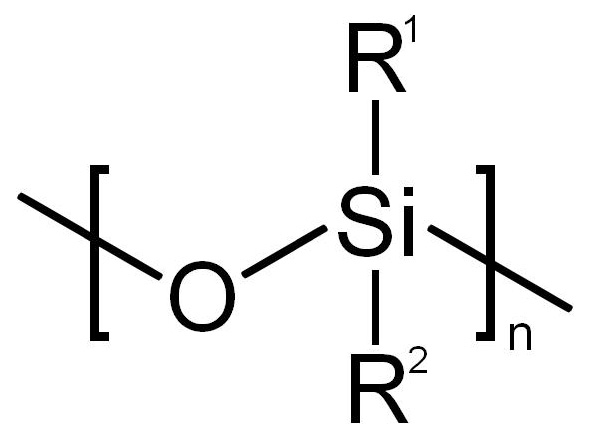
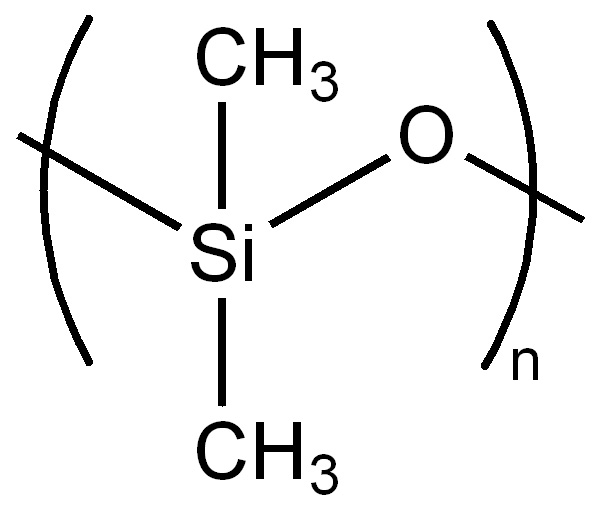
Figure 2
The siloxane “backbone” of silicone polymer molecules, shown (A) generically and (B) specifically with methyl (CH3) groups attached to produce the most common siloxane, polydimethylsiloxane (PDMS). This is the siloxane used to form both the silicone rubber and the silicone gel used in breast implants. Other organic groups can be attached instead of methyl to produce other siloxanes with other properties. Source: Wikimedia Commons.
PDMS is the basis for the both breast implant silicone gel and the silicone rubber sac or shell which contains the gel. The molecular weight of PDMS (or any polymer) is an average, and thus some PDMS molecules will be far shorter than the average – or even cyclic rather than linear. This is important to the behaviour of PDMS in breast implant gels as discussed later.
Silicones can be liquids, gels, elastomers (rubbers) and even hard plastics. Production of silicones starts with sand (fig. 3) and is accomplished by varying the -Si-O-Si- chain length, using different organic side groups, and chemically cross-linking the polymer chains. The siloxane backbone, due to its large bond angles and bond lengths (fig. 4) is much more flexible than polymers with a carbon backbone (e.g. polyethylene). As a result all silicones are rubbery to varying extents. Liquid PDMS also has especially peculiar mechanical properties. It runs and flows if poured slowly, and spreads under the influence of gravity. However, if deformed rapidly, the flexible polymer chains easily become entangled. As a result viscous forms of PDMS can be molded by hand into a ball – which will bounce if thrown against a hard surface. Silly Putty® is liquid PDMS whose viscosity has been increased by reacting it with boric acid.
The inorganic siloxane backbone also causes silicones and silicone-based materials to have other special properties. Although the -Si-O- linkage is flexible, it is extremely chemically stable, as is the bond between Si and O in quartz mineral (silica, SiO2). Thus silicones can be viewed as liquid or solid polymeric materials which have some properties of ceramics. These include:
– low thermal conductivity;
– high thermal stability – chemical and physical properties change little from −100 to +250 °C;
– high chemical resistance to attack by oxygen, ozone, and ultraviolet light.
For medical use, a consequence of thermal stability and resistance to chemical attack is that many silicone-based materials (e.g., silicone rubber) can be autoclave sterilised without altering their structure or properties.
Silicone breast implant shells are filled with either saline solution (fig. 5) or PDMS silicone gel (fig. 1) – or in some cases with both, in separate compartments. The general consensus is that post-surgical mechanical behaviour of implants filled with silicone gel is more like natural breast tissue [5]. Gels are defined as substantially cross-linked material systems, usually comprised of polymers (liquid or solid). Cross linking means that many of the starting units of the gel (e.g., polymer chains) are chemically attached to other units at various points, so that they form a 3-dimensional network. As a result, true gels exhibit no flow as long as their structure is intact [6]. Also, if a true gel is deformed by a non-damaging load, and the load is released, the gel specimen will in time return to its original dimensions.
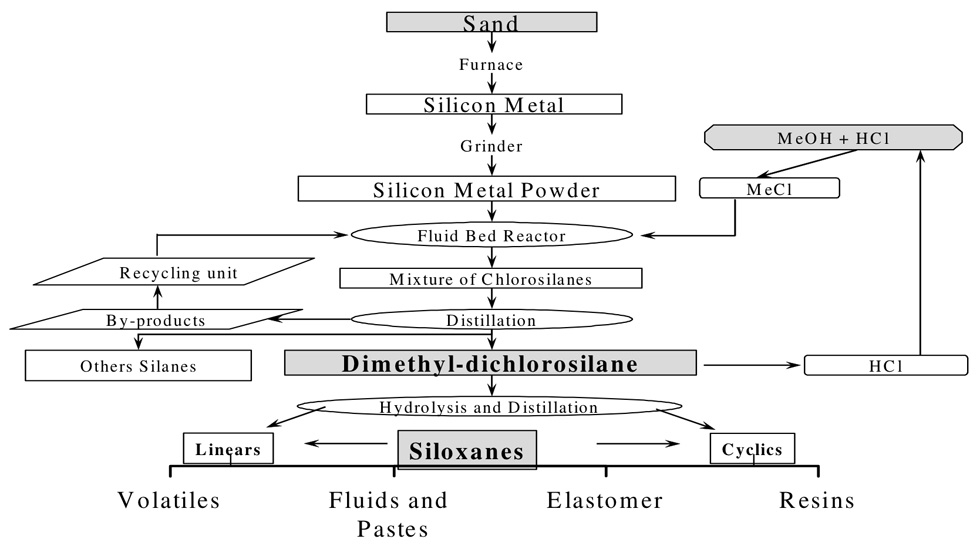
Figure 3
Flow chart of the process – starting with sand – for making siloxane-based materials, including silicone rubber and silicone gel used in breast implants. Source: Wikimedia Commons, © Naweedkhan.

Figure 4
Three-dimensional representation of polydimethylsiloxane molecular structure. The large bond angles and bond lengths result in polymers which tend to be much more flexible and stretchable than rubbers with a carbon “backbone” – i.e. -C-C-C-. Source: Wikimedia Commons.
When a polymer is described as cross-linked, it means a given batch of material has been exposed to a cross-linking method [7]. It does not mean that every molecule is cross linked or that cross-linking is uniform. This is a crucial factor in the behaviour of PDMS breast implant gels. The degree of cross linking is usually controllable, and increased cross-linking results in materials – including gels – which are stronger and stiffer.
Breast implants containing PDMS gels have been produced since the 1960s, and over the years gels with different amounts of cross-linking – and thus different properties – have been used [5].
PDMS gels with lower amounts of cross-linking may not strictly be gels but instead just rather viscous liquids. Due to the inherent incompleteness of cross-linking, PDMS gels (or viscous liquids) contain 1–2% PDMS molecules of extremely low molecular weight (ca. 3 to 20 siloxane units, molecular weights 20 to 1500) with either linear or cyclic structures [8]. These small PDMS molecules can pass (“bleed”) quite easily through silicone rubber membranes as described below. Also, their small size means that they can disperse through body tissues with relative ease. Increasing cross-linking of PDMS decreases the amount of these molecules that are free.
In general, the latest generation of PDMS breast implant gels are more highly cross-linked, thus minimising the amount of free low molecular weight molecules available to pass into the surrounding tissues through the silicone rubber shell [5]. However, even with the latest generation implants, low molecular weight PDMS molecules have been found in the breast tissues of implanted persons – even when the silicone rubber shell is intact [9]. In addition to low molecular weight PDMS, silicone gels can contain trace amounts of platinum – present because platinum is used as a catalyst to promote PDMS cross linking [1]. Platinum in amounts significantly greater than controls has also been found in the breast tissues of women with silicone rubber shells which are intact [9].
It is certainly conservative and appropriate to minimise the dispersion of foreign materials into the body from implants of any kind – except drug delivery devices. Also specific concerns have been voiced that low molecular weight PDMS – especially cyclic molecules – might mimic estrogens or CNS-active drugs [8]. In addition platinum can evoke toxic responses [10]. For example, cisplatin (cis-PtCl2(NH3)2), used in tumor chemotherapy, damages numerous types of non-tumorous cells.
Silicon (sic) rubber is a misnomer for silicone rubber, and the misuse appears in the media and even journal publications. The misuse should be avoided as there are industrial silicon rubbers (elastomers filled with silicon particles) [11]. The terms rubber and elastomer are generally interchangeable.
Figure 5
Silicone rubber breast implant shell, to be filled with saline during surgery. In-situ filling allows for custom volume adjustment. If later (e.g., years) after implantation the saline-filled shell ruptures, the saline disperses rapidly and harmlessly, but makes a highly obvious change in the recipient’s appearance.
© Natrelle. Reprinted with permission.
All current breast implants employ a PDMS silicone rubber sac or shell, although in some designs the surface is modified chemically or coated to control leakage or enhance/prevent tissue adhesion. The exceptional flexibility and extensibility of certain formulations of PDMS silicone rubber (compared to organic rubbers) contribute along with silicone gel to the overall ability of these implants to mechanically mimic breast tissue [5, 13].
Silicone rubbers were first formulated ca. 1940 [1, 12] and were in commercial production and industrial use before 1950. The purpose was to create flexible electrical insulating materials with high resistance to degradation at elevated temperatures or in hostile chemical environments. Thus it was the flexibility of the -Si-O-Si- linkage combined with its ceramic-like properties that made silicone elastomers attractive compared to most organic-based rubber insulators.
PDMS silicone rubbers are thus an old technology. Even their clinical use in breast implants dates back to the 1960s [2].
Since the technology is an old one, there are few recent journal papers devoted to mechanical and physical properties of PDMS silicone rubbers – except when proposed for some new use: for example in 1997 when PDMS rubbers was considered for use in creating micro-machined chemical sensors [14]. Besides providing a highly stable, flexible insulating material for use in chemical sensors, PDMS silicone rubbers were advantageous because of another property – high gas permeability. This also makes them attractive for contact lens and blood oxyenator applications.
In any case it is not possible to give exact physical or chemical properties for PDMS silicone rubber because there is no such thing as just “plain” PDMS silicone rubber.
Here is why: First, the liquid PDMS starting material can have a range of molecular weights. Then, a selected amount of “nano-particles” of amorphous “fumed” silica (SiO2) filler (fig. 6) is added to liquid PDMS to make higher-performance silicone rubber – e.g., for medical use [1]. This filler increases strength, tear-resistance and the amount the rubber can be stretched under tension before failure. After adding the particles, a PDMS silicone rubber is then formed by chemically cross-linking the formulation to various extents and in various ways. Thus PDMS silicone rubbers can have a wide range of structures and properties.
Finally, while it is possible to buy finished PDMS silicone rubber stock (e.g., sheets) and make things from it, that isnothow breast implant shells are made. The cross-linked, finished PDMS rubber in breast implants is created from liquid components during formation of the shell.
So the only meaningful way to determine composition, structure and properties of breast implant silicone rubber is to use specimens taken from a finished shell. Even then, the results only apply to that particular type of shell. And finally, the structure and properties may well differ from one part of the shell to another part due to differences in forming temperature, pressure, etc.
Colas and Curtis [2] provided a useful overview in 2004 quoted below. I have modified the quote by inserting reference numbers used in the present article rather than citing author names and year of publication:
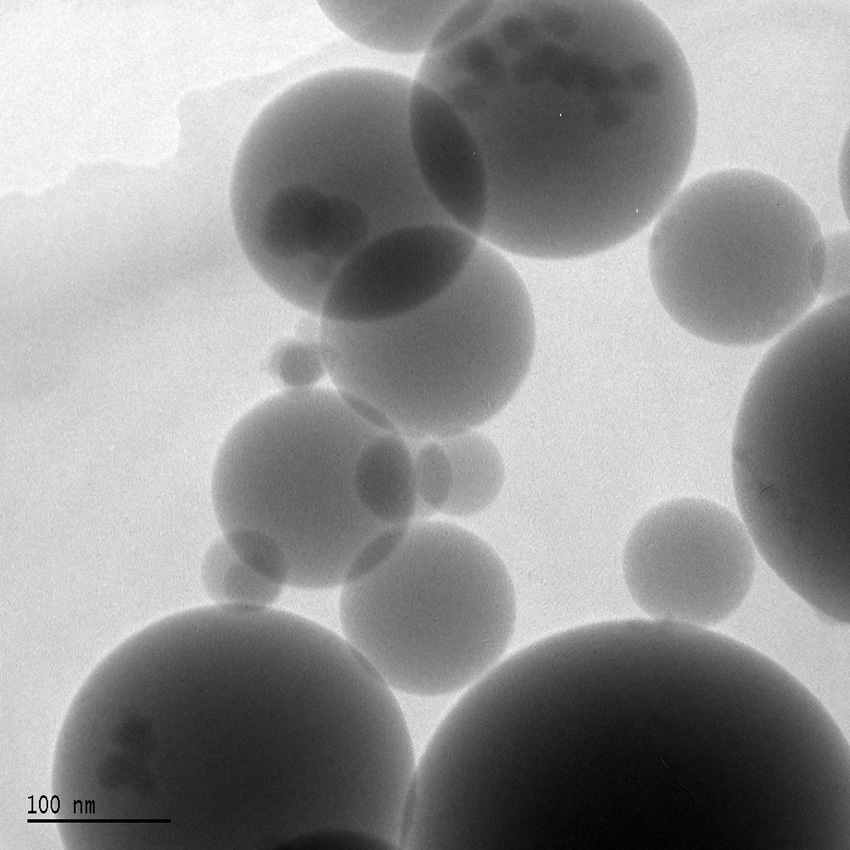
Figure 6
Amorphous “fumed” silica (SiO2) “nano-particles” (ca. 10–200 nm diameter) added to liquid PDMS silicone polymers before they are chemically cross-linked to turn them into silicone rubber. Adding the “nano-particles” results in silicone rubber with improved strength, tear-resistance and increased elongation before failure as tensile loading is increased. Source: Wikimedia Commons, © Silicaman.
“In the early 1990s, these popular devices became the subject of a torrent of contentious allegations regarding their safety. Although the legal controversy regarding silicone gel- filled implants continues in the United States, these medical devices are widely available worldwide and are available with some restriction in the United States. The controversy in the 1990s initially involved breast cancer, then evolved to auto- immune connective tissue disease, and continued to evolve to the frequency of local or surgical complications such as rupture, infection, or capsular contracture. Epidemiology studies have consistently found no association between breast implants and breast cancer [15–18]. In fact, some studies suggest that women with implants may have decreased risk of breast cancer [19, 20]. Reports of cancer at sites other than the breast are inconsistent or attributed to lifestyle factors [21]. The epidemiologic research on autoimmune or connective tissue disease has also been remarkably uniform and concludes there is no causal association between breast implants and connective-tissue disease [22–27]."
A widely accepted definition of biocompatibility is “the ability of a material to perform with an appropriate host response in a specific situation” [28]. Clearly, no material is universally biocompatible – i.e., elicits an appropriate host response in every form of external or internal body contact, in every tissue, regardless of the quantity of material to which the body is exposed or the length of time of the exposure. There is a pragmatic solution to determing biocompatibility, and to enabling selection of materials for clinical use. Biomaterials scientists have devised – and some regulatory agencies have adopted – a spectrum of simulated-use in-vitro and in-vivo animal tests. The nature and spectrum of the tests selected for a given use reflect the degree to which use might be dangerous to the host. The spectrum of tests and levels of acceptable performance increase and reach maximums for potentially life-threatening use (e.g., materials for artificial heart valves). According to the ISO (International Standards Organization) Materials Biocompatibility Matrix, breast implants materials are categorised as Implant Device/Tissue-Bone Contact/Permanent. As a result seven of the eight in vitro and in vivo “Initial Evaluation Tests” are required (all except hemocompatibility), plus two “Supplementary Evaluation Tests (chronic toxicity, carcinogenicity) [29].
Such simulated-use testing is validated by assessing clinical tissue effects of biomaterials by non-invasive means (e.g., imaging) and invasive means (e.g., tissue biopsy, autopsy). In my opinion, whether or not it is required by law, medical device producers should make sure that both the materials they obtain and their own final products made from them are properly tested for biocompatibility and pass the tests. In vitro and in vivo tests of this type [29] have been widely used by many producers for many years to evaluate silicone breast implant materials.
The clinical information cited in the previous section indicates that laboratory biocompatibility testing has been effective up to the present. Previous breast implant PDMS silicone gels and silicone rubbers have generally proved to be clinically biocompatible.
Biodurability is the inverse of biocompatibility. A material can be said to be biodurable if the host has a minimal effect on the functional properties of the material in a specific situation. As with biocompatibility, no implant material is likely to be universally biodurable – i.e. retain its functional properties in every form of external or internal body contact, in every tissue regardless of the severity of mechanical loading and the length of time of the exposure. Like biocompatibility testing, biodurability can be accomplished in the laboratory by in-vitro or in-vivo simulated-use testing. In vitro testing is generally focused on simulated-use mechanical loading in a simulated-use chemical environment, either for a fixed period or until mechanical failure. In any case, the materials are evaluated after exposure for changes in structure and properties.
While in-vitro biodurability testing of PDMS silicone breast implants is certainly done, results are not well-documented in the open literature. Much of this work is done by breast implant material and device producers, and considered a proprietary part of implant development and quality control.
Also, simulated-use is not actual use. It is important that biodurability be evaluated after clinical use. Fortunately, some assessments of long-term biodurability of clinically retrieved breast implant silicone gel and silicone rubber have been done and reported. In a key report [30] three different kinds of explanted silicone gel-filled PDMS silicone rubber shells with implantation times ranging from 3 months to 32 years were obtained for study. In all, 42 implants and 51 control implants were evaluated along with controls. Using specimens cut from the shells, mechanical properties (strength, stiffness, elongation to failure, tear-resistance) were determined. The authors also performed chemical extractions to determine shell PDMS molecular weight and low molecular weight extractables. In summary, they stated that:
“The investigation included the major types of gel-filled implants that were manufactured in the United States in a 30-year period... The silicone gel explants investigated in this study included some of the oldest explants of the various major types that have been tested to date. For assessment of long-term implantation effects, the data obtained in this study were combined with all known data from other institutions on the various major types of gel implants. The study also addressed the failure mechanisms associated with silicone gel breast implants. The results of the study demonstrated that silicone gel implants have remained intact for 32 years in vivo and that degradation of the shell mechanical and chemical properties is not a primary mechanism for silicone gel breast implant failure.”
Another study [31] of clinically retrieved silicone breast implant focused on looking for changes in molecular structure of both the shells and the gels using NMR (nuclear magnetic resonance) imaging. The authors stated that:
“Using NMR spectroscopy, as well as NMR relaxometry measurements (T2), no evidence of hydrolysis or other chemical degradation of the cross-linked silicone matrix was observed in specimens from an early breast implant model (Cronin) explanted after 32 years in vivo or a more recent Silastic1 II model after 13 years in vivo. In addition, no appreciable differences were seen in T2 relaxation times comparing explanted breast implants to suitably-matched non-implanted controls, further underscoring the biostability of the cross-linked silicone shell and gel. Our T2 data and resultant interpretations differ from a 2004 report by the NMR lab at the University of Münster, highlighting the importance of suitable non-implanted controls and sample preparation.”
The implants evaluated for biodurability in these studies were ones in common use, fabricated from silicone starting materials advertised as medical-grade. They constitute ample evidence that at least some widely-used PDMS silicone implant gel and rubber materials demonstrated substantial biodurability.
What the above studies do not provide is information on a key aspect of clinical biodurability. From a materials performance standpoint, the key information surgeons and prospective patients need is the cumulative likelihood over time that silicone implants will rupture.
Fortunately, the reports cited previously (e.g., in the quote from Colas and Curtis [2]) suggest that breast implant rupture and disease processes have not shown a high correlation. However, rupture can certainly have cosmetic (appearance) consequences. On the other hand, the literature [2, 5] suggests that cosmetic changes may occur slowly, with modern highly cross-linked gels tending to stay in place even after shell rupture.
Ruptures certainly do occur though, and this is why some favour saline-filled implants (see again fig. 5). With saline filling (1.) rupture is easily identified by sudden, readily apparent deflation, (2.) saline dispersal is biologically harmless, (3.) there is thus no anxiety related to dispersal of silicone gel and (4.) therefore the patient can decide for mostly cosmetic reasons whether to have further surgery to remove and possibly replace the implants.
The literature contains widely varying reports of the clinical rupture rate of PDMS silicone rubber breast implant shells. In a 2000 study [32] at least 55% of 687 implants were diagnosed as ruptured at ca. 11 years. In a 2003 study [33] based on over 500 implants in place 3 years or more, the authors estimated that ca. 16% would be ruptured by 10 years. In a 2006 study [34] based on 199 implants the authors concluded that 8% are ruptured at 11 years. It seems safe to conclude that the historic rupture rate is >10% at 10 years.
In 2007, two much larger, 10-year, multi-centre, yearly follow-up studies started that may provide a more comprehensive look at rupture rates and other consequences of breast implant surgery. They are taking place in the USA in cooperation with the US Food and Drug Administration [35]. A different commercial implant is being evaluated in each study. Both studies enrolled ca. 40,000 patients who received silicone-filled implants plus much smaller numbers with saline-filled implants as controls. Follow-up is proving to be difficult. In one study, the follow-up rate for the silicone-filled implants after two years post-implantation was ca. 60%. In the other, follow-up at three years was only ca. 21%.
There is a general professional and regulatory consensus that silicone-filled silicone rubber breast implants have previously had sufficient clinical biocompatibility and biodurability. For example:
In 2009 an international surgeons organisation, IQUAM (International Committee for Quality Assurance, Medical Technologies and Devices in Plastic Surgery) issued eight general recommendations concerning breast implants. The last one was a positive conclusion regarding clinical biocompatibility and biodurability: “IQUAM calls for the approval of silicone gel-filled breast implants for global clinical use and unrestricted availability to all patients.” [36]
The USA tends to go its own way in many things. However, in 2011 the US FDA also came to a positive conclusion concerning general clinical biocompatibility and biodurability [35]: “... the FDA believes that silicone gel-filled breast implants have a reasonable assurance of safety and effectiveness when used as labeled. Despite frequent local complications and adverse outcomes, the benefits and risks of breast implants are sufficiently well understood for women to make informed decisions about their use.”
In contrast to the relatively “settled” situation described above, a serious problem has emerged. Since 2010 there have been increasing concerns expressed by governmental agencies and health care providers about the biocompatibility and biodurability of breast implants produced by the now-defunct French company, Poly Implant Prosthese Company (PIP). In recent months the story has received increasing media coverage which has in turn raised public concern. As this article was being written in early February 2012, a formal criminal investigation was apparently under way. The news media had reported required appearances in French courts – and even arrests by French police – of former PIP employees.
In addition to potentially compromising the health of individual women, the dimensions of the problem make it potentially extremely serious socio-economically. According to the UK National Health Service [37] “More than 300,000 PIP implants have been sold globally in 65 countries over the past 12 years. Europe was a major market but more than half of the implants went to South America.” Various news media have described PIP as “once the world's third-largest global seller of breast implants.”
The questions which needed answering as this was being written were:
– whether PIP implants are producing more unfavourable clinical biologic responses than is typical for such implants – and if so, why?
– whether the silicone rubber shells of PIP implants are rupturing at a rate higher than normal for such implants – and if so why?
– if clinically serious problems exist, should some or all of the 300,000-plus PIP implants be removed and perhaps replaced? If so, who should pay the potential enormous cost?
The problem became official in France in March 2010. The AFSSAPS (Agence Française de Sécurité Sanitaire des Produits de Santé) issued a two-page announcement suspending marketing and use of PIP implants. The agency issued a follow-up statement in April 2011 [38]. The agency had concluded by then that PIP implants had significant heterogeneity in quality and fragility of the shells, and that the silicone gel in use had an irritant behaviour not found with other implants.
They also stated that there was a “highly variable rupture rate up to 10%” and “leakage of gel through the shell [...] with a rate up to 11%.” Further, they stated that “In case of rupture or leakage, storage of gel in axillary lymph nodes can cause pain and/or inflammation” and their removal should be considered. The French agency further recommended that women with PIP implants have an ultrasound scan every six months, and that any suspected rupture or leakage should lead to explantation of both the suspected prosthesis and its mate.
The French agency went further in a statement on 1 February 2012 [39] recommending that in accord with the proposal of the minister, and as a preventative, that all women with PIP implants should have them removed on a non-emergency basis, i.e.:
“Ce rapport conforte la recommandation des ministres de proposer à toutes les femmes, à titre préventif et sans caractère d’urgence, l’explantation des prothèses PIP.”
While completing my writing in early February 2012, I could not find via internet search whether any other countries except France had issued a blanket PIP implant removal recommendation. In early January 2012, the UK National Health Service (NHS) issued an interim report of an “Experts Group” they assembled to address the PIP implant problem [40]. The report stated that the NHS has already decided that PIP devices implanted at the expense of the NHS will be removed if the patient and her doctor decides it is necessary, and they will be replaced at NHS expense if desired. The Experts Group wrote that it endorses the offer and “It expects providers in the private sector to take similar steps.”
The Swiss (Swissmedic) regulatory recommendations [41] at the time this article was written were that:
“...women with silicone gel breast implants from the firm ‛PIP’ are recommended to consult their doctor (surgeon) for a check every six months. In the case of pain or any changes to the breast area or armpits, women concerned should have a medical examination without delay.
The removal of intact implants may be simpler than removing them as a result of tears or in the case of inflammation. Therefore, during the checks, women can also discuss the possibility of removing or replacing the implants before the filling material leaks, and without signs of inflammation. The risks and benefits should be considered on a case to case basis. Should filling material leak out of an implant pouch, or if there is any sign of inflammation in the breast area or the armpits, the expert societies recommend the removal of both implants.”
Swissmedic went on to say that Swiss women with PIP implants “can...be included via their doctor (surgeon) in the register for breast implants created by the Swiss Society for Plastic, Reconstructive and Aesthetic surgery (SGPRAC).”
What is causing this regulatory concern and action? There seems to be a general consensus in the documents cited above (issued by French, UK and Swiss agencies) that ruptures of PIP implants have occurred more frequently than the norm. Also, the documents imply that PIP implant silicone gel disperses more readily into tissues than is the case with implants from other producers and may have an increased potential to elicit an inflammatory response.
The fear is that these PIP implant biodurability and biocompatibility problems stem from the use of non medical grade silicone starting materials [40]:
“In March 2010 the French regulator, Agence Française de Sécurité Sanitaire des Produits de Santé (AFSSAPS), discovered that the manufacturer had been using industrial grade silicone instead of the medical grade specified for the CE mark. AFSSAPS revoked the CE mark...”
Investigative reporters were on the trail of this problem. There were allegations in the media during January 2012 [42] that to some extent three industrial silicone starting materials were used by PIP in producing their implants: Baysilone®, Silopren® and Rhodorsil®. The first, Baysilone®, is the trade name of a family of PDMS liquids produced by Bayer AG (Germany). No specific medical grades are mentioned in the product literature [43]. Silopren® is the trade name of a family liquid silicone rubbers also produced by Bayer AG and used to form solid silicone rubber objects. Some but not all of the Silopren® family are certified as medical grade [44]. Rhodorsil® is a family of PDMS liquids available from Bluestar Silicones (France). While the product literature [45] briefly mentions “Medical uses, excipient, active ingredient” in a long list of applications, I could not find any specific mention of a medical grade product.
The French regulatory agency, AFSSAPS, or other parties may eventually determine which, if any, non-medical grade silicones were used by PIP to produce their implants – and if they were used, whether and how this created clinical biocompatibility and biodurability problems. If non-medical grade materials were used, then it seems likely that liability will be resolved in court.
Archaeological and historical records indicate that humans have been permanently modifying their bodies for cosmetic and sometimes socio-religious reasons for thousands of years. Modifications include tattoos, scarring, piercing, male and female genital modifications, ear lobe and lip stretching, and reshaping the feet and head by binding. Some of these modifications continue at present. Also, modern surgery has made more costmetic alterations possible, including sub-dermal alterations with toxin botulin and collagen injections, hair transplantation, eyelid reshaping, face lifts, fat removal (e.g., liposuction) and of course breast reconstruction or augmentation.
In my opinion, cosmetic augmentation using silicone-based breast implants represents something of an extreme because of the size of the foreign device put in place, and the established fact that a significant number rupture over time. The public is in no position to judge the safety of having these large devices implanted or the consequences of rupture and must rely primarily on surgical advice. But surgeons do not create the implants, and they must rely on those who do to employ materials, designs and production principles which maximise implant biocompatibility and biodurability. Finally, regulatory agencies must try to assess all these things in order to meet their duty to the public.
Breast implants are clearly not perfect technology. They have limited biodurability that can lead to the need for further surgery. Beyond that, there is the larger question of general clinical failure of breast implantation – i.e., development for any reason of either a physical appearance or level of physical discomfort unacceptable to the patient, or an unexpected physiologic or biochemical response that poses a serious health threat.
Potential cosmetic breast implant patients must decide whether these risks are worth the reward. Up until the PIP incident, the question of breast implant material biocompatibility seems to have become resolved. Now it is again open. The PIP implants may also prove to be substandard in biodurability. Time and the regulatory processes underway may provide some answers. Let’s hope the result is a further increase in breast implant surgery safety and efficacy.
1 Colas A, Curtis J. Ch. 2.3 Silicone biomaterials: history and chemistry. In: Ratner B, Hoffman A, Schoen F, Lemons J (editors). Biomaterials Science: An Introduction to Materials in Medicine. 2nd Edition. New York: Elsevier; 2004. p. 80–6.
2 Colas A, Curtis J. Ch. 7.19 Medical applications of silicones. In: Ratner B, Hoffman A, Schoen F, Lemons J (editors). Biomaterials Science: An Introduction to Materials in Medicine. 2nd Edition. New York: Elsevier; 2004. p. 697–707.
3 (Unknown authors). Silicone, in Wikipedia the Free Encyclopedia, Wikimedia Foundation, San Francisco CA USA. (http://en.wikipedia.org/wiki/Silicone – vers. 2012.01.16).
4 (Unknown authors). Polydimethylsiloxane, in Wikipedia the Free Encyclopedia, Wikimedia Foundation, San Francisco CA USA. (http://en.wikipedia.org/wiki/Polydimethylsiloxane – vers. 2012.01.23).
5 (Unknown authors). Breast_implant, in Wikipedia the Free Encyclopedia, Wikimedia Foundation, San Francisco CA USA. (http://en.wikipedia.org/wiki/Breast_implant – vers. 2012.01.16)
6 (Unknown authors). Gel, in Wikipedia the Free Encyclopedia, Wikimedia Foundation, San Francisco CA USA. (http://en.wikipedia.org/wiki/Gel - vers. 2012.01.23)
7 (Unknown authors). Crosslinking, in Wikipedia the Free Encyclopedia, Wikimedia Foundation, San Francisco CA USA. (http://en.wikipedia.org/wiki/Crosslinking – vers. 2012.01.23).
8 Lykissa ED, Kala SV, Hurley JB, Lebovitz RM. Release of low molecular weight silicones and platinum from silicone breast implants. Anal Chem. 1997;69:4912–6.
9 Flassbeck D, Pfleiderer B, Klemens P, Heumann KG, Eltze E, Hirner AV. Determination of siloxanes, silicon, and platinum in tissues of women with silicone gel-filled implants. Anal Bioanal Chem. 2003;375:356–62.
10 US Government Depts. of Health & Human Services and Labor. Occupational Health Guideline for soluble platinum salts (as platinum). 1978.
11 Banks HT, Potter LK, Zhang Y. Stress-strain laws for carbon black and silicon filled elastomers. Proc. 36th IEEE Conf. on Dec. and Control;1997.
12 (Unknown authors). Silicone rubber, in Wikipedia the Free Encyclopedia, Wikimedia Foundation, San Francisco CA USA. (http://en.wikipedia.org/wiki/Silicone_rubber – vers. 2012.01.23).
13 Cronin TD, Gerow FJ (1963). Augmentation Mammaplasty: A New “natural feel” prosthesis. Excerpta Medica International Congress Series. 1963;66:41.
14 Lötters JC, Olthuis W, Veltink PH. Bergveld P. The mechanical properties of the rubber elastic polymer polydimethylsiloxane for sensor applications. J Micromech Microeng. 1997;7:145–7.
15 McLaughlin JK, Nyrén O, Blot WJ, Yin L, Josefsso, S, Fraumeni JF, et al. Cancer risk among women with cosmetic breast implants: a population-based cohort study in sweden. J Nat Cancer Inst. 1998;90(2):156.
16 Brinton LA, Lubin JH, Burich MC, Colton T, Brown SL, Hoover RN. Breast cancer following augmentation mammoplasty (United States). Cancer Causes Control 2000;11:819.
17 Mellemkjaer L, Kjøller K, Friis S, McLaughlin JK, Høgsted C, Winther JF, et al. Cancer occurrence after cosmetic breast implantation in Denmark. Int J Cancer. 2000;88:301.
18 Park AJ, Chetty U, Watson ACH. Silicone breast implants and breast cancer. Breast. 1998;7(1):22.
19 Deapen DM, Bernstein L, Brody GS. Are breast implants anticarcinogenic? A 14-year follow-up of the Los Angeles Study. Plast Reconstr Surg. 1997;99(5):1346.
20 Brinton LA, Malone KE, Coates RJ, Schoenberg JB, Swanson CA, Daling JR, et al. Breast enlargement and reduction: results from a breast cancer case-control study. Plast Reconstr Surg. 1996;97(2):269.
21 Herdman RC, Fahey TJ. Silicone breast implants and cancer. Cancer Investi 2001;19(8):821.
22 Hennekens CH, Lee IM, Cook NR, Hebert PR, Karlson EW, LaMotte F, Manson JE, Buring JE. Self-reported breast implants and connective-tissue diseases in female health professionals. A retrospective cohort study. JAMA. 1996;275(8): 616.
23 Sánchez-Guerrero J, Colditz GA, Karlson EW, Hunter DJ, Speizer FE, Liang MH. Silicon breast implants and the risk of connective-tissue diseases and symptoms. N Engl J Med. 1995;332(25):1666.
24 Gabriel SE, O’Fallon WM, Kurland LT, Beard CM, Woods JE, Melton LJ 3rd. Risk of connective- tissue diseases and other disorders after breast implantation. N Engl J Med. 1994;330(24):1697.
25 Nyrén O, Yin L, Josefsson S, McLaughlin JK, Blot WJ, Engqvist M, et al. Risk of connective tissue disease and related disorders among women with breast implants: a nation-wide retrospective cohort study in Sweden. Br Med J. 1998;316(7129):417.
26 Edworthy SM, Martin L, Barr SG, Birdsell DC, Brant RF, Fritzler MJ. A clinical study of the relationship between silicone breast implants and connective tissue disease. J Rheumatol. 1998;25(2): 254.
27 Kjøller K, Friis S, Mellemkjaer L, McLaughlin JK, Winther JF, Lipworth L, et al. Connective tissue disease and other rheumatic conditions following cosmetic breast implantation in Denmark. Arch Int Med. 2001;161:973.
28 Black J. Biological performance of materials. New York: Marcel Dekker; 1992.
29 ISO materials biocompatibility matrix, in assessing biocompatibility: A guide for medical device manufacturers. SGS Life Science Services, Fairfield NJ USA, 22 p. 2007.
30 Brandon HJ, Jerina KL, Wolf CJ, Young VL. Biodurability of retrieved silicone gel breast implants. Plastic & Reconstructive Surgery. 2003;111(7):2295–306.
31 Taylor RB, Eldred DE, Kim G, Curtis JM, Brandon HJ, Klykken PC. Assessment of silicone gel breast implant biodurability by NMR and EDS techniques. J Biomedical Materials Res. 2008;85A:684–91.
32 Brown SL, Middleton MS, Berg WA, Soo MS, Penello G. Prevalence of rupture of silicone gel breast implants revealed on MR imaging in a population of women in Birmingham, Alabama. Am J Radiology. 2000;175:1057–64.
33 Hölmich LR, Friis S, Fryzek JP, Vejborg IM, Carsten C, Sletting S, et al. Incidence of silicone breast implant rupture. Arch. Surgery. 2003;138:801–6.
34 Heden P, Nava MB, Tetering JPB, Magalon G, Fourie LR, Brenner RJ, et al. Prevalence of rupture in inamed silicone breast implants. Plastic & Reconstructive Surgery 2006;118:303.
35 (no author named) FDA update on the safety of silicone gel-filled breast implants. 2011. Center for Devices & Radiological Health, U.S. Food & Drug Adminstration.
36 Neuhann-Lorenz C, Fedeles J, Eisenman-Klein M, Kinney B, Cunningham BL. Eighth IQUAM Consensus Conference Position Statement: Transatlantic innovations, April 2009. Plastic & Reconstructive Surgery 2011;127:1368–75.
37 (No author named). PIP breast implants – latest from the NHS. 2 February 2012. http://www.nhs.uk/news/.
38 (No author named). Breast implants with silicone based gel filling from Poly Implant Prothèse company: Update of tests results. 14 April 2011. Medical Devices Evaluation Direction, Vigilance Department. Agence Française de sécurité sanitaire des produits de santé. Republique Française.
39 (No author named). Xavier Bertrand et Nora Berra, ministres chargés de la Santé, ont reçu les conclusions du rapport sur les prothèses mammaires Poly Implant Prothèse, réalisé par la DGS et l’AFSSAPS. 1 February 2012. http://travail-sante.gouv.fr.
40 Keough B. Pip breast implants: Interim report of the experts group. 6 January 2012. NHS Medical Directorate, Department of Health, United Kingdom.
41 (No author named). Defective “PIP” silicone-filled breast implants, Rueckruf_PIP_Silikon-Brustimplantate_(Update_dfe)_2011-12-23.doc, Swissmedic - Hallerstr. 7 - Postfach - CH-3000 Bern.
42 (No author named). Fuel additive in banned PIP breast implants – report. BBC News Europe. 2 January 2012. http://www.bbc.co.uk/news/world-europe-16384708.
43 (No author named). Bayer silicones Baysilon® fluids M. No date given. Bayer AG Inorganics Business Group, Silicones Business Unit, Baysilone Marketing Section. 51368 Leverkusen DE.
44 High Tear Strength Silopren® LSR4600 Series. May 2010. Momentive performance materials, Inc. Columbus OH USA.
45 (No author named). Bluestar Silicones, Oil 47. No date given. Bluestar Silicones France SAS. Lyon France.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.