Pregnancy mediated improvement of rheumatoid arthritis
DOI: https://doi.org/10.4414/smw.2012.13644
Frauke
Förger, Inka
Vallbracht, Klaus
Helmke, Peter Matthias
Villiger, Monika
Ostensen
Summary
QUESTION UNDER STUDY: To investigate pregnancy related changes of rheumatoid factor (RF) isotypes and anti-citrullinated protein antibodies (ACPA) and their association with disease activity and therapy in patients with rheumatoid arthritis.
METHODS: In 22 rheumatoid arthritis patients disease activity (DAS28-CRP), therapy as well as the levels of rheumatoid factor isotypes (IgG, IgA, IgM) and ACPA were analysed longitudinally within 4 months before conception, once at each trimester and at 6,12 and 24 weeks postpartum. In addition, immunoglobulin isotypes (IgG, IgA, IgM) were measured in the pregnant rheumatoid arthritis patients as well as in 29 healthy pregnant women at the time points mentioned above.
RESULTS: Our results showed that pregnancy significantly reduced systemic levels of the immunoglobulin isotypes IgG and IgA, but did not change the levels of IgG-RF, IgA-RF nor those of IgG-ACPA using assays with different antigen preparations. However, patients with active disease before and during pregnancy showed significantly higher levels of ACPA as compared to patients with low disease activity during pregnancy. Significantly more patients with low disease activity during pregnancy received pregnancy compatible disease modifying antirheumatic drugs or TNF-inhibitor therapy within four months before conception (P = 0.025).
CONCLUSIONS: Our results suggest that treatment should be adjusted to pregnancy compatible drugs preconceptionally and continued until conception and beyond to allow for stable inactive disease during gestation and control the levels of autoantibodies.
Effect of therapy on disease activity and autoantibodies
Introduction
Pregnancy induces a change of the immune system in order to allow tolerance towards the semi-allogeneic foetus. This natural immunomodulation leads to amelioration of the disease in most patients with rheumatoid arthritis (RA) during pregnancy [1]. With more effective treatment in the last decade, more RA patients enter pregnancy with inactive disease. Both, the increasing percentage of patients with inactive disease and the use of more objective disease activity measurements gave lower percentages of a relevant improvement during pregnancy. In the Dutch prospective PARA study, the group of inactive patients remained stable during pregnancy whereas 49% of RA patients entering pregnancy with active disease defined by a DAS28-CRP of >3.2 showed a EULAR response during gestation [2].
Studies on the humoral immune system during pregnancy in RA patients and healthy women showed a decrease of the immunoglobulin isotypes IgG and IgA but not of IgM [3–5]. Similarly, the rheumatoid factor (RF) as measured by the Waaler-Rose assay decreased at late pregnancy in a small cohort of RA patients [5]. By contrast, in the Dutch PARA study, no change of RF or anti-citrullinated protein antibodies (ACPA) could be seen from pre-pregnancy until the third trimester [6].
RF and ACPA are two distinct biomarkers in RA that are mostly used in combination to increase the diagnostic value [7, 8]. Among the RF isotypes, the IgM-RF is the most prevalent. However, the detection of IgG-RF and IgA-RF were shown to predict more erosive disease [9]. High levels of RF are associated with rheumatic nodules. ACPA which were shown to be important in the pathogenesis of RA often precede the onset of symptoms and predict more aggressive disease [8, 10, 11]. Several studies have demonstrated that an effective therapy with disease modifying antirheumatic drugs (DMARD) or biologics is associated with a decrease of RF and ACPA [12–15].
In the present study, we tested the hypothesis that low disease activity during pregnancy corresponds with low levels of RF and ACPA as well as with the use of antirheumatic therapy.
Patients and methods
Patients
We prospectively studied 22 pregnant patients with RA once before conception, once in each trimester (gestational week 10–12, 20–22 and 30–32) and at 6, 12 and 24 weeks postpartum. The pre-pregnancy blood sampling took place up to 4 months before the wish to conceive. All patients fulfilled the American College of Rheumatology criteria [16]. In addition, in two RA patients paired sera of maternal peripheral blood and cord blood were obtained at delivery. For comparison of the immunoglobulin isotypes, sera from 29 healthy women who were recruited among healthy volunteers (by F. Förger and M. Østensen) were analysed at the same time points during and after pregnancy as mentioned before. No woman was included twice. The study was approved by the ethical committee of the University of Berne. All patients gave written informed consent.
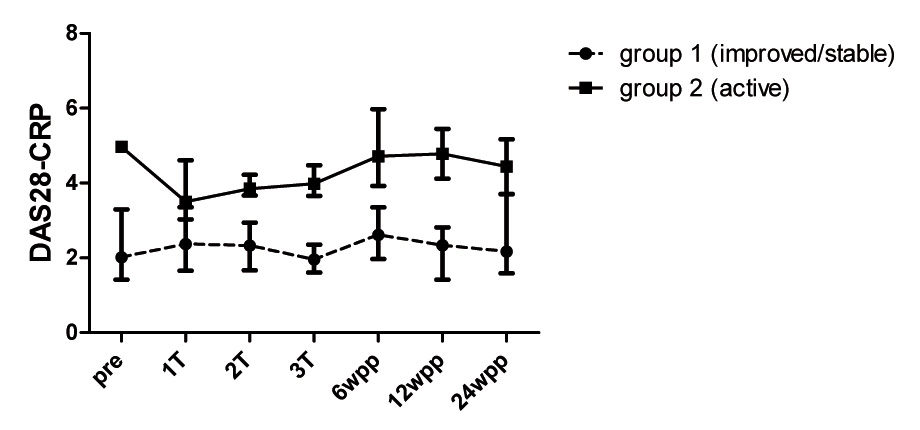
Figure 1
Disease activity of RA patients during and after pregnancy. Disease activity measured before (pre), once at each trimester (1T, 2T, 3T) and after pregnancy (6, 12 and 24 wpp: weeks postpartum) by the DAS28-CRP in 16 RA patients with low disease activity during pregnancy (group 1,dashed line) and in 6 RA patients with persistent active disease (group 2, black line). Values are medians with interquartile range.
Disease activity measurements of RA patients was performed (by F. Förger and M. Østensen) using the DAS28-CRP with three variables consisting of a swollen joint count, a tender joint count and the C-reactive protein (CRP) level (mg/l) that performed best in pregnant RA patients [17]. RA patients were categorised into two different groups depending on disease activity during pregnancy: Group 1 consisted of RA patients with decreasing disease activity during pregnancy reaching a DAS28-CRP <3.2 at the third trimester or with stable low disease activity (DAS28-CRP <3.2) at all time points during pregnancy. Group 2 consisted of RA patients with persistent active disease (DAS28-CRP >3.2) throughout pregnancy.
Immunoglobulin and autoantibody measurements
In all sera, levels of the rheumatoid factor (RF) isotypes IgG, IgA and IgM, the anti-citrullinated protein antibodies (ACPA) as well as the immunoglobulin isotypes IgG, IgA and IgM were measured. All RF isotypes were analysed by a commercially available ELISA (AESKULISA RF-AGM, Aesku Diagnostics, Wendelheim, Germany). For the detection of ACPA we used three commercially available ELISAs with different preparations of the citrullinated antigen: (1) Anti-CCP Mark2 (CCP2) assay uses a second generation cyclic citrullinated synthetic peptide to detect IgG anti-CCP antibodies (Immunoscan RA assay, Euro-Diagnostica, Malmö, Sweden), (2) AESKULISA RA/CP Detect (RA/CP) (Aesku Diagnostics, Wendelheim, Germany) is an ELISA for the detection of IgG autoantibodies to synthetic citrullinated peptides of human immunoglobulin G, and (3) QUANTA Lite CCP3 IgG (CCP3) ELISA (INOVA, San Diego, USA) determines IgG anti-CCP3 antibodies against a third generation cyclic citrullinated synthetic peptide. RA/CP for the detection of IgA and IgM autoantibodies was developed by the manufacturer for the ACPA analysis in mother-child pairs (Aesku Diagnostics, Wendelheim, Germany). All ELISAs were carried out according to the manufacturer’s instructions. Positive results for each ELISA were defined as follows: for all RF isotypes >15 U/ml, for anti-CCP Mark2 >25 U/ml, for AESKULISA RA/CP Detect >15 U/ml and for the CCP3 IgG ELISA >20 U/ml. Sera reaching the upper limit of the measurement range were not further diluted. The immunoglobulin isotypes were determined by nephelometry (Beckman Coulter) with the following reference ranges: for IgG 2.6–7.8 g/l, for IgA <0.16 g/l, for IgM 0.03–0.24 g/l.
Statistics
Longitudinal changes of the antibody levels within patients were compared using the Wilcoxon test. Differences of antibody levels between the subgroups of RA patients or between patients and controls were analysed using the Mann Whitney U test. The presence or absence of medication or autoantibody results in the two groups of RA patients was compared using the Fisher exact test. Statistical analysis was performed using SPSS statistics 17.0 software package. A two-sided significant difference was assumed in case of P <0.05.
Results
Disease activity and medication
Of the 22 RA patients included in the study, 16 (73%) patients experienced improvement or persistent low disease activity during pregnancy reaching a DAS28-CRP below 3.2 at the third trimester (fig. 1, group 1). Among these low active patients, 13 had a DAS28-CRP below 2.6. By contrast, 6 (27%) patients had persistent active disease throughout pregnancy (fig. 1, group 2). Disease activity differed significantly between group 1 and group 2 (P <0.001). At six weeks postpartum, a DAS28-CRP >3.2 could be seen in all patients with persistent active disease during pregnancy and in 5 out of 16 patients with low or inactive disease during gestation. As for medication, significantly more patients of group 1 received pregnancy compatible DMARDs therapy such as sulfasalazine and antimalarials or TNF-inhibitors within the four months before conception (P = 0.025). Likewise, more patients of group 1 remained on sulfasalazine and antimalarials during gestation (table 1).
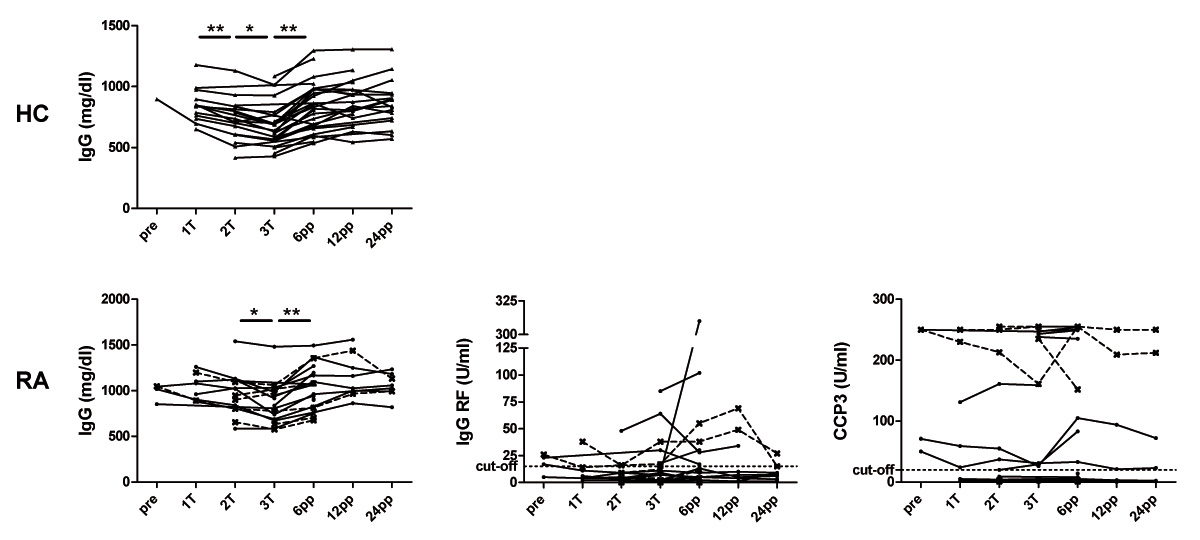
Figure 2
Immunoglobulin levels, IgG-RF and ACPA during and after pregnancy in healthy and rheumatoid arthritis patients.Levels of the immunoglobulin IgG, IgG-RF and IgG-ACPA (anti-CCP3 ELISA) in healthy women (HC, n = 29) as well as in patients with rheumatoid arthritis (RA, n = 22) analysed before conception (pre), at the 1st (1T), 2nd (2T) and 3rd (3T) trimester as well as 6, 12 and 24 weeks postpartum (wpp). RF and ACPA levels are shown in RA patients with persistent active disease (dashed lines) and in those with low disease activity during pregnancy (black lines). * P <0.05, ** P <0.01.
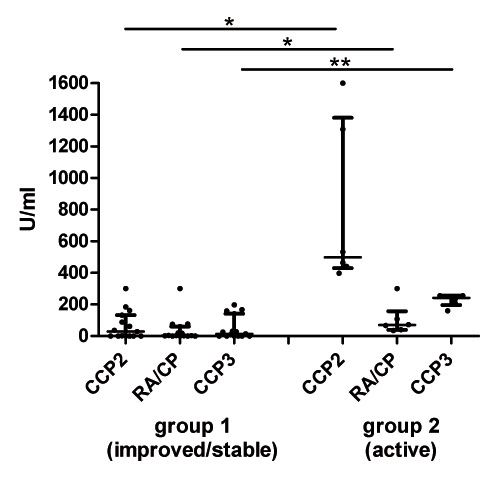
Figure 3
Lower ACPA levels in RA patients with inactive or ameliorated disease during pregnancy. ACPA levels measured by three different ACPA ELISA (CCP2 ELISA, RA/CP ELISA and CCP3 ELISA) at the third trimester in RA patients with low disease activity during pregnancy (group 1, n = 16) and in those with persistent active disease during pregnancy (group 2, n = 6). Lines show median and interquartile range; * P <0.05, ** P <0.01.
Presence of RF isotypes and ACPA
To analyse whether the detection of ACPA in our cohort of pregnant patients would depend on the difference of antigen preparation, three different ACPA tests were used. More pregnant RA patients were positive for any of the ACPA assays (CCP2: n = 12, RA/CP: n = 11, CCP3: n = 12) than for any of the RF isotypes (IgG-RF: n = 7, IgA-RF: n = 5, IgM-RF: n = 9). Among twelve pregnant RA patients who were negative for all RF isotypes, six were positive for at least one of the ACPA tests used.
No significant difference with regard to prevalence of positive or negative results for RF isotypes or ACPA could be found between the two RA groups. However, in the group of low disease activity during pregnancy, slightly more patients tended to be negative for both RF isotypes and ACPA (RA/CP or CCP3) as compared to the group of persistent active disease (group 1: 6/16 (38%), group 2: 2/6 (33%), data not shown).
Levels of immunoglobulin, RF and ACPA
In all patients as well as in healthy controls, the IgG concentrations showed the most pronounced decrease during pregnancy reaching a minimum at the third trimester and a postpartum rise (fig. 2). Changes of IgA levels during and after pregnancy could also be seen in healthy controls and RA patients whereas IgM levels showed minor longitudinal variations (supplementary figure). From the second trimester until six weeks postpartum, RA patients showed significantly higher IgG and IgA levels as compared to healthy controls (P <0.05). Levels of IgG, IgA or IgM did not differ between RA patients with low disease activity (group 1) and those with active disease (group 2).
As opposed to the gestational modulations of IgG in RA patients, neither the levels of IgG-RF (fig. 2) nor those of IgA- or IgM-RF (supplementary figure) revealed any significant changes during pregnancy and postpartum. Similar results could be seen for the longitudinal analysis of the different ACPA assays that all tested for the IgG isotype against citrullinated peptides. However, pregnant RA patients with persistent active disease during pregnancy (group 2) had higher ACPA levels as compared to pregnant RA patients with inactive disease (group 1) (fig. 2 and 3). This difference could not be found for any of the RF isotypes.
Transplacental transfer of RF and ACPA
In two RA patients, ACPA of the IgG isotype and total immunoglobulin isotypes IgG, IgA, IgM were measured at term in maternal serum and in cord blood. As expected, total IgG levels of the newborns exceeded those of the mothers (table 2). ACPA of the IgG isotype were found in the cord blood of the newborns at similar or higher concentrations as compared to those found in maternal sera. By contrast, only a small concentration for IgG-RF could be found in the newborn of one RF positive patient. Cord blood IgM levels were minimal and IgA levels not detectable. Neither RF nor ACPA of the IgA or IgM isotype could be detected in cord blood samples.
|
Table 1: Patient’s characteristics and medication before, during and after pregnancy. |
| |
Group 1
(improved/stable)
|
Group 2
(active)
|
| |
n = 16 |
n = 6 |
|
Age at pregnancy (median, range), years
|
30.5 (23–40) |
33.5 (25–38) |
|
Disease duration
|
| At study entry, median years (range) |
3.5 (0.83–15) |
4 (0.3–11) |
|
Autoantibodies
|
|
|
| RF (IgM) present at diagnosis / unknown, n (%) |
10 (63) / 0 |
4 (67) / 0 |
| RF (IgM) present during pregnancy, n (%) |
7 (44) |
2 (33) |
| ACPA present at diagnosis / unknown, n (%) |
5 (31) / 11 (69) |
1 (17) / 5 (83) |
| ACPA present during pregnancy, n (%)# |
9 (56) |
4 (67) |
|
Medication
|
|
Sulfasalazine*, n (%)
|
| |
Prior to wish to conceive |
7 (44) |
3 (50) |
| |
Pre-pregnancy+ |
8 (50) |
2 (33) |
| |
Pregnancy (1st, 2nd, 3rd trimester) |
6 (38), 5 (31), 4 (25) |
1 (17), 1 (17), 1 (17) |
| |
Postpartum++ |
6 (38) |
2 (33) |
|
Antimalarials, n (%)
|
| |
Prior to wish to conceive |
8 (50) |
2 (33) |
| |
Pre-pregnancy + |
4 (25) |
|
| |
Pregnancy (1st, 2nd, 3rd trimester) |
2 (13), 3 (19), 2 (13) |
0, 0, 0 |
| |
Postpartum++ |
2 (13) |
|
|
Methotrexate, n (%)
|
| |
Prior to wish to conceive |
4 (25) |
4 (67) |
| |
Pre-pregnancy+ |
|
|
| |
Pregnancy (1st, 2nd, 3rd trimester) |
0, 0, 0 |
0, 0, 0 |
| |
Postpartum++ |
|
|
|
Leflunomide, n (%)
|
| |
Prior to wish to conceive |
2 (13) |
|
| |
Pre-pregnancy+ |
|
|
| |
Pregnancy (1st, 2nd, 3rd trimester) |
0, 0, 0 |
0, 0, 0 |
| |
Postpartum++ |
|
|
|
TNF-inhibitors, n (%)
|
| |
Prior to wish to conceive |
1 (6) |
1 (17) |
| |
Pre-pregnancy+ |
2 (13) |
|
| |
Pregnancy (1st, 2nd, 3rd trimester) |
0, 0, 0 |
0, 0, 0 |
| |
Postpartum++ |
|
1 (17) |
|
Oral prednisone /-solone, n (%)
|
| |
Prior to wish to conceive |
10 (63) |
5 (83) |
| |
Pre-pregnancy + |
2 (13) |
3 (50) |
| |
Pregnancy(1st, 2nd, 3rd trimester) |
4 (25), 6 (38), 4 (25) |
3 (50), 4 (67), 5 (83) |
| |
Postpartum++ |
5 (31) |
6 (100) |
|
NSAID, n (%)
|
| |
Prior to wish to conceive |
15 (94) |
3 (50) |
| |
Pre-pregnancy+ |
3 (19) |
2 (33) |
| |
Pregnancy (1st, 2nd, 3rd trimester)+++ |
3 (19), 2 (13), 1 (6) |
2 (33), 3 (50), 3 (50) |
| |
Postpartum++ |
3 (19) |
1 (17) |
|
No medication, n (%)
|
| |
Prior to wish to conceive |
|
|
| |
Pre-pregnancy+ |
1 (16) |
1 (17) |
| |
Pregnancy (1st, 2nd, 3rd trimester) |
4 (25), 2 (25), 7 (44) |
2 (33), 1 (17), 0 |
| |
Postpartum++ |
5 (31) |
|
| *Sulfasalazin: in combination with folic acid; +pre-pregnancy: within four months before conception; ++ postpartum: at 6 weeks postpartum; NSAID: non-steroidal anti-inflammatory drug; +++NSAID pregnancy: until gestational week 32; preg: pregnancy; # positive in any of the three ACPA tests performed. |
|
Table 2: RF, ACPA and immunoglobulin levels in two mother-child pairs. |
|
Mother-child pairs
|
IgG-RF
(U/ml)
|
IgG-ACPA(CCP3)
(U/ml)
|
IgG-ACPA (RA/CP)
(U/ml)
|
total IgG
(g/l)
|
IgA-RF
(U/ml)
|
IgA-ACPA(RA/CP)
(U/ml)
|
total IgA
(g/l)
|
IgM-RF
(U/ml)
|
IgM-ACPA (RA/CP)
(U/ml)
|
total IgM
(g/l)
|
|
No 1
|
Maternal serum |
1707 |
167 |
238 |
7.03 |
558 |
449 |
2.31 |
4911 |
1453 |
0.83 |
| CB serum |
34 |
143 |
143 |
8.99 |
nd |
nd |
nd |
nd |
nd |
0.06 |
|
No 2
|
Maternal serum |
nd |
100 |
nd |
6.06 |
nd |
nd |
1.15 |
nd |
1324 |
0.81 |
| CB
serum |
nd |
110 |
nd |
9.41 |
nd |
nd |
nd |
nd |
nd |
0.06 |
| No: number; CB: cord blood; nd: not detected |
Discussion
In this study, we compared longitudinal changes of immunoglobulin isotypes, RF isotypes and ACPA during pregnancy and postpartum in a group of RA patients with well controlled disease during gestation as compared to a group of patients with persistent active disease. Our results showed that pregnancy significantly reduced systemic levels of the immunoglobulin isotypes IgG and IgA, but did not change the levels of IgG-RF, IgA-RF nor those of IgG-ACPA using assays with different antigen preparations. However, the group of pregnant RA patients who entered and continued with active disease displayed higher levels of ACPA as compared to the group of low active disease. Interestingly, more patients with low active disease during pregnancy were on pregnancy compatible DMARD therapy or on TNF-inhibitors before conception.

Supplementary Figure
Levels of IgA and IgM as well as IgA-RF and IgM-RF during and after pregnancy. Sera of healthy controls (HC, n = 29) and RA patients (n = 22) were analysed before conception (pre), at the 1st (1T), 2nd (2T) and 3rd (3T) trimester as well as 6, 12 and 24 weeks postpartum (wpp) for IgA (A) and IgM (B). In RA patients, longitudinal changes of the immunoglobulin levels were compared with those of the IgA-RF (A) and the IgM-RF (B). No significant difference was found between RA patients with persistent active disease (dashed lines) and those with low disease activity during pregnancy (black lines). * P <0.05, ** P <0.01.
The gestational DMARD therapy and the pre-conceptional use of DMARD and TNF-inhibitors most probably supported the disease ameliorating effect of pregnancy in most of our RA patients and suppressed levels of ACPA. A decreasing effect on RF and ACPA has previously been shown by DMARD like methotrexate, sulfasalazine, antimalarials or minocycline [12–14]. Similarly, studies with TNF-inhibitors or B cell depletion showed a more rapid and uniform decrease of RF whereas the effect on ACPA was either undetectable or occurring only after 6 months of follow-up [15, 18–21]. Whereas levels of RF are more closely related to disease activity, this association remains controversial for ACPA titers [12, 22]. This might be related with the slow response of ACPA titers upon treatment. The continuation of treatment in RA patients who plan to become pregnant might therefore be of particular importance. In the group of inactive disease, 2 patients were on TNF-inhibitors and 12 patients were on DMARD before conception of whom six continued DMARD treatment throughout pregnancy. By contrast, in the group of persistent active disease only two patients received immunosuppressive therapy before conception and only one continued therapy during pregnancy.
The pregnancy related decrease of the IgG has also been observed in previous studies [4, 5, 23]. The decrease of IgG is most likely related to specific transplacental transport mechanisms, not to plasma dilution or change in B cell function [24]. IgG, especially IgG1, is actively transported from the mother to the foetus via neonatal Fc receptors (nFcR) in the placenta. IgG levels of the mature newborns therefore exceed those of the mothers as also found in two mother-child pairs in the present study and by the detection of RF- and ACPA IgG antibodies in cord blood. However, these autoantibodies have no ill-effect in the newborn and get catabolised within the first six months after birth as known for other transplacental transported antibodies [25, 26]. The decreased levels of IgA and IgM that we detected at the third trimester as compared to 6 weeks postpartum are most likely due to the plasma dilution during pregnancy since no receptor depended transplacental transport is known for these immunoglobulins. However, a slow rate of passive diffusion across the placenta is suggested for the transplacental transport of IgA [23].
In spite of transplacental transport, maternal serum levels of RF and ACPA did not show significant changes during pregnancy which is in line with previously published data [6]. Since pregnancy itself does not compromise the number and function of B cells allowing for intact immune responses to vaccines and stable vaccination titers [27, 28], an increased production of these autoantibodies by autoreactive B cells is most likely. The augmented B cell function in our cohort of pregnant RA patients was also reflected by higher levels of total IgG and total IgA as compared to healthy controls.
The main limitation of our study is the small number of patients as compared with the Dutch PARA study [6]. The PARA study could show that RA patients who fulfilled the EULAR response criteria during pregnancy were more often seronegative for both RF and ACPA. In our small cohort of patients with inactive disease during pregnancy defined by DAS28-CRP <3.2, lower levels of ACPA as compared to the levels in the persistent active group indirectly add to this finding. We did not analyse the EULAR response criteria which can only be applied for patients who enter pregnancy with active disease having a DAS28-CRP >3.2. Yet, we counsel patients when they plan pregnancy and advise them to enter pregnancy in an inactive status and therefore tend to continue a pregnancy compatible DMARD therapy.
In agreement with previous studies our results confirm that entering pregnancy with low disease activity is a relevant factor for stable low disease activity during gestation and even for a reduced risk for a postpartum flare [2, 29]. By contrast, high disease activity at conception portends a less favourable expectation for disease improvement. In spite of the small study population and no randomisation to treatment in the two groups, the results support continuing pregnancy compatible drugs until conception and beyond to control disease activity, the levels of autoantibodies and to support the disease ameliorating effect of pregnancy.
Acknowledgments:We wish to thank Manuela Tham, Martina Oppermann and Michael Horn for kindly performing the different autoantibody and immunoglobulin measurements. Furthermore we acknowledge Aesku Diagnostics and GA Generic Assays for donating part of the reagents.
References
1 Ostensen M, Villiger PM. The remission of rheumatoid arthritis during pregnancy. Semin Immunopathol. 2007;29(2):185–91.
2 de Man YA, Dolhain RJ, van de Geijn FE, Willemsen SP, Hazes JM. Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study. Arthritis Rheum. 2008;59(9):1241–8.
3 Malek A, Sager R, Kuhn P, Nicolaides KH, Schneider H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996;36(5):248–55.
4 Ben-Hur H, Gurevich P, Elhayany A, Avinoach I, Schneider DF, Zusman I. Transport of maternal immunoglobulins through the human placental barrier in normal pregnancy and during inflammation. Int J Mol Med. 2005;16(3):401–7.
5 Ostensen M, Lundgren R, Husby G, Rekvig OP. Studies on humoral immunity in pregnancy: immunoglobulins, alloantibodies and autoantibodies in healthy pregnant women and in pregnant women with rheumatoid disease. J Clin Lab Immunol. 1983;11(3):143–7.
6 de Man YA, Bakker-Jonges LE, Goorbergh CM, Tillemans SP, Hooijkaas H, Hazes JM, et al. Women with rheumatoid arthritis negative for anti-cyclic citrullinated peptide and rheumatoid factor are more likely to improve during pregnancy, whereas in autoantibody-positive women autoantibody levels are not influenced by pregnancy. Ann Rheum Dis. 2010;69(2):420–3.
7 Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69(9):1580–8.
8 Vallbracht I, Rieber J, Oppermann M, Forger F, Siebert U, Helmke K. Diagnostic and clinical value of anti-cyclic citrullinated peptide antibodies compared with rheumatoid factor isotypes in rheumatoid arthritis. Ann Rheum Dis. 2004;63(9):1079–84.
9 Bas S, Genevay S, Meyer O, Gabay C. Anti-cyclic citrullinated peptide antibodies, IgM and IgA rheumatoid factors in the diagnosis and prognosis of rheumatoid arthritis. Rheumatology. (Oxford) 2003;42(5):677–80.
10 De Rycke L, Peene I, Hoffman IE, Kruithof E, Union A, Meheus L, et al. Rheumatoid factor and anticitrullinated protein antibodies in rheumatoid arthritis: diagnostic value, associations with radiological progression rate, and extra-articular manifestations. Ann Rheum Dis. 2004;63(12):1587–93.
11 Kroot EJ, de Jong BA, van Leeuwen MA, Swinkels H, van den Hoogen FH, van’t Hof M, et al. The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis Rheum 2000;43(8):1831–5.
12 Mikuls TR, O'Dell JR, Stoner JA, Parrish LA, Arend WP, Norris JM, et al. Association of rheumatoid arthritis treatment response and disease duration with declines in serum levels of IgM rheumatoid factor and anti-cyclic citrullinated peptide antibody. Arthritis Rheum. 2004;50(12):3776–82.
13 Danis VA, Franic GM, Rathjen DA, Laurent RM, Brooks PM. Circulating cytokine levels in patients with rheumatoid arthritis: results of a double blind trial with sulphasalazine. Ann Rheum Dis. 1992;51(8):946–50.
14 Ronnelid J, Wick MC, Lampa J, Lindblad S, Nordmark B, Klareskog L, et al. Longitudinal analysis of citrullinated protein/peptide antibodies (anti-CP) during 5 year follow up in early rheumatoid arthritis: anti-CP status predicts worse disease activity and greater radiological progression. Ann Rheum Dis. 2005;64(12):1744–9.
15 Moller B, Aeberli D, Eggli S, Fuhrer M, Vajtai I, Vogelin E, et al. Class-switched B cells display response to therapeutic B-cell depletion in rheumatoid arthritis. Arthritis Res Ther. 2009;11(3):R62.
16 Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24.
17 de Man YA, Hazes JM, van de Geijn FE, Krommenhoek C, Dolhain RJ. Measuring disease activity and functionality during pregnancy in patients with rheumatoid arthritis. Arthritis Rheum. 2007;57(5):716–22.
18 Alessandri C, Bombardieri M, Papa N, Cinquini M, Magrini L, Tincani A, et al. Decrease of anti-cyclic citrullinated peptide antibodies and rheumatoid factor following anti-TNFalpha therapy (infliximab) in rheumatoid arthritis is associated with clinical improvement. Ann Rheum Dis. 2004;63(10):1218–21.
19 Bobbio-Pallavicini F, Alpini C, Caporali R, Avalle S, Bugatti S, Montecucco C. Autoantibody profile in rheumatoid arthritis during long-term infliximab treatment. Arthritis Res Ther. 2004;6(3):R264–72.
20 Cambridge G, Leandro MJ, Edwards JC, Ehrenstein MR, Salden M, Bodman-Smith M, et al. Serologic changes following B lymphocyte depletion therapy for rheumatoid arthritis. Arthritis Rheum. 2003;48(8):2146–54.
21 Klaasen R, Cantaert T, Wijbrandts CA, Teitsma C, Gerlag DM, Out TA, et al. The value of rheumatoid factor and anti-citrullinated protein antibodies as predictors of response to infliximab in rheumatoid arthritis: an exploratory study. Rheumatology. (Oxford) 2011;50(8):1487–93.
22 Miriovsky BJ, Michaud K, Thiele GM, O’Dell JR, Cannon GW, Kerr G, et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease activity in men with rheumatoid arthritis. Ann Rheum Dis. 2010;69(7):1292–7.
23 Malek A, Sager R, Schneider H. Transport of proteins across the human placenta. Am J Reprod Immunol. 1998;40(5):347–51.
24 Aagaard-Tillery KM, Silver R, Dalton J. Immunology of normal pregnancy. Semin Fetal Neonatal Med. 2006;11(5):279–95.
25 Guzman-Enriquez L, Avalos-Diaz E, Herrera-Esparza R. Transplacental transfer of human antinuclear antibodies in mice by injection of lupus IgG in pregnant animals. J Rheumatol. 1990;17(1):52–6.
26 Vasiliauskas EA, Church JA, Silverman N, Barry M, Targan SR, Dubinsky MC. Case report: evidence for transplacental transfer of maternally administered infliximab to the newborn. Clin Gastroenterol Hepatol. 2006;4(10):1255–8.
27 Luppi P. How immune mechanisms are affected by pregnancy. Vaccine. 2003:21(24):3352–7.
28 Kuhnert M, Strohmeier R, Stegmuller M, Halberstadt E. Changes in lymphocyte subsets during normal pregnancy. Eur J Obstet Gynecol Reprod Biol. 1998;76(2):147–51.
29 Musiej-Nowakowska E, Ploski R. Pregnancy and early onset pauciarticular juvenile chronic arthritis. Ann Rheum Dis. 1999;58(8):475–80.



