Stem cells for heart valve regeneration
DOI: https://doi.org/10.4414/smw.2012.13622
Benedikt
Weber, Maximilian Y
Emmert, Simon P.
Hoerstrup
Summary
Heart valve tissue engineering holds the potential to overcome limitations of currently used heart valve prostheses. It involves the isolation and expansion of autologous patient cells, the subsequent seeding of these cells onto an appropriate scaffold material, the in vitro incubation and the in vivo implantation of the derived tissue-engineered construct into the patient from whom the cells were taken. While vascular-derived cells require harvest of intact donor tissue and show limited expansion capacities, the use of stem or progenitor cells may overcome these limitations and expand the versatility of the concept of heart valve tissue engineering. Possible sources include cells isolated from blood, bone marrow, adipose tissue, amniotic fluid, chorionic villi, umbilical cord and induced pluripotent stem cells. Here we review different stem cell sources with particular regard to cellular phenotypes and their suitability for application in heart valve tissue engineering.
Introduction
In valvular heart ailments the global disease load is overwhelming and will substantially increase in the near future. Approximately 290,000 heart valve replacements are performed globally per year and the number of patients requiring heart valve replacement is expected to triple by the year 2050 [1]. Despite the major advances in surgical treatment, valvular heart disease thus remains one of the outstanding causes of morbidity and mortality round the globe [1–3]. Although the currently used valvular prostheses provide adequate short-term performance, prosthesis-related complications are reported in up to 50% of heart valve recipients 10–15 years after the operation [4]. While mechanical prostheses require lifelong anticoagulation therapy, bioprosthetic substitutes are inherently prone to progressive calcification and valvular degeneration. None of the currently used non-viable valve replacements hold potential for remodelling, regeneration and/or growth.
Cardiovascular tissue engineering has therefore evolved as an interdisciplinary field applying the principles of engineering to the development of biological substitutes that can restore, maintain or improve these impaired or diseased cardiovascular structures [1–3, 5]. According to this pre-definition two basic strategies have been developed to generate living autologous valvular replacements [5]. The first requires an in-vitro phase generating the valvular substitute ex vivo. This “classical” tissue engineering paradigm includes isolation and expansion of autologous patient cells, subsequent seeding of these cells onto an appropriate scaffold material, in-vitro tissue formation and, finally, implantation of the construct into the patient from whom the cells were taken. This paradigm, which is further referred to as the ‘in vitro tissue engineering’ approach, has been employed as the major approach for heart valve tissue engineering over the last decade and primarily aims at full development of native-like tissue substitutes ex vivo. The second approach of “in situ heart valve tissue engineering” circumvents the extensive in vitro tissue culture phase by straight implantation of polymeric or natural tissue-derived heart valve matrices that are pre-seeded with autologous cells and principally aim at potential host cell attraction and subsequent remodelling in vivo. This approach has aroused major interest in the last few years and also brought the initiation of first human clinical trials for vascular grafts in the United States [6–7].
Stem cells as a potential cell source for cardiovascular tissue engineering
Initially, heart valve tissue engineering involved the isolation of cells harvested from vascular donor tissues, including peripheral arteries, where mixed vascular cell populations were obtained for seeding [1–2, 8]. Out of those, two principal cell lines could be isolated: endothelial cells forming a confluent endothelial cell lining on the surface with antithrombogenic features, and myofibroblast-like cells responsible for the extracellular matrix development of the bioengineered constructs [9]. While isolation of these cells was technically simple and these cells have repeatedly demonstrated substantial tissue formation capacity in vitro, the use of vascular-derived cells is also associated with certain fundamental shortcomings. Cell harvesting prior to seeding requires the sacrifice of intact vascular donor tissues, which constitutes a considerable limitation, in particular for paediatric applications. In addition, cardiovascular risk factors and co-morbidities such as diabetes mellitus and atherosclerosis may cause endothelial dysfunction [10] and thus influence the consistence of vessel-derived cell layers. To avoid the sacrifice of healthy human vascular structures and circumvent additional surgical interventions, several different stem cell sources have been investigated with regard to their use in cardiovascular tissue engineering, in particular for heart valves. The common defining properties of stem cells are (1.) self-renewal in clonogenic conditions and (2.) potential for differentiation into several lineages [11]. Hence, besides avoiding laceration of intact vascular donor tissue, stem and/or progenitor cells could also be associated with several advantages, i.e. (1.) they have the potential to differentiate into multiple cell lineages in vivo under a certain biochemical and mechanical stimulation, (2.) they display unique immunological characteristics allowing persistence in allogeneic settings, but also mediating the attraction of reparative host immune and/or progenitor cells, and (3.) they exhibit extensive in vitro proliferation capacity demonstrating their stem cell nature [2–3].
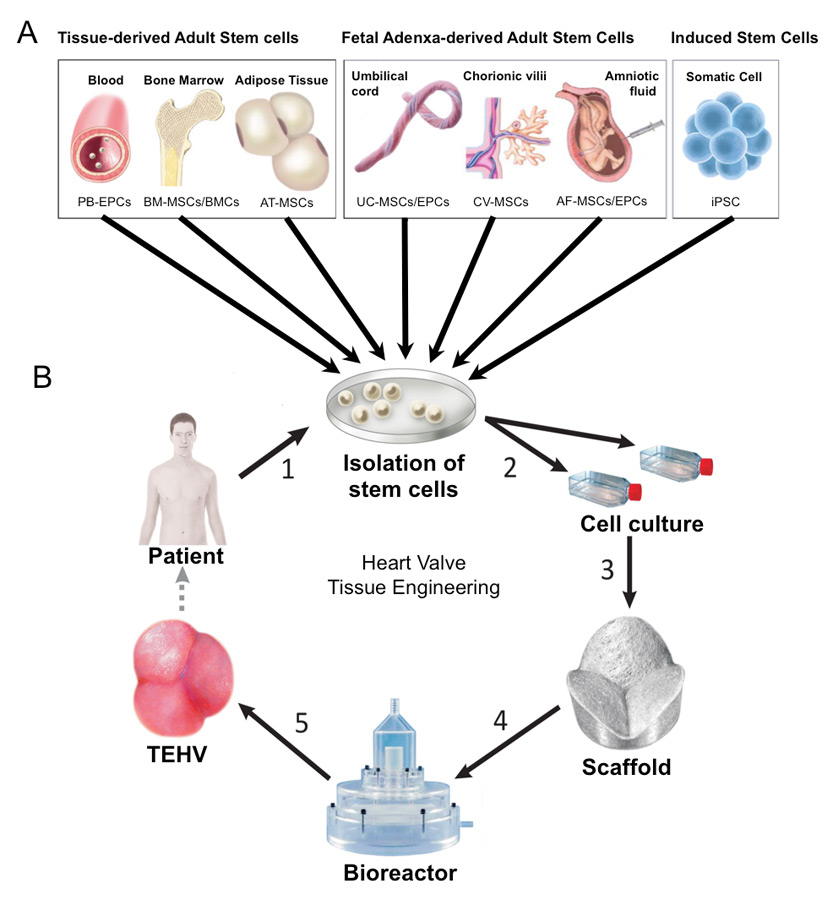
Figure 1
Available stem cells for heart valve tissue engineering. (A: Donor tissues and stem cell sources, B: Introduction of the cells into the in vitro heart valve tissue engineering procedure; 1: cell isolation, 2: cell expansion, 3: seeding of cells onto the scaffold, 4: dynamic conditioning of the construct in a bioreactor system, 5: possible implantation of the deriving construct into the patient).
In particular, in paediatric patients prenatally harvested stem cells derived from the foetus could allow a novel therapeutic concept of “paediatric heart valve tissue engineering” in the future. This approach primarily aims at the fabrication of living, autologous, growing replacements for the repair of congenital malformations even before or shortly after birth. For this purpose cells are ideally obtained during pregnancy or perinatally from the foetal “adnexa”. Several recent investigations have addressed this issue of finding the appropriate prenatal cell source for this novel concept. In particular, umbilical cord-derived cells, amniotic fluid-derived cells and chorionic villi-derived cells are among the most promising candidates under investigation. Besides these foetus-derived adult stem cells, several other stem cell sources have been investigated with regard to their potential use in heart valve tissue engineering (fig. 1; table 1). In the following we provide a comprehensive overview and summary of the potential stem cells used and the principal techniques involved.
|
Table 1: Available stem cell sources for heart valve tissue engineering. |
|
Stem cell source
|
Isolated phenotypes
|
Reference
|
| Bone marrow |
Mesenchymal stem cells
|
[15–17] |
| |
Bone marrow mononuclear cells
|
[23] |
| Blood |
Endothelial progenitor cells
|
[17] |
| Adipose tissue |
Mesenchymal stem cells
|
[26, 27] |
| Amniotic fluid |
Mesenchymal stem cells
|
[34, 35] |
| |
Endothelial progenitor cells
|
[34, 35] |
| Chorionic villi |
Mesenchymal stem cells
|
[38] |
| Umbilical cord matrix |
Mesenchymal stem cells
|
[44] |
| Umbilical cord blood |
Mesenchymal stem cells
|
[40] |
| |
Endothelial progenitor cells
|
[44] |
Available sources of stem cells for heart valve tissue engineering
Bone marrow-derived mesenchymal stem cells
Aside from stem and/or progenitor cells of different haematopoietic lineages, the bone marrow is known to be a source of cells fulfilling criteria for stem cells of non-haematopoietic tissue. These bone marrow-derived non-haematopoietic stem cells have been widely employed as a potential cell source for cardiovascular regeneration in general [12]. While these cells are considered multipotent, they are also referred to as either “mesenchymal stem cells” (MSCs), because of their ability to differentiate into different phenotypes of the mesoderm germ layer, including fat, bone and cartilage, or as “marrow stromal cells” as they appear to arise from the complex array of supporting structures found in the marrow stroma [13].
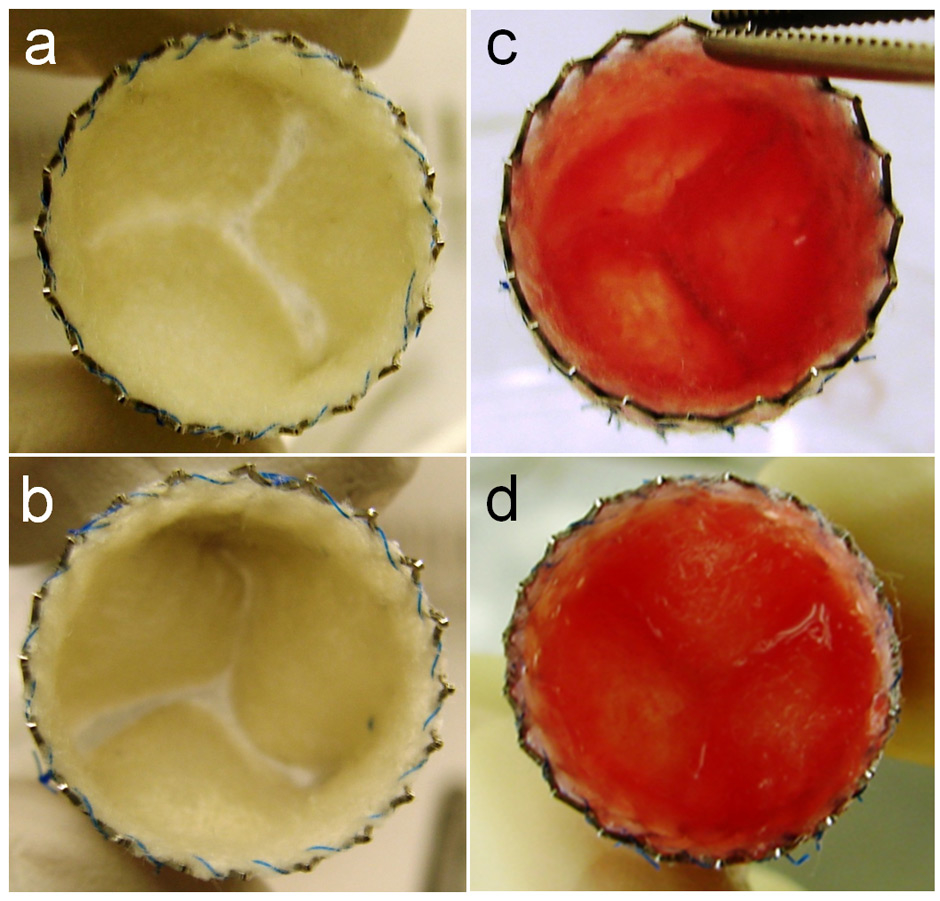
Figure 2
Bone marrow mononuclear cell-based tissue engineered heart valve prior to implantation into an animal model. (Reprinted from [23]: Weber B, Scherman J, Emmert MY, Gruenenfelder J, Verbeek R, Bracher M, et al. Injectable living marrow stromal cell-based autologous tissue engineered heart valves: first experiences with a one-step intervention in primates. Eur Heart J. 2011;32(22):2830–40. © Oxford University Press, Oxford, UK, with kind permission.)
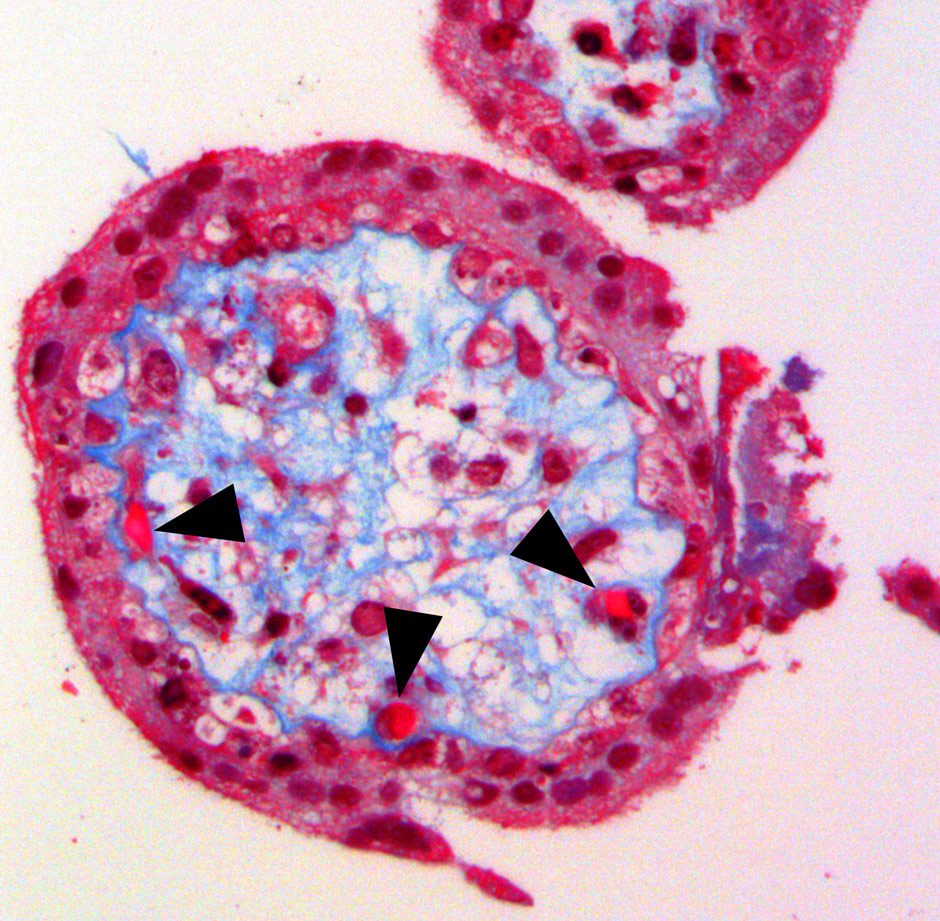
Figure 3
Histomorphology of a chorionic villus showing the outer trophoblastic coverage surrounding the inner foetal mesenchyme also comprising the foetal capillaries (black arrows). (Reprinted from: Weber B, Zeisberger SM, Hoerstrup SP. Prenatally harvested cells for cardiovascular tissue engineering: Fabrication of autologous implants prior to birth. Placenta. 2011;32(Suppl. 4):S316–9. © Elsevier, Oxford, UK, with kind permission).
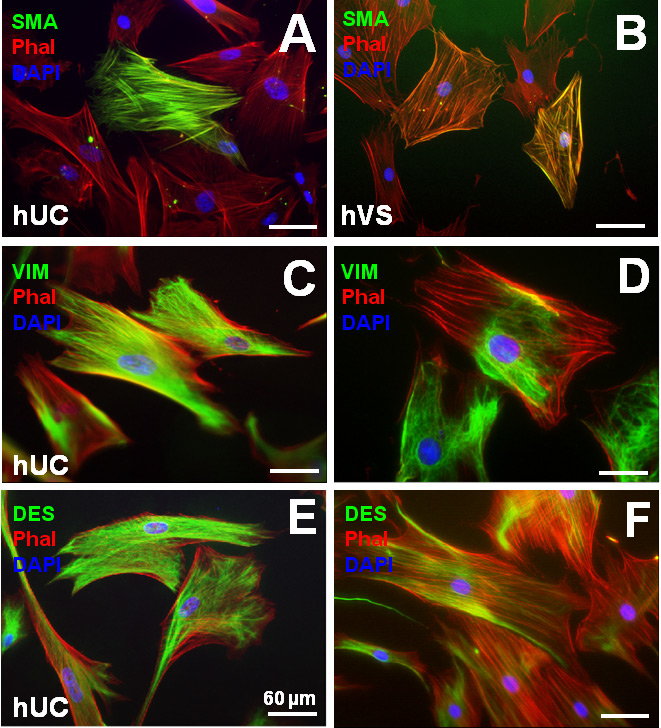
Figure 4
Human umbilical cord Wharton’s jelly-derived myofibroblast-like cells (WMFs) revealed smooth muscle-like phenotypes with positive stainings for vimentin (C), desmin (E) and partially for α-sma (A) similar to mature vascular-derived myofibroblasts (B, D, F). (Reprinted from [48]: Weber B, Schoenauer R, Papadopulos F, Modregger P, Peter S, Stampanoni M, et al. Engineering of living autologous human umbilical cord cell-based septal occluder membranes using composite PGA-P4HB matrices. Biomaterials. 2011;32(36):9630–41. ©Elsevier, Oxford, UK, with kind permission.)
These cells have also been investigated as a possible cell source for the in vitro fabrication of cardiovascular substitutes in vitro [14]. Hoerstrup et al. [15] then successfully used human bone marrow-derived MSCs (BM-MSCs) for the fabrication of living trileaflet heart valves in vitro, while Sutherland et al. [16] also demonstrated initial satisfactory in vivo performance as pulmonary valve substitutes. Recently, the combination of autologous BM-MSC-derived tissue engineered heart valves with a minimally invasive stent-based implantation technique has also been investigated in vivo in the ovine model [17].
Bone marrow-derived mononuclear cells
Apart from using adherent fractions of bone marrow cells, represented by the multipotent MSCs, the option of using unsorted bone marrow mononuclear cells (BMCs) in total for the technique of in situ tissue engineering has recently had a major impact on the field of cardiovascular tissue engineering, and also given promising initial clinical results in Japan [18]. This was further stimulated by the recent initiation of a clinical trial in the USA [6–7], mainly aiming at autologous cell-based vascular repair in infants with congenital heart defects.
This principle of using BMCs for cardiovascular tissue engineering was first described by Shin’oka et al. and has been extensively assessed in experimental [19–20] as well as clinical [18, 21] investigations. Initially it was hypothesised that the contained fraction of multipotent bone marrow-derived stem cells (BM-MSCs) would proliferate and constitute new tissues on the bioengineered constructs [19]. However, Roh et al. [20] showed that initially seeded BMCs disappear from the scaffolds within days and no differentiation of mesenchymal stem cells into valvular interstitial cells may be observed. In fact, they showed that the potential underlying molecular mechanism is a BMC-mediated inflammation-driven process of vascular remodelling via a paracrine role of pre-seeded BMCs, which is consistent with multiple studies reporting that (trans-) differentiation of bone marrow-derived mesenchymal stem cells in vivo is deemed rare [22–23].
While Shin’oka et al. mainly focused on the repair of blood vessels [18–21], Weber et al. [23] recently for the first time applied the BMC-based in situtechnique to the repair of heart valves (fig. 2). After seeding of matrices with autologous BMCs in vitro, the tissue engineered heart valves were implanted in vivo and evaluated in a large clinically relevant animal model with promising initial results.
Adipose tissue-derived mesenchymal stem cells
Adipose tissue has been shown to contain mesenchymal stem cells that can differentiate into various phenotypes in vitro [24]. As to the high availability of this source of tissue and because it can be obtained in fairly large quantities with low invasive techniques (e.g., liposuction), adipose tissue-derived multipotent stem cells (ADSCs) have been considered as a potential alternative to bone marrow-derived mesenchymal stem cells in the future [25]. Besides their mesenchymal phenotype, several studies demonstrated that ADSCs show characteristics of endothelial progenitor cells (EPCs), express endothelial specific markers in vitro when cultured with VEGF, and hold the potential to differentiate into endothelial cells in vivo, which also seems important with regard to heart valve tissue engineering applications [26]. In a first attempt Colazzo et al. [27] investigated the use of ADSCs for heart valve tissue engineering in vitro with promising initial results concerning the formation of collagen and elastin.
Peripheral blood-derived endothelial progenitor cells
Multiple investigations have demonstrated that the presence of endothelium on cardiovascular surfaces may significantly reduce the risk of coagulation and inflammatory complications as well as playing an intricate role for proper valvular functionality in general [28]. Hence in order to improve the functional capacities, the tissue engineered heart valve constructs are usually covered with a layer of autologous endothelial cells (ECs [29]). Fully matured ECs have been successfully isolated from different vascular donor tissues and used in heart valve tissue engineering [2–3, 8–9]. However, despite the importance of ECs, mature vascular-derived ECs have been associated with several shortcomings including a relatively slow expansion rate and limited proliferation capacity [30]. Beyond that, the harvest of mature ECs from donor vessels requires an invasive harvest procedure, potentially associated with an evident risk for the donor, finding a source of rapidly proliferating and ready-to-use ECs not requiring harmful surgical harvest procedures would therefore be advantageous.
First discovered in human peripheral blood by Asahara et al. [31], endothelial progenitor cells (EPCs), have been investigated as a possible alternative source of ECs. Isolated from the blood, these progenitor cells constitute a highly attractive alternative cell source of ECs, as they can easily be isolated from peripheral blood, bone marrow and umbilical cord blood ([2–3]; for umbilical cord-blood derived EPCs see also below).
In a first attempt, Schmidt et al. isolated EPCs from adult peripheral blood in an ovine model, which were then seeded onto tissue engineered heart valves and re-implanted in vivo [17].
Amniotic fluid-derived mesenchymal stem cells
Amniotic fluid represents an attractive foetal cell source for the concept of paediatric heart valve tissue engineering since it renders possible prenatal access to foetal cells from all three germ layers via a low-risk procedure [32]. Several studies have shown promising initial results based on human amniotic fluid-derived mesenchymal stem cells in relation to non-cardiovascular tissue engineering [33].
In 2007, Schmidt et al. demonstrated the feasibility of generating living autologous heart valve leaflets in vitro using human amniotic fluid as a single cell source. This also involved isolation of mesenchymal-like progenitor cells using the MACS-sorting technique [34]. To expand the versatility and clinical applicability of this cell source also, cryopreserved amniotic fluid-derived mesenchymal cells were investigated as a potential life-long cell source [35]. These experiments showed that freshly isolated as well as cryopreserved amniotic fluid-derived cells can be successfully used to fabricate endothelialised tissue engineered heart valves in vitro. In spite of these promising results, several concerns possibly related to this cell source, such as carcinogenicity (given the high plasticity), long-term expansion capacity and stable growth behaviour need to be investigated in future studies.
Amniotic fluid-derived endothelial progenitor cells
Besides the mesenchymal cell lineages, several groups have also reported on the successful isolation of endothelial-like progenitor lineages sharing several similarities of mature endothelial cells [32, 34–36]. Schmidt et al. combined these endothelial phenotypes with the previously described amniotic fluid-derived MSCs for the fabrication of autologous endothelialised functional heart valve leaflets [34–35].
Chorionic villi-derived mesenchymal stem cells
The human placenta is composed of a maternal and a foetal portion. The foetal part, the chorionic villi (fig. 3), contain extraembryonic mesenchymal tissue embedded between the trophoblastic cells. These mesenchymal cells can be isolated by chorionic villus sampling – a prenatal biopsy technique for antenatal genetic diagnostics. Isolated from a rather early developmental stage, these cells have been shown to exhibit profound stem cell-like properties [37]. Schmidt et al. [38] first demonstrated the feasibility of this approach for heart valve tissue engineering by using chorionic villi-derived mesenchymal cells for the fabrication of viable heart valve leaflets in vitro, with promising results. Importantly, this extra-embryonic mesenchyme also contains foetal capillary endothelial cells, which might also be isolated using cell sorting techniques as part of future therapeutic concepts.
Umbilical cord blood-derived mesenchymal stem cells
Blood isolated from the umbilical cord pre- and/or perinatally represents a known reserve for adherently growing, fibroblast-like cells, which render a similar immunophenotype as BM-MSCs characterised by the expression of CD45-, CD13+, CD29+, CD73+, CD105+ [3, 39]. In the light of these properties of UCB-derived MSCs and the plethora of UCB-units already banked worldwide, UCB could represent a potential future cell source for the isolation of high numbers of MSCs required for heart valve tissue engineering. Sodian et al. recently demonstrated the fabrication of tissue engineered autologous heart valves from CD133+ umbilical cord blood-derived cells with stem-like properties as a single cell source [40].
However, recent investigations somewhat dampened existing hopes by making it clear that, when using unseparated frozen CB units outgrowth of adherent cells from the mononuclear fraction was poor and adherence to conventional culture flasks seemed difficult [41].
Umbilical cord blood-derived endothelial progenitor cells
Several studies have shown the feasibility of using human umbilical cord blood-derived EPCs to generate constant neo-endothelial phenotypes on tissue engineered cardiovascular replacements [42–43]. In 2006, Schmidt et al. also demonstrated the fabrication of biologically active living heart valve leaflets when using prenatally available human umbilical cord-derived cells as the only cell source [44]. Matrix stromal cells as well as umbilical cord blood-derived EPCs were seeded onto biodegradable scaffolds and cultured in a bio-mimetic system. The leaflets showed mature layered tissue formation with functional neo-endothelia and extracellular matrix production comparable with that of native tissues, which convincingly demonstrates the feasibility of using umbilical cord-derived progenitor cells including EPCs for coverage of tissue engineered heart valve leaflets in vitro.
Besides their use for surface endothelialisation, the outstanding ease of harvesting and the favourable expansion capacities of UCB-EPCs have also stimulated research on their transdifferentiation into more mesenchymal/myofibroblast-like phenotypes in order to provide new strategies to guide tissue formation in engineered heart valves and to render cord blood a feasible single source for heart valve regeneration in the future.
Umbilical cord matrix-derived mesenchymal stem cells
The human umbilical cord represents a fundamental component of the foetal circulation and is composed of two arteries and one vein. These vessels are embedded in mucoid fetal mesenchymal tissue, which is often referred to as “Wharton’s jelly”. Wang et al. [45] for the first time identified mesenchymal cells within this matrix/jelly-tissue expressing significant amounts of mesenchymal stem cell (MSC) markers, suggesting the existence of mesenchymal cells with stem-like properties. Subsequently, the presence of these MSCs, termed umbilical cord matrix stromal cells (UCMSCs, fig. 4), including their potential for multilineage differentiation, has been confirmed, also indicating that these UCMSCs may represent a promising cell source for cell-based therapies involving cardiovascular tissue engineering [46–47].
Several studies demonstrated the successful in vitro generation of living cardiovascular patches [42, 48], blood vessels [43] and biologically active living heart valve leaflets in vitro based on UCMSCs [44]. In 2006, Sodian et al. [49] also demonstrated the use of cryopreserved human umbilical cord cells (CHUCCs) for the in vitro fabrication of tissue engineered heart valves expanding the versatility of this cell source.
Induced pluripotent stem cells
Embryonic stem cells represent the “gold standard” for stem cell research given their high differentiation potential with the ability to differentiate into all three germ layers. However, the ethical concerns associated with their embryo-destructive harvesting as well as the induction of tumour formation and immune-rejection in vivo have so far limited their clinical use [50]. In their investigation Takahashi et al. [51] introduced different combinations of embryonic-development specific genes into mouse embryonic fibroblasts and determined relevant factors necessary to render a cell with differentiation potential into all three germ layers. These factors could then be defined as the minimum number of factors – mainly including OCT-3/4, Sox-2, c-myc and KLF-4 – required for the generation of induced pluripotent stem cells (iPS). The principal idea behind this cellular reprogramming represents the possibility of taking terminally differentiated cells, including e.g. skin fibroblasts, and providing them with capacities of pluripotent stem cells, including the possibility of giving rise to any tissue of the body. Contrary to pluripotent cells derived from the embryoblast of the blastocyst, iPS do not raise ethical concerns associated with embryo-destruction and may ultimately circumvent immunological rejection.
In the first attempt, Hibino et al. [52] fabricated cell sheets from induced puripotent stem cells in a murine model, which could be used for vascular tissue engineering. In spite of the major hopes raised by these developments, the potential carcinogenicity of virally transduced cells remains a major obstacle for a clinical translation of this concept. Even in non-virally transduced lines the occurrence of genome instability as well as evident epigenetic memory of iPS are of concern and currently limit the clinical feasibility of this approach [53]. In addition, recent studies revealed that vascular cells derived from pluripotent cells present with heterogeneous phenotypes and consideration of in vivo developmental patterning might be beneficial for future in vivo differentiation approaches [54]. Besides the creation of iPS, the concept of direct reprogramming of one differentiated cell type into another [55–56], which circumvents the fully pluripotent differentiation state, may also have a major impact on the field of vascular and valvular regeneration.
Alternative stem cell sources for future applications
In addition to the sources mentioned above, several further stem cells have been identified that may principally hold potential as a reserve of cells for heart valve tissue engineering applications in the future. This includes cells from other foetal and/or embryonic tissues such as cells from the amniotic membrane. Also the spectrum of chorionic villi-derived cells might be broadened by the isolation of the endothelial fraction present in the foetal mesenchyme-derived capillaries of secondary or tertiary chorionic villi. In addition, foetal progenitor cells have been found in the maternal circulation, which may also be used for autologous foetal cell-based therapies in the future.
For adult applications, several further stem cell sources have not yet been applied to the heart valve tissue engineering concept and might harbour great potential. This includes transdifferentiated mesenchymal cells from endothelial progenitor lines or blood-vessel-derived MSCs.
Limitations
When dealing with stem cells there is a potential risk that an uncontrolled growth or genetic alterations of these immature cells might lead to tumour formation in vivo. In embryonic stem cells as well as iPS this risk is most evident, but also the risks of using other (non-pluripotent) stem cells have not yet been fully evaluated. In addition, in the use of biodegradable scaffolds, local inflammation and systemic toxicity due to degradation products of biodegradable scaffolds represents a possible problem.
Hence, prior to clinical implementation of the stem cell-based heart valve tissue engineering concept, systematic investigation in large animal studies is necessary to evaluate long-term performance as well as principal safety of this technology. In addition, systematic comparison of the in vivo fate and function of TEHVs with the currently used standard heart valve prosthesis technologies seems indispensable.
Conclusions
In summary, several different stem / progenitor cell sources are available and have been successfully integrated into the tissue engineering workflow in vitro (table 1). This includes cells from the bone marrow, blood, adipose tissue, amniotic fluid, placenta and umbilical cord. Besides these ‘adult’ stem cell sources, novel technologies exist to ‘reprogram’ a terminally differentiated somatic cell into a stem-like cell by integrating specific genes into the cellular genome, certainly representing a novel, highly promising option for the future. Besides the plethora of existing studies on the use of these various cell sources for different bioengineering applications, studies on a detailed comparison of these sources with regard to factors such as expansion capacity, collagen formation, glycosaminoglycan production, genomic stability or extracellular matrix remodelling, would be of fundamental importance. Although these comparative approaches are an indispensable prerequisite for a future clinical translation, there are as yet few studies on this aspect.
In addition, in vivo studies exist which provide us with the first insight into the in vivo behaviour of the stem cell-based therapeutic constructs. Although having these initial impressions on remodelling and maturation of the bioengineered tissues in vivo, knowledge on the actual mechanisms involved is still very limited. In addition, little is known of the in vivo fate of the stem cells and the potential disparities between the different cell sources. This seems important as it may ultimately determine which stem cell source might be most advantageous for future clinical translation.
Overall, heart valve tissue engineering represents a promising approach for functional autologous cell-based replacements based on fully biodegradable materials. While the in vitro heart valve tissue engineering approach offers more mature implants with better immediate functionality, the in situ approach offers a less complex alternative with potentially higher clinical feasibility. Therefore, paediatric patients in particular would benefit from these replacement structures suited to repair of congenital heart defects. But a constantly increasing number of older patients with acquired heart valve disease could also benefit from living replacement material with regenerative capacities and antithrombogenic surfaces. In this regard stem cells could offer an easily accessible cell source with favourable expansion capacities. Nevertheless, prior to final clinical implementation of the heart valve tissue engineering concept, several issues will have to be overcome on the experimental level to ensure the safety of this promising technology.
References
1 Yacoub MH, Takkenberg JJ. Will heart valve tissue engineering change the world? Nat Clin Pract Cardiovasc Med. 2005;2(2):60–1.
2 Weber B, Hoerstrup SP. Regenerating heart valves. In: Regenerating the heart: stem cells and the cardiovascular system; 1st edition; Springer – New York, 2011.
3 Schmidt D, Hoerstrup SP. Tissue engineered heart valves based on human cells. Swiss Med Wkly. 2006;136(39-40):618–23.
4 Kobayashi J. Stentless aortic valve replacement: an update. Vasc Health Risk Manag. 2011;7:345–51.
5 Langer R, Vacanti JP (1993) Tissue engineering. Science. 260:920–6.
6 Dolgin E. Taking tissue engineering to heart. Nat Med. 2011;17(9):1032–5.
7 Vogel G. Tissue engineering. Mending the youngest hearts. Science. 2011;333(6046):1088–9.
8 Mol A, Bouten CV, Baaijens FP, Zünd G, Turina MI, Hoerstrup SP. Tissue engineering of semilunar heart valves: current status and future developments. J Heart Valve Dis. 2004;13(2):272–80.
9 Hoerstrup SP, Sodian R, Daebritz S, Wang J, Bacha EA, Martin DP, et al. Functional living trileaflet heart valves grown in vitro. Circulation. 2000;102(19 Suppl 3):III44–9.
10 De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130(5):963–74.
11 Weiner LP. Definitions and criteria for stem cells. Methods Mol Biol. 2008;438:3–8.
12 Vassalli G, Moccetti T. Cardiac repair with allogeneic mesenchymal stem cells after myocardial infarction. Swiss Med Wkly. 2011;141:w13209.
13 Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–4.
14 Kadner A, Hoerstrup SP, Zund G, Eid K, Maurus C, Melnitchouk S, et al. A new source for cardiovascular tissue engineering: human bone marrow stromal cells. Eur J Cardiothorac Surg. 2002;21(6):1055–60.
15 Hoerstrup SP, Kadner A, Melnitchouk S, Trojan A, Eid K, Tracy J, et al. Tissue engineering of functional trileaflet heart valves from human marrow stromal cells. Circulation. 2002;106(12 Suppl 1):I143–50
16 Sutherland FW, Perry TE, Yu Y, Sherwood MC, Rabkin E, Masuda Y, et al. From stem cells to viable autologous semilunar heart valve. Circulation. 2005;31;111:2783–91.
17 Schmidt D, Dijkman PE, Driessen-Mol A, Stenger R, Mariani C, Puolakka A, et al. Minimally-invasive implantation of living tissue engineered heart valves: a comprehensive approach from autologous vascular cells to stem cells. J Am Coll Cardiol. 2010;3;56(6):510–20.
18 Hibino N, McGillicuddy E, Matsumura G, Ichihara Y, Naito Y, Breuer C, et al. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg. 2010;139(2):431–6, 436.e1–2.
19 Matsumura G, Miyagawa-Tomita S, Shin’oka T, Ikada Y, Kurosawa H. First evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. Circulation. 2003;108:1729–34.
20 Roh JD, Sawh-Martinez R, Brennan MP, Jay SM, Devine L, Rao DA, et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A. 2010;107:4669–74.
21 Shin’oka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129:1330–8.
22 Zentilin L, Tafuro S, Zacchigna S, Arsic N, Pattarini L, Sinigaglia M, et al. Bone marrow mononuclear cells are recruited to the sites of VEGF-induced neovascularization but are not incorporated into the newly formed vessels. Blood. 2006;107:3546–54.
23 Weber B, Scherman J, Emmert MY, Gruenenfelder J, Verbeek R, Bracher M, et al. Injectable living marrow stromal cell-based autologous tissue engineered heart valves: first experiences with a one-step intervention in primates. Eur Heart J. 2011;32(22):2830–40.
24 Pansky A, Roitzheim B, Tobiasch E. Differentiation potential of adult human mesenchymal stem cells. Clin Lab. 2007;53(1-2):81–4
25 Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28.
26 Colazzo F, Chester AH, Taylor PM, Yacoub MH. Induction of mesenchymal to endothelial transformation of adipose-derived stem cells. J Heart Valve Dis. 2010;19(6):736.
27 Colazzo F, Sarathchandra P, Smolenski RT, Chester AH, Tseng YT, Czernuszka JT, et al. Extracellular matrix production by adipose-derived stem cells: implications for heart valve tissue engineering. Biomaterials. 2011;32(1):119–27.
28 El-Hamamsy I, Balachandran K, Yacoub MH, Stevens LM, Sarathchandra P, Taylor PM, et al. Endothelium-dependent regulation of the mechanical properties of aortic valve cusps. J Am Coll Cardiol. 2009;53(16):1448–55.
29 Kasimir MT, Weigel G, Sharma J, Rieder E, Seebacher G, Wolner E, et al. The decellularized porcine heart valve matrix in tissue engineering: platelet adhesion and activation. Thromb Haemost. 2005;94(3):469–70.
30 Alsberg E, von Recum HA, Mahoney MJ. Environmental cues to guide stem cell fate decision for tissue engineering applications. Expert Opin Biol Ther. 2006;6:847–66.
31 Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–7.
32 Parolini O, Soncini M, Evangelista M, Schmidt D. Amniotic membrane and amniotic fluid-derived cells: potential tools for regenerative medicine? Regen Med. 2009;4(2):275–91.
33 Kaviani A, Guleserian K, Perry TE, Jennings RW, Ziegler MM, Fauza DO. Fetal tissue engineering from amniotic fluid. J Am Coll Surg. 2003;196(4):592–7.
34 Schmidt D, Achermann J, Odermatt B, Breymann C, Mol A, Genoni M, et al. Prenatally fabricated autologous human living heart valves based on amniotic fluid derived progenitor cells as single cell source. Circulation. 2007;11;116(11 Suppl):I64–70.
35 Schmidt D, Achermann J, Odermatt B, Genoni M, Zund G, Hoerstrup SP. Cryopreserved amniotic fluid-derived cells: a lifelong autologous fetal stem cell source for heart valve tissue engineering. J Heart Valve Dis. 2008;17(4):446–55.
36 De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, Perin L, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25(1):100–6.
37 Poloni A, Rosini V, Mondini E, Maurizi G, Mancini S, Discepoli G, et al. Characterization and expansion of mesenchymal progenitor cells from first-trimester chorionic villi of human placenta. Cytotherapy. 2008;10(7):690–7.
38 Schmidt D, Mol A, Breymann C, Achermann J, Odermatt B, Gössi M, et al. Living autologous heart valves engineered from human prenatally harvested progenitors. Circulation. 2006;114(1 Suppl):I125–31.
39 Erices A, Conget, P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109(1):235–42.
40 Sodian R, Schaefermeier P, Abegg-Zips S, Kuebler WM, Shakibaei M, Daebritz S, et al. Use of human umbilical cord blood-derived progenitor cells for tissue-engineered heart valves. Ann Thorac Surg. 2010;89(3):819–28.
41 Buchheiser A, Liedtke S, Looijenga LH, Kögler G. Cord blood for tissue regeneration. J Cell Biochem. 2009;108(4):762–8.
42 Schmidt D, Mol A, Neuenschwander S, Breymann C, Gössi M, Zund G, Turina M, et al. Living patches engineered from human umbilical cord derived fibroblasts and endothelial progenitor cells. Eur J Cardiothorac Surg. 2005;27(5):795–800.
43 Schmidt D, Asmis LM, Odermatt B, Kelm J, Breymann C, Gössi M et al. Engineered living blood vessels: functional endothelia generated from human umbilical cord-derived progenitors. Ann Thorac Surg. 2006;82(4):1465–71.
44 Schmidt D, Mol A, Odermatt B, Neuenschwander S, Breymann C, Gössi M et al. Engineering of biologically active living heart valve leaflets using human umbilical cord-derived progenitor cells. Tissue Eng. 2006;12(11):3223–32.
45 Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, et al. Mesenchymal stem cells in Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22:1330–37.
46 Kögler G, Sensken S, Airey JA, Trapp T, Müschen M, Feldhahn N, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–35.
47 Weiss ML, Anderson C, Medicetty S, et al. Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem Cells. 2008;26:2865–74.
48 Weber B, Schoenauer R, Papadopulos F, Modregger P, Peter S, Stampanoni M, et al. Engineering of living autologous human umbilical cord cell-based septal occluder membranes using composite PGA-P4HB matrices. Biomaterials. 2011;32(36):9630–41.
49 Sodian R, Lueders C, Kraemer L, Kuebler W, Shakibaei M, Reichart B, et al. Tissue engineering of autologous human heart valves using cryopreserved vascular umbilical cord cells. Ann Thorac Surg. 2006;81(6):2207–16.
50 Forsberg M, Hovatta O. Challenges for the therapeutic use of pluripotent stem derived cells. Front Physiol. 2012;3:19.
51 Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;25;126(4):663–76.
52 Hibino N, Duncan DR, Nalbandian A, Yi T, Qyang Y, Shin’oka T, et al. Evaluation of the use of an induced pluripotent stem cell sheet for the construction of tissue-engineered vascular grafts. J Thorac Cardiovasc Surg. 2012;1:11.
53 Drews K, Jozefczuk J, Prigione A, Adjaye J. Human induced pluripotent stem cells – from mechanisms to clinical applications. J Mol Med (Berl). 2012 May 30.
54 Cheung C, Bernardo AS, Trotter MW, Pedersen RA, Sinha S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat Biotechnol. 2012;30(2):165–73.
55 Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142(3):375–86.
56 Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, et al. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol. 2011;13(3):215–22.



