Biomechanics and pathomechanisms of osteoarthritis
DOI: https://doi.org/10.4414/smw.2012.13583
Christian
Egloff, Thomas
Hügle, Victor
Valderrabano
Summary
Today, the most frequent chronic musculoskeletal disorder and the leading cause of disability in the elderly is osteoarthritis (OA). Approximately 43 million people in the United States and 15% of the world population are affected. Due to demographic changes, the incidence of OA is rapidly increasing, leading to an ascending socioeconomical and personal burden. Despite the exact cause of OA remains unknown, the pathogenic role of biomechanical dysfunction in OA is well established. For weight-bearing joints altered loading mechanisms, increased mechanical forces and changed biomechanics are significant contributing factors for initiation and progression of OA. Thus, OA is a disease of the whole joint, including muscles, tendons, ligaments, synovium and bone. This review focuses on the influence of biomechanics on the pathogenesis and progression of OA. We notably illustrate the pathological bioreactivity of soft tissues, subchondral bone and joint inflammation. Procedures, conservative or surgical, which actively alter the biomechanics of the lower limb, are promising strategies to treat symptoms as well as to influence disease progression in OA.
Introduction
Osteoarthritis (OA) is one of the most common causes of disability in the world. Epidemiological studies estimate around 43 million affected patients in the United States alone and about 15% of the world population [1, 2]. The incidence is estimated to 100,000 new cases per year [3]. The risk of mobility impairments caused by knee OA alone is greater than due to any other medical condition in people over 65 [4]. It leads to social, psychological and economical burdens in patients with substantial financial consequences [5]. In France the cumulated health costs resulting from OA almost doubled within 10 years (from 1993–2003) [1]. A further increase has to be expected due to the increasing prevalence of obesity and ascending life expectancy of our population [6].
The role of biomechanics in the development and progression of OA, especially of the lower limb has become integral in current knowledge of this disease.
In this review we focus on the influence of different biomechanical factors on the pathogenesis and progression of OA, underlining the pathological bioreactivity of soft tissues, subchondral bone and subsequent joint inflammation. We discuss current conservative and surgical intervention strategies aimed to reduce biomechanical loads and thereby preventing or slowing down the vicious cycle of OA. To date our knowledge is still limited concerning pathobiomechanical loads and its impact on joint homeostasis leading to destructive OA. We concentrate our perspective on the lower extremity; as the hip, knee and ankle are the joints which are highly weight bearing and the most commonly affected sites by OA [3].
Pathobiomechanics of osteoarthritis
OA is regarded as a whole joint disease with a multifactorial etiology, including increased mechanical stress, ligament derangements, cartilage degradation, subchondral bone changes and muscular impairments (fig. 1). Furthermore, secondary synovial inflammation plays a role in OA, notably in the early stage [7]. Several studies pointed out the importance of mechanical factors in the destructive cascade of this disease [8–10].
Epidemiological studies of the last decades tried to define risk factors such as age, genetic predisposition, obesity, joint congruency, increased mechanical stress and greater bone density [11]. OA may evolve as a consequence after an antecedent incidence, such as intraarticular fractures and ligament lesions, systemic diseases like rheumatoid arthritis, hemochromatosis, haemophilia, post infectious arthritis or osteochondrosis dissecans, or as a result of a congenital or developmental anatomic abnormality [10].
OA occurs when the dynamic steady state between destructive forces and repair mechanisms destabilises the joint homeostasis [12, 13]. This imbalance is thought to be the driving force in this progressive disease and may produce pain and disability, although many patients with obvious radiographic findings don’t complain of any symptoms related to OA [14]. OA is most common in weight bearing joints such as the hips, knees and the ankle but it can occur in any synovial joint of the body.
Bone and cartilage
OA of the hips and knees are mostly the result of a slow and degenerative process [15], whereas clinical treatments studies of ankle OA showed a close coherence with a precedent trauma and are therefore classified as post-traumatic osteoarthritis [10, 16, 17]. This phenomenon is thought to be caused by the different and unique anatomical and biomechanical tissue characteristics of the hip, knee and ankle. Anatomically the contact areas are larger in the hip and the knee compared to the ankle (at 500-N load: ankle, 350 mm2; hip, 1100 mm2; knee, 1,120 mm2) [18–20]. Therefore, the ankle is exposed to higher loading pressures per mm2. Interestingly, the histological images of ankle cartilage show a higher proteoglycan density, lower matrix degradation and higher compressive stiffness [21].
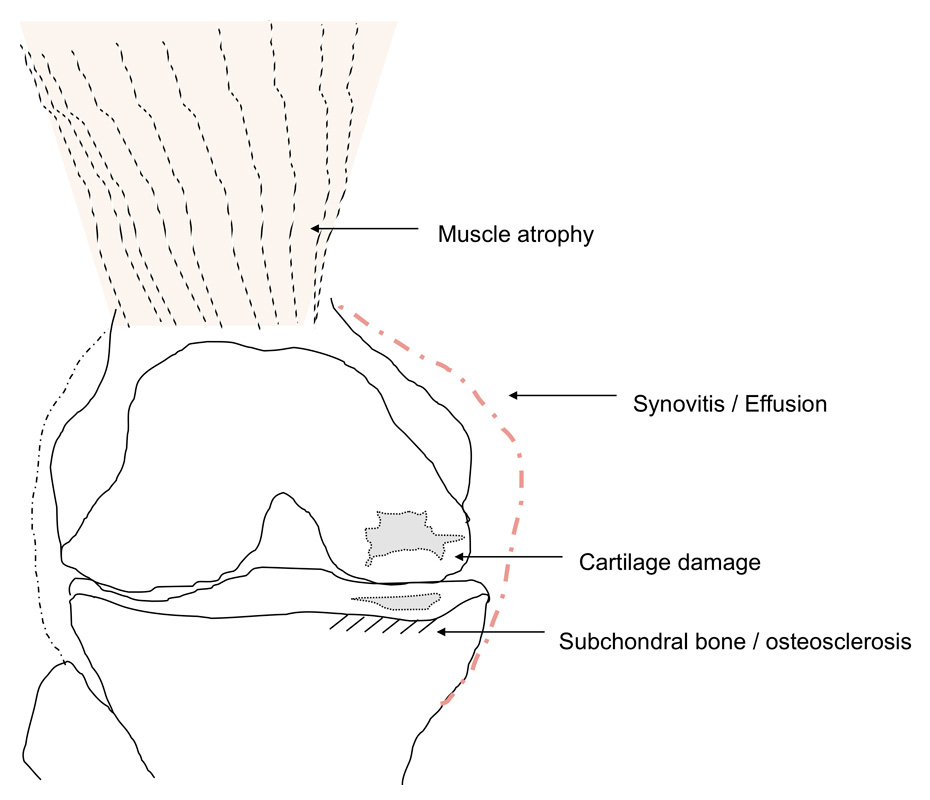
Figure 1
Schematic illustration of OA as a “whole joint disease” with structures involved in OA pathology. Muscle atrophy can cause OA, but is also seen as a consequence of OA, e.g., pain-induced. Synovitis with secretion of proinflammatory cytokines into the joint space correlates with pain and radiological progression. Cartilage degradation is the hallmark of OA. Subchondral bone pathology is also observed in OA, ultimately leading to osteosclerosis.
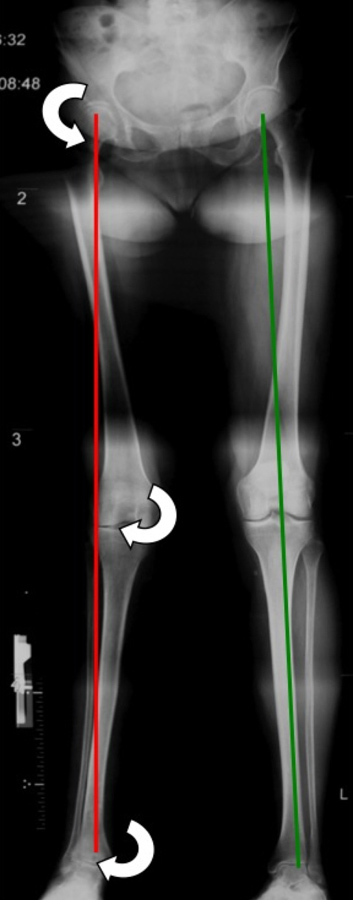
Figure 2
Long leg film with right side genu valgum and advanced ankle arthritis. The white arrows indicate the abduction moments on each joint resulting from the deviated mechanical axis.
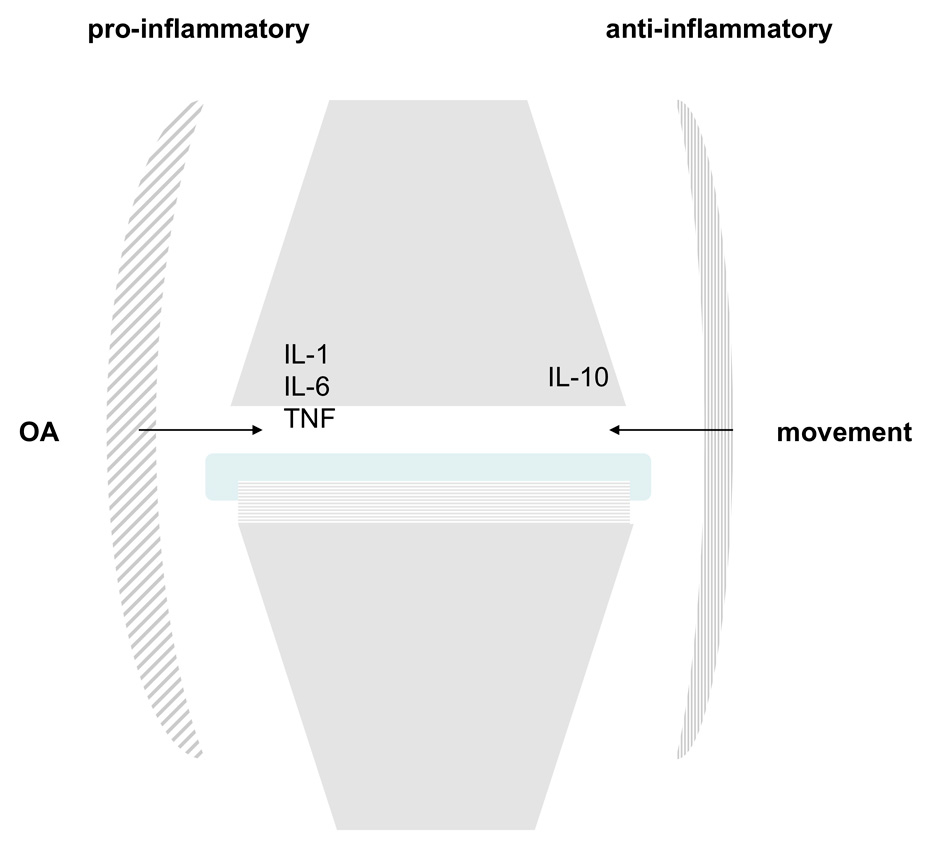
Figure 3
Cytokine expression in OA and movement. Synovitis related to OA leads to the secretion of proinflammatory cytokines such as IL-1,6 or TNF-alpha. Under normal circumstances, joint movement induces IL-10, a potent anti-inflammatory cytokine.
Biomechanically the knee joint bears higher shear forces than the hip or ankle joint as it incorporates sliding, rotating and rolling motions during movements [21]. These tissue properties combined with the different kinematics may be an explanation why the ankle joint is more resistant to degenerative OA than the hip or knee joint.
The role of bone density in OA is currently debated. Typically, a reduced bone density of the subchondral bone is observed in the early stages of OA [22]. However, at a later stage, subchondral bone sclerosis and higher bone density are seen radiologically. Subchondral osteosclerosis assessed by bone-densitometry (BMD) showed that subchondral BMD indeed might predict cartilage defect development [23]. Functional computed tomography like CT-osteoabsorptiometry (CT-OAM) also enabled visualization of the load-dependent reaction of subchondral bone mineralisation density in advanced OA [24]. The newly organised subchondral bone also contains new vessels and nerve fibers, which are likely involved in OA pathogenesis and pain sensation, respectively [25]. Cartilage and bone receive and dissipate contact loads associated with movements and weight bearing, and are therefore continuously challenged biomechanically. Recent data support the view that cartilage and bone do communicate over the calcified tissue barrier through molecular crosstalk. This interaction is crucial for the subchondral bone–articular cartilage unit and seems to show specific changes in the development of OA [25–27]. Currently it is not clear whether subchondral bone changes occur as cause or consequence of cartilage damage.
Pathobiomechanics: the fracture
Although it is virtually impossible to establish a specific time of disease onset in degenerative OA, in secondary OA patients recall a precedent injury taking into responsibility for disease initiation. For example, an intraarticular fracture implicates an incongruence of the joint line followed by axis deviation and altered load distribution across the joint. Recurrent ankle sprains or ankle fractures are very often consistent with osteochondral lesions (OCL). During arthroscopy, Taga and colleagues found chondral lesions of 89% in 9 patients with acute ankle injuries and 95% in 22 patients with chronic ankle injuries. [28]. Hintermann et al. reported in their investigation on 148 patients that 66% of those with lateral ankle instability had cartilage lesions after one or more ankle sprains, as did 98% of those with instability on the medial side [29]. Following recurrent unphysiological loading of an instable joint, the shearing forces cause chondrocyte deformation up to irreversible damage and lead to chondrocyte apoptosis [30]. They affect the biomechanical protection (gliding and shock absorption) of the joint and the contact pressures around the OCL rise combined with pain and a local inflammatory reaction [9, 31]. These stimuli let the lesion size grow, and the initial small and local damage turns into a joint-wide OA.
Pathobiomechanics: ligament Instability and muscle weakness
Besides the initial cartilage damage, i.e. through injury, the contribution of ligaments, muscles and nerves to the mechanical environment have become central to pathobiomechanical investigation. Chronic ligament laxity is frequently observed in ankle OA [17]. It has been postulated that instability of the talus leads to a pathologic range of translation and rotation, which increase the shear forces on the cartilage surface. Furthermore chronic ligament instability, on the medial or lateral side of the ankle, enhances the progressive varus or valgus malalignment of the hindfoot, which worsens the pressure distribution and accelerates the progression of OA.
Other contributing factors are muscle weakness and somatosensory deficits which are consistently accompanied by OA [32]. Muscle weakness is one of the first and most frequent symptoms in OA [33]. As observed in animal models and humans, the loss of the anterior cruciate ligament (ACL) is associated with muscle atrophy, changes in somatosensory activation patterns (i.e., force generation and motor unit recruitment) and gait kinematics [32].
However, while muscle weakness and atrophy accompanies OA it is still not clear whether it is caused by OA or precedes it. The observational work of Slemenda et al. link muscle weakness to narrowed joint space, increased knee pain and elevated development of OA in elderly women [34]. Furthermore, decreased isokinetic quadriceps muscle strength in women have been found to be an indication of increased lower limb loading during gait cycle [35]. Shown in an animal model, there is evidence that prolonged and repetitive rapid and heavy loading of joints result in deformation of articular cartilage and chondrocytes and might promote the initiation and progression of OA [30]. In patients with endstage OA of the ankle, Valderrabano et al. showed significant loss of muscle strength in dorsiflexion and plantar flexion of the ankle (–35.1% and –36.2% respectively), measured with surface electromyography compared to age and gender matched normal subjects [36].
Muscle overload on the other hand has never been shown to cause OA in humans. Few data exist, obtained from an animal model, indicating that excessive eccentric muscle contractions exceeding a certain threshold lead to increased chondrocyte death [37].
These observational data from humans support the evidence from animal models that muscle weakness may be an independent risk factor for OA and might be the link between other risk factors such as age, obesity, sex or joint injury.
Pathobiomechanics: inflammation in OA
Joint inflammation is a well recognised feature of OA, notably in the early stage [7]. Inflammation in OA can be triggered by malalignment, overuse, trauma, crystal formation, trauma or idiopathy. Clinically, synovial inflammation is demonstrated by joint effusion and pain, often well responding to steroid infiltration. Arthroscopy studies revealed synovitis in approximately 50% of the cases [38]. MRI currently is the best diagnostic modality to assess synovitis and correlates with pain and radiological progression of OA [39]. Pathobiologically, synovitis leads to the secretion of proinflammatory cytokines such as tumor necrosis factor (TNF) –alpha, interleukin (IL)-1 or 6. This impaired cytokine balance in the synovial fluid leads to the induction of proteinases such as metalloproteinases or aggrecanase with subsequent cartilage degradation and an inflammatory reaction once the fluid has contact with the subchondral bone, e.g., by subchondral cyst formation. The current consensus based on in-vitro mechanical loadings experiments is that injurious compression leads to proteoglycan depletion, destruction of the collagen network and cartilage degradation [40]. In response, proinflammatory products are released and are postulated to activate the synovium and to cause synovitis [7, 41]. Apart from synovitis also the intraarticular fat body secretes profibrotic cytokines such as IL-6 [42]. Interestingly, movement of a joint induces the expression of IL-10, which is a potent anti-inflammatory cytokine [43] (fig. 3). This indicates that not only the induction of inflammation, but also the lack of resolution of inflammation might be of importance in OA. Possibly an impaired biomechanical process of a joint will lack this anti-inflammatory reaction in form of IL-10 expression.
The role of mechanics and the kinematic chain
The concept of biomechanics includes the assembly of the structural reaction of joint tissues to mechanical stimuli. This interplay has gained much attention in the current understanding of pathogenesis of OA [9, 12]. A joint represents the connective unit between two bones or functional segments. Joints connected serially act as a kinematic chain. This construct allows motion and simultaneously provides stability, congruency and shock absorption. Alignments, adduction moments and muscle balancing are the key determinants for optimal load reduction and distribution as well as to guarantee painless gliding: hence unphysiological loading patterns on one joint may influence the adjacent levels as well.
All joints are exposed to biomechanical loading but most scientific data result from the lower extremity, especially from the knee joint, because it is the most commonly affected joint by OA and it has been extensively examined by joint kinematic testing, gait analysis and other investigations [44–46].
Adequate mechanical loading provides the essential stimulus to maintain physiological joint homeostasis, whereas excessive mechanical stress as well as unloading the joint is crucial for the disease onset and progression [31, 47]. Over the last two decades it has been shown that altered joint biomechanics of the knee, such as loss of cruciate ligaments, removal of menisci, posttraumatic cartilage damage, changes in bone alignments, unloading through casting and overloading through intense exercise may cause disease initiation and progression of cartilage degradation [8, 9, 12].
Mechanical axis
The mechanical axis or alignment of the lower extremity is defined as a line drawn from the centre of the femoral head to the centre of the talus. The hip-knee-ankle alignment is best assessed with long leg films (fig. 2). This line passes close to the centre of the tibial head between the eminentia tibiae, approximately 1° in varus (neutral alignment 0–2°) [48]. Therefore, the medial compartment of the knee sustains 60–70% of the load [46, 48]. This physiologically imbalanced dispensation may be a predisposing factor to medially accentuated tibio-femoral OA. A varus-aligned knee is described as “bow-legged” and a valgus-aligned knee is described as “knocked-knee". This valgus or varus alignment have been described to influence the load distribution across the articular joint surface [49]. This asymmetry reduces the area of load bearing and amplifies the resultant load to the remaining joint surface. The deviation of the mechanical axis does strongly correlate with radiographic joint space narrowing, subchondral cyst formation, bone sclerosis, and functional decline in OA [49].
Dynamic loads
Static loads have a significant impact for OA pathophysiology, but they do not reflect loads generated during locomotion. Movements, angulation and normal gait produce dynamic loads on joints up to five times body weight in the ankle and up to three times in the knee relative to standing [50]. Especially shearing forces are greatest during gait and ambulation. Therefore, it is most important to calculate these forces in order to assess dynamic loading during physiologic activity and its influence on OA. Dynamic loading can be measured using intraarticular pressure devices, but they are less established in humans, as direct interventions to control loading patterns are difficult to perform. However, as observed in animal models, malalignment leads to excessive dynamic loads followed by cartilage destruction and progressive OA [8, 9].
Indirect methods include gait analysis that is noninvasive and easily reproducible in humans and are therefore widely accepted [46, 51]. During gait cycles video cameras and ground reaction force plates summarise pressure and movements and transform these data to external “moments” relative to internal joint loads. In mechanics a “moment” is the tendency of force to twist or rotate an object. It is valued mathematically as the product of the force and the moment (lever) arm [52]. In varus alignment this “adduction moment” (AdM) represents a varus torque on the knee joint and is determined by the ground reaction force (GRF, force generated by the foot touching the ground) and by the distance of the GRF vector from the center of the knee joint. If any of these parameters alter, the AdM is gravely affected. For example, a genu varus has a mechanical axis, which runs medial to the joint centre; therefore the lever arm increases resulting in an AdM of greater magnitude. Yang et al. showed in a recent study in humans that maximum values of stress and strain on articular cartilage of the knee are in line with the peak adduction moment during the stance phase of gait. At the early and late stance phase (heel strike to foot flat and toe off, respectively) a valgus moment is present together with maximum compressive stress on the lateral knee compartment. During mid stance phase (i.e., 25–75% of gait cycle) a varus moment occurs to the knee in line with maximum compressive stress on the medial knee compartment [53]. Moreover, they showed that a preexisting varus alignment of the knee increase the compressive stresses compared to subjects with normal or valgus knee alignment. In the only longitudinal study, Miyazaki et al. found that for every one unit increase in the peak AdM, there was a 6.5-fold increase in the risk of medial compartment disease progression on X-ray in a cohort of 74 patients [54]. Moreover, several authors found that the AdM is directly associated with radiographic findings, cartilage loss and pain [49, 55].
The AdM has become a widely accepted marker to measure joint loading. At the same time its limitations are given as it only reflects the load at a specific time of gait. Differences during gait, walking speed and stance phase are not represented. This comes into consideration because obese and older patients with OA walk slower and have longer stance phases, so their total time weight bearing is longer than normal [51, 56]. Up to now the identification of distinct prognostic factors for progression of OA of the lower limb has been difficult with the strongest evidence being for static malalignment [49]. However, even though AdM has been shown to predict medial knee OA, a recent study by the same group showed that reducing the AdM by conservative means even after one year, did not provide any structural or symptomatic benefits compared to a untreated control group [57]. These findings arouse the suspicion that AdM is an important determinant but not the only one in the multifactorial disease of OA.
As mentioned before, the contribution of muscles in load absorption and distribution throughout the kinematic chain of the lower limb play an integral role. Various analyses of gait have found that the quadriceps, hamstrings and the gastrocnemius muscles are able to produce an internal valgus moment and provide stabilisation against external adduction moments [53, 58]. In patients with medial OA and an varus alignment these muscle show altered activity level and is probably an attempt to unload the medial knee compartment [53]. This underlines the complexity to calculate the total loads running across a joint and the resultant effect on the articular surfaces.
Osteoarthritis treatment options
To date a medical cure for OA, a “restitutio ad integrum”, does not exist, nor do disease modifying OA drugs (DMOADs) comparable to disease modifying anti-rheumatic drugs (DMARs). Treating patients suffering from OA consists of mainly treating symptoms, providing joint stability and trying to postpone end stage OA. Notwithstanding, inflammation in OA is a more and more recognised target for therapies in form of biologics. Within the variety of treatment options, surgical interventions should only take place if conservative strategies have failed to relief symptoms (fig. 4). The aim is to restore ligament balancing, to realign mechanical axes and to re-establish the natural biomechanics of the joint. If joint preserving procedures, joint replacement or joint fusions (arthrodesis) is the optimal treatment needs to be decided on a individual base [59].
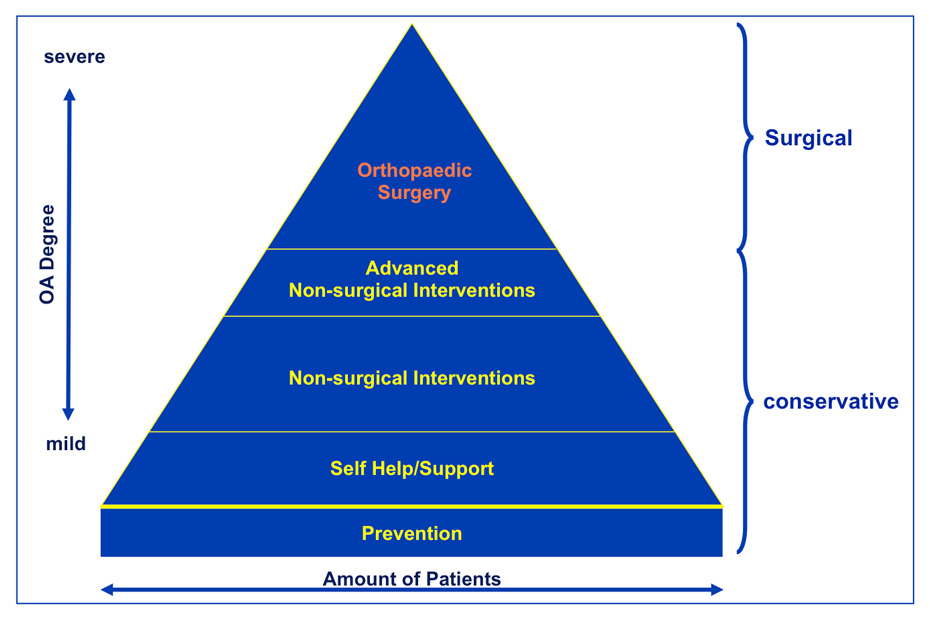
Figure 4
Treatment algorhythm for OA patients.
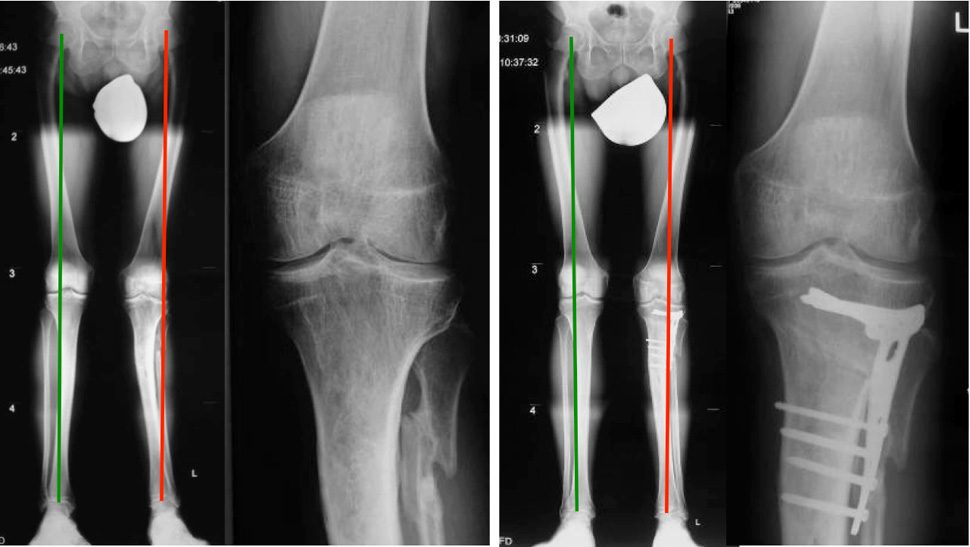
Figure 5
Patient before and after realignment surgery with an open wedge osteotomy of the proximal tibia.
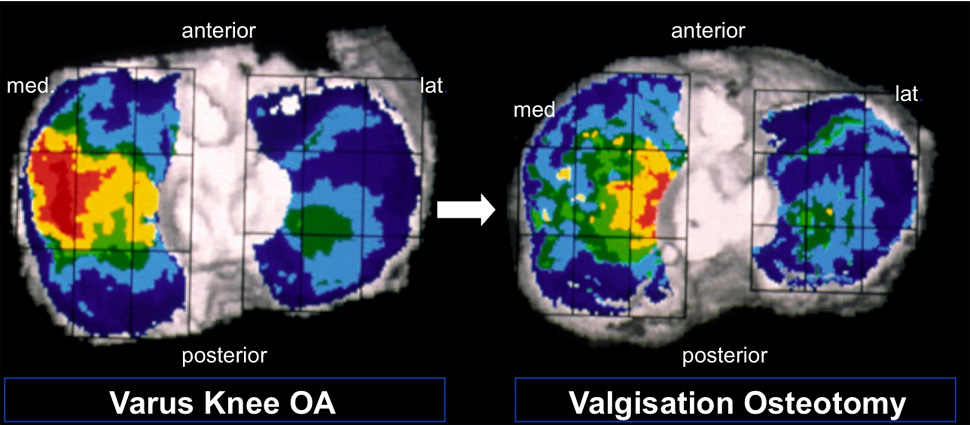
Figure 6
CT-OAM before and 2 years after valgisation osteotomy of the proximal tibia. Areas with high subchondral bone density are coloured in red and yellow (© Madry H, van Dijk CN, Mueller-Gerbl M. The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):419–33. Reprinted with kind permission).

Figure 7
Multi-level surgery: 53 year old patients with rheumatoid and psoriasis arthritis, consistent of two total hip arthroplasties, two total knee arthroplasties, realignment surgery of the right ankle, and two total ankle replacements.
Conservative treatment strategies
Conservative treatment strategies include footwear interventions, braces, gait modifications, muscle strengthening and weight loss. Barefoot walking has been shown to reduce the knee adduction moment by 7–13% compared to normal shoes and up to 23% compared to high-heeled shoes in healthy women [60, 61]. The use of lateral wedge insoles in patients with OA led to a immediate reduction of pain during walking and a reduction of the knee adduction moment by 4–14% [57]. However, this study did not show any influence on disease progression over a 12-month period.
Valgus knee braces and gait modifications with a toe-out gait have been shown to reduce knee adduction moment significantly [62, 63]. Despite the fact that such strategies are simple, they need consequent adherence by the patient to be effective.
Obesity by itself enhances not only the load to the joints of the lower limb during stance, but it also magnifies the effects of malalignment during walking [56, 64]. Diet and weight loss are important therapeutic measures, because they result in a direct reduction of load exerted on the knee during locomotion [65].
Studies on specific muscle training in humans, such as strengthening of the quadriceps and the hip abductors muscles showed a significant decrease of the knee adduction moment, less cartilage loss of the patello-femoral joint and a reduction of pain, but have failed to show any protective effects for malaligned or lax knees [34, 66, 67].
Neuromuscular training aims to improve sensomotor control and functional stability. The long-term results of a prospective cohort study of 100 ACL deficient patients who were treated using supervised neuromuscular rehabilitation showed remarkably low incidence of OA after 15 years [68]. These results underline the current understanding that strong muscles and neuromuscular control is needed to absorb altered knee loads seen in patients with these defects.
Joint preserving surgery
In many cases of mechanically induced OA of the lower limb, cartilage defects are regionally limited with partially preserved areas.
The symptomatic stage in knee and ankle OA typically occurs when patients are in their economically important active middle ages, because trauma is the predominant cause of their OA [20, 28]. Controversy on the recommended therapy is related to increasing life expectancy and high activity demands of these patients [59, 69]. Joint sparing osteotomies are generally used in selected patients, for example for unicompartimental knee OA and varus or valgus ankle OA, to unload the affected joint area as well as for hip OA to improve joint congruency and coverage [70, 71]. Young, active patients may benefit from joint preservation surgery to postpone arthroplasty.
The efficacy of realignment surgery to restore almost normo-biomechanics has been shown to relieve pain and improve function [71]. The orthopaedic procedure includes correction osteotomy, with either open or closing wedge, on the proximal tibia for the knee and on the distal tibia for the ankle, followed by an arthroscopy for cartilage and meniscal surgery, if needed (fig. 5). The shifted and normalised mechanical axis allows addressing the osteochondral lesions with orthobiologics and viscosupplementation [71]. There are a variety of procedures available for the management of OCL’s: the surgeon may treat the articular cartilage, i.e., autologous chondrocytes implantations (ACI), the bone with microfracturing, or both with transplantation of osteochondral allografts (OATS) or mosaicplasty [72, 73]. Lately collaborations with tissue engineering broad the horizons of orthopaedic researchers with synthetic scaffolds like autologous osteochondral plugs [74].
Using CT-OAM Mueller-Gerbl et al. were able to show remodelling of the subchondral bone density after realignment surgery of the proximal tibia [24]. These data indicate that realignment surgery is a powerful tool to realign biomechanical loading forces across the joint with subsequent bone reaction (fig. 6).
Total joint replacement
In cases of advanced OA with totally destroyed articular surfaces, these highly specialised procedures are not applicable. Here total joint replacements (TJR) are the treatment of choice. The use of high quality materials, anatomical modular systems and minimal invasive surgical procedures allow patient-tailored solutions, efficient and quick rehabilitation programmes and long durability of materials [51, 75, 76].
Total joint replacements of the knee and the ankle may preserve the neighbouring joints from mechanical overload and wear, but correction of deformity and adjusting of ligament balance are limited [59, 70]. Infections, failure of prosthesis and loss of bone stock may challenge the treating surgeon with revision arthroplasty and imminent fusion. Joint fusion especially in the ankle may enable a higher activity level, but degeneration rates of the neighbouring joints are elevated up to 50% after 7–8 years and 100% after 22 years [77, 78].
Compared to ankle OA these issues are less threatening in knee and hip OA because arthroplasty of these joints have better long-term outcomes and good functional results. Buechel and colleagues reported 97% and 83% survival rates after 10 and 16 years of total knee replacement [75]. Even better results are documented in hip arthroplasty with 90% survival rate after a minimum of 30 years with revision as end point [76].
Severe OA accompanied with profound deformities are very often not limited to one joint only. To restore normal biomechanics the therapy of choice is to address every level of the kinematic chain. Otherwise the destruction of the adjacent joints proceeds, resulting in further aberrant mechanical alignments and advanced wear of the replaced joint. Multi-level surgery might provide a solution in these severe cases (fig. 7). Although extensive preoperative planning and precise patient selection are crucial, because these highly invasive procedures demand a great deal of the patient and require a close guidance and adequate patient compliance.
Conclusion
OA and biomechanics are inescapably linked together. However, the contribution of biomechanical factors to aetiology, pathogenesis and to disease progression require further research in order to reduce the enormous socioeconomic and personal impact of this disease. To this end, modern treatment pathways including the collaboration of basic sciences e.g., tissue engineering and pathology, diagnostics, biomarker research, conservative treatment strategies and orthopaedic surgery are necessary to guarantee an optimal, individually adapted treatment plan with respect to joint biomechanics and biomechanical reactivity.
Literature
1 Solignac M. COART France 2003 report on new socioeconomic data on osteoarthritis in France. Presse Med. 2004;33(9 Pt 2):S4–6.
2 Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35.
3 (CDC) CfDCaP. Prevalence and Impact of chronic joint symptoms – seven states. MMWR – Morb Mortal Wkly Rep. 1998;47:345–51.
4 Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84(3):351–8.
5 Gupta S, Hawker GA, Laporte A, Croxford R, Coyte PC. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology (Oxford). 2005;44(12):1531–7.
6 Arias E. United States life tables, 2002. Natl Vital Stat Rep. 2004;53(6):1–38.
7 Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6(11):625–35.
8 Herzog W, Adams ME, Matyas JR, Brooks JG. Hindlimb loading, morphology and biochemistry of articular cartilage in the ACL-deficient cat knee. Osteoarthritis Cartilage. 1993;1(4):243–51.
9 Sun HB. Mechanical loading, cartilage degradation, and arthritis. Ann N Y Acad Sci. 2010;1211:37–50.
10 Valderrabano V, Horisberger M, Russell I, Dougall H, Hintermann B. Etiology of ankle osteoarthritis. Clin Orthop Relat Res. 2009;467(7):1800–6.
11 World Health Organization. The Bone and Joint Decade. http://www.boneandjointdecade.org. 2001.
12 Helminen HJ, Saamanen AM, Jurvelin J, Kiviranta I, Parkkinen JJ, Lammi MJ, et al. The effect of loading on articular cartilage. Duodecim. 1992;108(12):1097–107.
13 Eyre DR. Collagens and cartilage matrix homeostasis. Clin Orthop Relat Res. 2004;(427 Suppl):S118–22.
14 Bagge E, Bjelle A, Eden S, Svanborg A. Osteoarthritis in the elderly: clinical and radiological findings in 79 and 85 year olds. Ann Rheum Dis. 1991;50(8):535–9.
15 Gunther KP, Sturmer T, Sauerland S, Zeissig I, Sun Y, Kessler S, et al. Prevalence of generalised osteoarthritis in patients with advanced hip and knee osteoarthritis: the Ulm Osteoarthritis Study. Ann Rheum Dis. 1998;57(12):717–23.
16 Thomas RH, Daniels TR. Ankle arthritis. J Bone Joint Surg Am. 2003;85-A(5):923–36.
17 Valderrabano V, Hintermann B, Horisberger M, Fung TS. Ligamentous posttraumatic ankle osteoarthritis. Am J Sports Med. 2006;34(4):612–20.
18 Brown TD, Shaw DT. In vitro contact stress distributions in the natural human hip. J Biomech. 1983;16(6):373–84.
19 Kimizuka M, Kurosawa H, Fukubayashi T. Load-bearing pattern of the ankle joint. Contact area and pressure distribution. Arch Orthop Trauma Surg. 1980;96(1):45–9.
20 Ihn JC, Kim SJ, Park IH. In vitro study of contact area and pressure distribution in the human knee after partial and total meniscectomy. Int Orthop. 1993;17(4):214–8.
21 Treppo S, Koepp H, Quan EC, Cole AA, Kuettner KE, Grodzinsky AJ. Comparison of biomechanical and biochemical properties of cartilage from human knee and ankle pairs. J Orthop Res. 2000;18(5):739–48.
22 Intema F, Hazewinkel HA, Gouwens D, Bijlsma JW, Weinans H, Lafeber FP, et al. In early OA, thinning of the subchondral plate is directly related to cartilage damage: results from a canine ACLT-meniscectomy model. Osteoarthritis Cartilage. 2010;18(5):691–8.
23 Dore D, Quinn S, Ding C, Winzenberg T, Cicuttini F, Jones G. Subchondral bone and cartilage damage: a prospective study in older adults. Arthritis Rheum. 2010;62(7):1967–73.
24 Madry H, van Dijk CN, Mueller-Gerbl M. The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):419–33.
25 Walsh DA, McWilliams DF, Turley MJ, Dixon MR, Franses RE, Mapp PI, et al. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology (Oxford). 2010;49(10):1852–61.
26 Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230–7.
27 Sanchez C, Pesesse L, Gabay O, Delcour JP, Msika P, Baudouin C, et al. Regulation of subchondral bone osteoblast metabolism by cyclic compression. Arthritis Rheum. 2011.
28 Taga I, Shino K, Inoue M, Nakata K, Maeda A. Articular cartilage lesions in ankles with lateral ligament injury. An arthroscopic study. Am J Sports Med. 1993;21(1):120–6; discussion 6–7.
29 Hintermann B, Boss A, Schafer D. Arthroscopic findings in patients with chronic ankle instability. Am J Sports Med. 2002;30(3):402–9.
30 Abusara Z, Seerattan R, Leumann A, Thompson R, Herzog W. A novel method for determining articular cartilage chondrocyte mechanics in vivo. J Biomech. 2011;44(5):930–4.
31 Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7(5):619–36.
32 Herzog W, Longino D. The role of muscles in joint degeneration and osteoarthritis. J Biomech. 2007;40 Suppl 1:S54–63.
33 Palmieri-Smith RM, Thomas AC, Karvonen-Gutierrez C, Sowers MF. Isometric quadriceps strength in women with mild, moderate, and severe knee osteoarthritis. Am J Phys Med Rehabil. 2010;89(7):541–8.
34 Slemenda C, Heilman DK, Brandt KD, Katz BP, Mazzuca SA, Braunstein EM, et al. Reduced quadriceps strength relative to body weight: a risk factor for knee osteoarthritis in women? Arthritis Rheum. 1998;41(11):1951–9.
35 Mikesky AE, Meyer A, Thompson KL. Relationship between quadriceps strength and rate of loading during gait in women. J Orthop Res. 2000;18(2):171–5.
36 Valderrabano V, von Tscharner V, Nigg BM, Hintermann B, Goepfert B, Fung TS, et al. Lower leg muscle atrophy in ankle osteoarthritis. J Orthop Res. 2006;24(12):2159–69.
37 Horisberger M, Fortuna R, Leonard TR, Valderrabano V, Herzog W. The influence of cyclic concentric and eccentric submaximal muscle loading on cell viability in the rabbit knee joint. Clin Biomech (Bristol, Avon). 2011.
38 Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis – results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 2005;13(5):361–7.
39 Krasnokutsky S, Belitskaya-Levy I, Bencardino J, Samuels J, Attur M, Regatte R, et al. Quantitative magnetic resonance imaging evidence of synovial proliferation is associated with radiographic severity of knee osteoarthritis. Arthritis Rheum. 2011;63(10):2983–91.
40 Oliviero F, Ramonda R, Punzi L. New horizons in osteoarthritis. Swiss Med Wkly. 2010;140:w13098.
41 Goldring MB, Otero M, Plumb DA, Dragomir C, Favero M, El Hachem K, et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cell Mater. 2011;21:202–20.
42 Klein-Wieringa IR, Kloppenburg M, Bastiaansen-Jenniskens YM, Yusuf E, Kwekkeboom JC, El-Bannoudi H, et al. The infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype. Ann Rheum Dis. 2011;70(5):851–7.
43 Helmark IC, Mikkelsen UR, Borglum J, Rothe A, Petersen MC, Andersen O, et al. Exercise increases interleukin-10 levels both intraarticularly and peri-synovially in patients with knee osteoarthritis: a randomized controlled trial. Arthritis Res Ther. 2010;12(4):R126.
44 Jackson BD, Wluka AE, Teichtahl AJ, Morris ME, Cicuttini FM. Reviewing knee osteoarthritis – a biomechanical perspective. J Sci Med Sport. 2004;7(3):347–57.
45 Hassan BS, Doherty SA, Mockett S, Doherty M. Effect of pain reduction on postural sway, proprioception, and quadriceps strength in subjects with knee osteoarthritis. Ann Rheum Dis. 2002;61(5):422–8.
46 Andriacchi TP. Dynamics of knee malalignment. Orthop Clin North Am. 1994;25(3):395–403.
47 Torzilli PA, Grigiene R, Huang C, Friedman SM, Doty SB, Boskey AL, et al. Characterization of cartilage metabolic response to static and dynamic stress using a mechanical explant test system. J Biomech. 1997;30(1):1–9.
48 Cooke TD, Sled EA, Scudamore RA. Frontal plane knee alignment: a call for standardized measurement. J Rheumatol. 2007;34(9):1796–801.
49 Tanamas S, Hanna FS, Cicuttini FM, Wluka AE, Berry P, Urquhart DM. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61(4):459–67.
50 Harrington IJ. A bioengineering analysis of force actions at the knee in normal and pathological gait. Biomed Eng. 1976;11(5):167–72.
51 Valderrabano V, Nigg BM, von Tscharner V, Stefanyshyn DJ, Goepfert B, Hintermann B. Gait analysis in ankle osteoarthritis and total ankle replacement. Clin Biomech (Bristol, Avon). 2007;22(8):894–904.
52 Roberts A. Statics and Dynamics with a Background in Mathematics2003.
53 Yang NH, Nayeb-Hashemi H, Canavan PK, Vaziri A. Effect of frontal plane tibiofemoral angle on the stress and strain at the knee cartilage during the stance phase of gait. J Orthop Res. 2010;28(12):1539–47.
54 Miyazaki T, Wada M, Kawahara H, Sato M, Baba H, Shimada S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis. 2002;61(7):617–22.
55 Bennell KL, Bowles KA, Wang Y, Cicuttini F, Davies-Tuck M, Hinman RS. Higher dynamic medial knee load predicts greater cartilage loss over 12 months in medial knee osteoarthritis. Ann Rheum Dis. 2011;70(10):1770–4.
56 Sharma L, Lou C, Cahue S, Dunlop DD. The mechanism of the effect of obesity in knee osteoarthritis: the mediating role of malalignment. Arthritis Rheum. 2000;43(3):568–75.
57 Bennell KL, Bowles KA, Payne C, Cicuttini F, Williamson E, Forbes A, et al. Lateral wedge insoles for medial knee osteoarthritis: 12 month randomised controlled trial. BMJ. 2011;342:d2912.
58 Shelburne KB, Torry MR, Pandy MG. Contributions of muscles, ligaments, and the ground-reaction force to tibiofemoral joint loading during normal gait. J Orthop Res. 2006;24(10):1983–90.
59 Valderrabano V, Pagenstert G, Horisberger M, Knupp M, Hintermann B. Sports and recreation activity of ankle arthritis patients before and after total ankle replacement. Am J Sports Med. 2006;34(6):993–9.
60 Kerrigan DC, Lelas JL, Karvosky ME. Women’s shoes and knee osteoarthritis. Lancet. 2001;357(9262):1097–8.
61 Shakoor N, Block JA. Walking barefoot decreases loading on the lower extremity joints in knee osteoarthritis. Arthritis Rheum. 2006;54(9):2923–7.
62 Self BP, Greenwald RM, Pflaster DS. A biomechanical analysis of a medial unloading brace for osteoarthritis in the knee. Arthritis Care Res. 2000;13(4):191–7.
63 Jenkyn TR, Hunt MA, Jones IC, Giffin JR, Birmingham TB. Toe-out gait in patients with knee osteoarthritis partially transforms external knee adduction moment into flexion moment during early stance phase of gait: a tri-planar kinetic mechanism. J Biomech. 2008;41(2):276–83.
64 Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109(1):18–24.
65 Messier SP, Gutekunst DJ, Davis C, DeVita P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52(7):2026–32.
66 Amin S, Baker K, Niu J, Clancy M, Goggins J, Guermazi A, et al. Quadriceps strength and the risk of cartilage loss and symptom progression in knee osteoarthritis. Arthritis Rheum. 2009;60(1):189–98.
67 Thorp LE, Wimmer MA, Foucher KC, Sumner DR, Shakoor N, Block JA. The biomechanical effects of focused muscle training on medial knee loads in OA of the knee: a pilot, proof of concept study. J Musculoskelet Neuronal Interact. 2010;10(2):166–73.
68 Ageberg E, Pettersson A, Friden T. 15-year follow-up of neuromuscular function in patients with unilateral nonreconstructed anterior cruciate ligament injury initially treated with rehabilitation and activity modification: a longitudinal prospective study. Am J Sports Med. 2007;35(12):2109–17.
69 Hardeman F, Londers J, Favril A, Witvrouw E, Bellemans J, Victor J. Predisposing factors which are relevant for the clinical outcome after revision total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2011.
70 Paley D, Pfeil J. Principles of deformity correction around the knee. Orthopade. 2000;29(1):18–38.
71 Pagenstert GI, Hintermann B, Barg A, Leumann A, Valderrabano V. Realignment surgery as alternative treatment of varus and valgus ankle osteoarthritis. Clin Orthop Relat Res. 2007;462:156–68.
72 Valderrabano V, Leumann A, Rasch H, Egelhof T, Hintermann B, Pagenstert G. Knee-to-ankle mosaicplasty for the treatment of osteochondral lesions of the ankle joint. Am J Sports Med. 2009;37(Suppl 1):105S–11S.
73 Bedi A, Feeley BT, Williams RJ, 3rd. Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92(4):994–1009.
74 Scotti C, Wirz D, Wolf F, Schaefer DJ, Burgin V, Daniels AU, et al. Engineering human cell-based, functionally integrated osteochondral grafts by biological bonding of engineered cartilage tissues to bony scaffolds. Biomaterials. 2010;31(8):2252–9.
75 Buechel FF, Sr. Long-term followup after mobile-bearing total knee replacement. Clin Orthop Relat Res. 2002(404):40–50.
76 Callaghan JJ, Templeton JE, Liu SS, Pedersen DR, Goetz DD, Sullivan PM, et al. Results of Charnley total hip arthroplasty at a minimum of thirty years. A concise follow-up of a previous report. J Bone Joint Surg Am. 2004;86-A(4):690–5.
77 Coester LM, Saltzman CL, Leupold J, Pontarelli W. Long-term results following ankle arthrodesis for post-traumatic arthritis. J Bone Joint Surg Am. 2001;83-A(2):219–28.
78 Takakura Y, Tanaka Y, Sugimoto K, Akiyama K, Tamai S. Long-term results of arthrodesis for osteoarthritis of the ankle. Clin Orthop Relat Res. 1999(361):178–85.






