Modelling HIV infection and therapies in humanised mice
DOI: https://doi.org/10.4414/smw.2012.13618
Marc
Nischang, Gustavo
Gers-Huber, Annette
Audigé, Ramesh
Akkina, Roberto F
Speck
Summary
The human immunodeficiency virus (HIV) type-1 is a human-specific virus. The lack of a widely available small-animal model has seriously hampered HIV research. In 2004, a new humanised mouse model was reported. It was based on the intrahepatic injection of human CD34+ cord blood cells into newborn, highly immunodeficient mice. These mice develop a lymphoid system of human origin and are highly susceptible to HIV infection and showed disseminated infection, persistent viraemia and characteristic helper CD4+ T-cell loss. Here, we will briefly review the various existing humanised mouse models and highlight their value to the study of HIV infection.
The HIV situation globally
The UNAIDS report on the Global AIDS Epidemic 2010 optimistically announced that the HIV pandemic had peaked in the preceding two years. Nevertheless, the numbers are shocking: an estimated 33.3 million humans – 0.8% of all adults 15–49 years old – are infected, and more than 1.8 million people died in 2009 (http://www.unaids.org/globalreport/Global_report.htm). More hopefully, the number of people newly infected with HIV declined by nearly one-fifth over the last decade (1999, 3.1 million; 2009, 2.6 million). This decline is based on more widely applied “safer sexual practices” and reductions in mother-child transmission. The UNAIDS vision is Zero New Infections, Zero Discrimination, Zero HIV-associated Deaths. The goal is to halt and reverse the spread of HIV.
A human-specific virus: a challenge for in vivo studies
HIV specifically infects human cells. Even cells from chimpanzee, a very close relative of humans, are only somewhat permissive to HIV infection [1]. Human host factors are critical for the virus throughout its entire replication cycle (fig. 1). For example, to enter a cell and begin its replication cycle, HIV engages a receptor complex of CD4 and a chemokine receptor, either CCR5 or CXCR4 [2, 3]. However, expressing human CD4 on murine cells does not make them permissive to HIV. Other human-specific factors, such as the human chemokine receptors, are needed.
Over the last three decades, human transgenes essential for HIV replication were expressed together in rodent cells, but the cells were still not permissive [4]. Furthermore, human transgenes were expressed in rodents in an attempt to generate HIV small-animal models. These models confirmed the human-specific nature of HIV and the in vitro data. No replication was observed in mice expressing human CD4 [5], CD4 and CCR5 [6] and Cyclin CDK9 [7], and rats transgenic for human CD4 and CCR5 replicated HIV only at very low levels for limited times [8, 9].
Other in vivo studies attempted to create models based on creating chimeric HIV strains. This approach relies on engineering a distinct HIV gene in a species-specific retrovirus, which despite the HIV transgene, replicates vigorously in the original species (e.g., simian-immunodeficiency virus [SIV] engineered to express the HIV envelope [SHIV]) [10]. The use of SHIV in monkeys allowed key questions about immune responses to vaccine constructs expressing various HIV gene encoded proteins to be addressed [10]. However, use of monkeys as animal models is restricted to specific questions with a narrow focus and cannot recapitulate the overall complexity of HIV, since the biological properties between SHIV, SIV and HIV are quite distinct.
Finally, HIV-encoded gene products were expressed entirely as transgenes in mice [11–15]. These studies provided insight into the pathogenic potential of HIV gene products. However, they were expressed universally at high levels, and it is difficult to assess the significance of the resulting data since the dynamic nature of true HIV replication is lacking.
The requirements for a mouse model to study HIV infection
Faithfully modelling any human disease in an animal is difficult. Does the model replicate enough key features of the disease to allow us to conduct experiments?
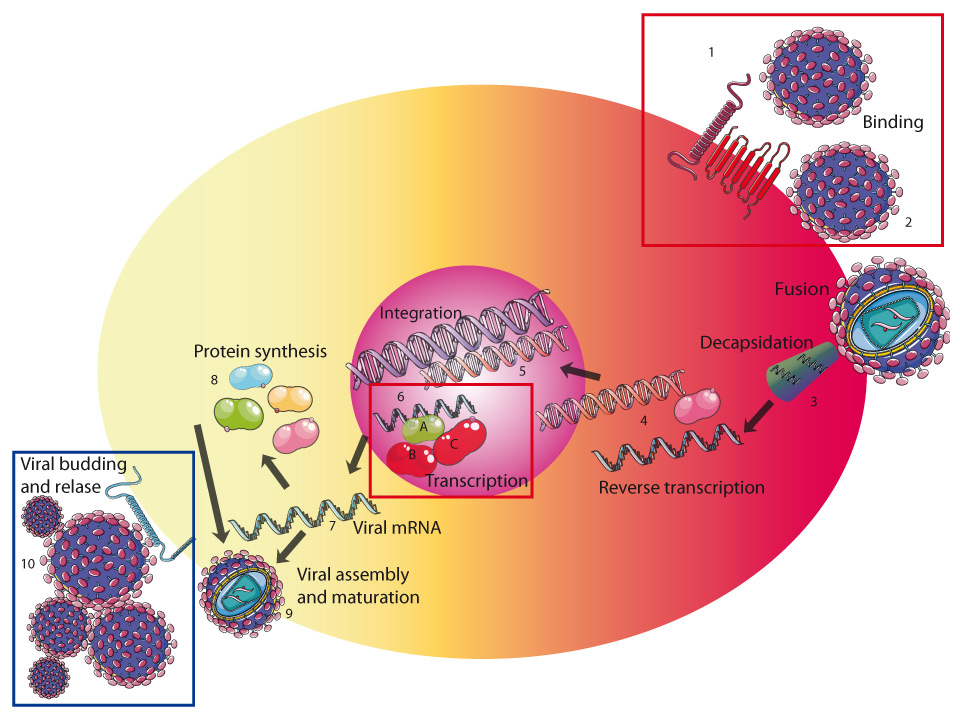
Figure 1
HIV-1 needs critical host factors for efficient replication. HIV binds to the HIV receptor complex of the human CD4 cell-surface molecule and a co-receptor, either CCR5 or CXCR4, via the HIV envelope glycoprotein 120 (HIV env gp120). After conformational changes in the HIV env gp41, viral host cell membrane fusion occurs (2). The next steps are the decapsidation (3) and release of the HIV RNA from the virus particle. Reverse transcription generates a viral complementary DNA (cDNA) based on the viral RNA template and using HIV’s own reverse transcriptase (4). Once the cDNA is generated, the preintegration complex (PIC) is assembled, nuclear trafficking and integration of the viral cDNA into the host genomic DNA follow (5). Efficient transcription and elongation require formation of P-TEFb (positive transcription elongation factor b) consisting of Tat (6A), human cyclin-dependent kinase 9 (CDK-9) (6B) and cyclin T1 (6C), which binds to the nascent HIV transcripts. Fully or partially spliced HIV mRNA (7) is used to translate viral proteins. Unspliced HIV RNA is packaged into newly generated virions. Assembly of HIV proteins and RNA and budding takes place at the cellular membrane (9). HIV release is inhibited by murine tetherin at the cellular membrane because murine tetherin is insensitive to the viral protein Vpu, which inhibits human tetherin by directing its proteasomal degradation (blue frame). Human host factors critical for HIV replication are CD4, CCR5, CXCR4 and cyclin T1 (red frame). Additional human specific factors probably exist.
Many of the key features of HIV infection are known. The main route of HIV transmission is vaginal or rectal intercourse. In acute HIV infection, a massive productive infection causes cell death in the lymphatic system, most prominently in the gastrointestinal tract. About 3–5 weeks after acute HIV infection, the levels of HIV RNA decline and the specific anti-HIV CD8+ T-cell response begins. Unlike in acute infection, fewer than 1% of CD4+ T cells are productively infected in the chronic phase [16], a number that cannot fully explain the progressive immunodeficiency. Poorly understood bystander effects seem to contribute to the overall cell loss [17], and sustained immune activation triggers it [18]. Combined antiretroviral treatment (cART) has been very successful in suppressing HIV RNA levels to below the limit of detection in about 90% of treated patients [19] and has resulted in a marked reduction of morbidity and mortality [20]. However, cART does not cure HIV. A small portion of HIV remains silent in long-lived cells, such as the quiescent memory CD4+ T-cells [21]; these cells form a latent reservoir of HIV. Besides finding simpler and more efficient treatment strategies, major efforts are now aimed at eradicating the latently infected cells to eventual cure HIV, and to develop novel gene therapy approaches and vaccination strategies. Other efforts are focused on orally administered pre-exposure prophylactic measures using anti-retroviral drugs and finding effective topical microbicides that can prevent sexual transmission.
Thus, the requirements for a HIV mouse model include the following:
– Permissiveness to replication-competent HIV with distinct co-receptor usage (i.e., CCR5- or CXCR4-tropic HIV strains), resulting in high-level viraemia, systemic viral dissemination and histopathology reminiscent of HIV disease in humans.
– Supporting long-term chronic infection, allowing monitoring of HIV infection over time.
– Susceptibility to natural transmission modes of HIV, including vaginal and rectal routes.
– Displaying gradual depletion of CD4+ T-cell numbers during HIV infection.
– Activation of the immune system to lead to HIV-specific immune responses.
– Establishment and maintenance of an HIV latent reservoir.
– Allow development and testing of anti-HIV therapeutic and prevention strategies.
Humanised mice in general
The generation of humanised mice involves either the expression of human transgenes or the transplantation of human tissue into immunodeficient mice. However, as mentioned above, even constitutive expression of multiple human transgenes has not rendered mice fully permissive to HIV infection.
The human-PBL-SCID and foetal thy/liv SCID mouse model
Transplantation of human tissue into immunodeficient mice without rejection was first reported in the early 1980s. This became possible with the identification of a spontaneous mutation of the Prkdc gene in mice, which results in the complete lack of T and B cells and consequently in severe combined immunodeficiency (C.B.-17 SCID/SCID [SCID]; descriptions of the various mouse strains, see box) [22]. The Prkdcgene encodes for the catalytic subunit of a DNA-dependent protein kinase that is needed for V(D)J recombination in developing T and B lymphocytes. The two early humanised (Hu) mouse models were the foetal thymus/liver (thy/liv) SCID-hu mouse [23, 24] and the hu-PBL-SCID (PBL, peripheral blood leucocytes) mouse [25].
The foetal thy/liv SCID-hu mouse model is based on surgical placement of foetal thymus/liver tissue under the renal capsule. At 4–6 months post-implantation, foetal thymus/liver tissue forms a conjoint organoid that resembles human thymus and sustains T-cell lymphopoiesis for over a year [23]. The system is susceptible to HIV infection, but in the absence of robust peripheral human leukocyte reconstitution, samplings to analyse the infected human cells are mainly restricted to the engrafted conjoint organoid. Also there is no multilineage human haematopoiesis in this model (table 1).
The hu-PBL-SCID mouse model is based on the intraperitoneal injection of human PBL [25] and is susceptible to HIV infection [26]. However, within days, human PBL injected into mice react against the murine disparity with a vigorous activation: their proliferation rate increases, and the CCR5 chemokine receptor and HLA-DR are upregulated [27–29], resulting in xeno-reactive T-cells [30]. Mice with significant blood T-lymphocyte chimerism suffer from high levels of graft-versus-host disease (GVHD) and mortality. Mice with no or transient T-cell chimerism have a low incidence [31]. Use of this model is limited mostly by the lack of de novo development of continuously differentiating human cells, activation status of the xenoreactive T cells and the GVHD (table 1).
|
Table 1: Compilation of the various humanised mouse models. |
| Humanised mouse model |
Engraftment |
Cellular composition in reconstituted hu mice |
Supports HIV infection with |
Advantages |
Disadvantages |
|
hu-PBL SCID [25]
|
N/A |
T and B cells |
CCR5 and CXCR4-tropic strains§ [26, 28, 62] |
Easy to generate (i.e. good access to PBLs)
Can immediately be used after transfer of PBLs |
No multilineage haematopoiesis
Limited time frame for experiments [62]
Strong activation of T-cells [27, 28]
Emergence of xeno-reactive T-cells (GvHD) [30] |
|
Thy/Liv SCID hu [23]
|
N/A |
T cells
Single positive double positive and double negative Thymocytes |
CCR5-* and CXCR4-tropic strains [24, 58, 59] |
Organoid of foetal thymus/liver tissue with sustained T-cell lymphopoiesis
Valuable to study certain pathogenic aspects (see text) |
Surgical skills needed
Human foetal tissue needed
No multilineage haematopoiesis
Sampling mainly restricted to the organoid since lack of solid peripheral reconstitution
Lack of CCR5 expression on intrathymic T progenitor cells |
|
Rag2-/- γc -/-[32]
|
++ |
T and B cells
Monocytes
Macrophages
NK cells
DCs |
CCR5- and CXCR4-tropic strains [68, 69, 71] |
Long-term multilineage haematopoiesis
Specific antibody response to recall antigens [32]
Suited to study HIV pathogenesis [118, 131, 132], HIV latency [90], gene therapy and novel anti-HIV treatment approaches [90] |
Delay between transplantation of human CD34+ cells and development of lymphoid system of ~15 weeks. |
|
NOG [38] or NSG [33]
|
+++ |
T and B cells
Monocytes
Macrophage
NK cells
DCs |
CCR5- and CXCR4-tropic strains [40, 70, 131, 132] |
Higher reconstitution levels as compared to Rag mice [39, 41]
Suited for studying HIV pathogenesis [119, 123], HIV treatment [95, 98] and latency and gene therapy approaches [102, 107] |
Sensitive to irradiation |
|
NOD/SCID ¶
-hu BLT [42]
And
NOD/SCID γc -/- (NSG) BLT [133]
|
+++ |
T and B cells
Monocytes
Macrophages
NK cells
DCs |
CCR5- and CXCR4-tropic strains [43, 77, 80, 88, 108, 115] |
Generation of adaptive immune responses [115]
Suited for studying HIV pathogenesis [43, 115], anti-HIV treatment [77, 80, 108], HIV latency [88] as well as novel gene therapies |
Two step procedure for generating BLT mice
Surgical skills needed
Human foetal tissue needed |
| N/A = not applicable
+ = good engraftment
++ = high engraftment
+++ = very high engraftment
§ Infection using CXCR4 tropic HIV-1 strains only successful shortly after transfer of human PBLs.
* Controversial data concerning the permissiveness of foetal thy/liv SCID mice to infection with CCR5 tropic HIV-1 strains.
¶ For simplicity reasons, we put together these two subtly different models; in fact, BLT mouse using NSG background show a superior engraftment as compared to NOD/SCID BLT mice. |
New approaches for generating humanised mice
In 2004, a novel humanised mouse model was reported. It was based on transplanting human CD34+ haematopoietic progenitor cells (CD34+ cells) directly into the liver of newborn immunodeficient mice (Rag2-/- γc
-/-) [32]. By 10 weeks after transplantation, the mice develop a lymphoid system of human origin with T cells, B cells, NK cells, monocytes and dendritic cells. Notably, the T cells display a pattern of naive and memory cells and a Vβ repertoire similar to that of humans. The mouse mounts a specific antibody response against model antigens, such as pneumococcal and tetanus toxoid antigens, but the response is much weaker than that in humans (table 1).
This model is a significant step toward humanisation. Importantly, the mice lack the γc chain, which results in even more drastic immunodeficiency as compared with SCID mice. The γc chain is an essential component of the IL-2, -4, -7, -9, - 15 and -21 receptors. Its absence severely compromises the development of immune cells, including NK-cell development, and thus their rejection potential against transplanted xenogeneic tissue. It also makes the mice less susceptible to lymphoma development. Indeed, the NOD-SCID IL-2Rγ-null mice are much more useful than the NOD-SCID mice for transplanting human tissue [33]. Notably, NOD-SCID IL-2Rγ-null mice show a similar degree of immunodeficiency as Rag1 or 2-/- γc -/- knock-out mice; they have been developed by crossing of SCID mice with non-obese diabetic (NOD) mice and mice deficient in the gamma c (γc) chain of the IL-2 receptor [34].
Modifications for improving the engraftment of human haemato-lymphopoietic tissue have been investigated, including the use of human foetal liver derived CD34+ cells, cultivating the CD34+ cells with a cytokine cocktail before transplantation [35], pre-conditioning the mice with busulfan instead of irradiation [36, 37], the use of different mouse strains, such as NOD/shi-scid/γcnull (NOG) [38] or NOD/SCID/γc -/- (NSG) mice [39], the transplantation of CD34+ cells intravenously or into the bone marrow or the transplantation of CD34+ cells at older age of the mice [39]. (NSG and NOG mice are nearly identical except for the modification of the γc chain receptor; in both strains, triggering through the γc chain receptor is disabled: in NSG mice the receptor is completely knocked down, and in NOG mice the intracytoplasmic tail is truncated). NOG mice are especially vulnerable to developing lymphomas after irradiation; however, they yield similar engraftment results even when not irradiated [40]. Very importantly, the lifespans of humanised mice, except of irradiated NOG mice, appear to be similar to those of wildtype mice; the mice eventually die due to infirmity. NSG mice transplanted at birth with haematopoietic progenitor cells either from human foetal liver or from human cord blood gave the better engraftment than the Balb/c-Rag1-/- and C.B-17-scid/bg mice [41]. Similar data have been reported by Brehm et al. [39].
The BLT mouse deserves special mention [42]. BLT is an acronym for bone marrow liver thymic. In this model, foetal liver/thymus is placed under the renal capsule in 6–8-week-old immunodeficient mice as with standard SCID-hu mice. However, after 3 weeks, the mice are sub-lethally irradiated, and autologous human CD34+ cells are transplanted into the mice. These cells home to the bone marrow and also migrate to the scaffold generated by the initial transplantation of the human foetal liver/thymus tissue. In the BLT mice, engraftment of human lymphoid tissue is highly efficient, even to the gastro-intestinal tract [43]. The innate and adaptive immune responses appear to be generally more complete in the BLT mice than in humanised mice generated by transplanting human CD34+ cells alone [42]: BLT mice generate a human MHC-restricted T-cell response to Epstein Barr virus (EBV) and activated Vβ2-TCR+ T-cells when dendritic cells present the superantigen toxic shock syndrome toxin 1 (TSST-1). Notably, TSST-1 specifically activates and induces the TCR Vβ2+ cells to proliferate. Generating an adaptive immune response is facilitated by educating the human T-cells in an autologous thymic microenvironment. This is not the case in the other humanised mouse models, which have xenogeneic mouse thymic environments. To overcome this limitation, immunodeficient mice were generated expressing the human HLA class I genes [44]. Here, mice transplanted with HLA-matched cord blood cells supported the in vivo differentiation of functionally mature human cytotoxic lymphocytes associated with a wide spectrum of functional human T-cell subsets. The mice mounted an EBV-specific immune response upon challenge as quantified by tetramer staining and enzyme-linked immunospot (ELISPOT) assay.
Thus, introducing human HLA-class I transgenes significantly improved the humanisation of the mice. Similarly, a new report demonstrated expression of class II (HLA-DR4) in NOD- Rag1 -/- /γc -/- mice and consequent improvement in T- and B-cell development and function [45]. Additional human transgenes critical for haematopoiesis have been introduced into the mouse strain backgrounds, and this action should result in a lymphoid system that even more closely approximates the human lymphoid system.
Humanised mice have also been used to study (1.) haematopoietic development, (2.) a variety of microorganisms, including EBV [42, 46], herpes simplex virus [47], Dengue fever [48, 49], influenza [50] and Salmonella typhi [51, 52], (3.) sepsis [53] and iv) virus-induced tumours [54, 55].
Irrespective of the strain, immunodeficient mice are prone to opportunistic infections and must be kept in optimized hygienic animal care facilities. Whether the humanisation protects mice from infections is not known.
|
Box
|
|
Human host factors
|
Definition
|
Function
|
| CD4 |
Cluster of differentiation 4 |
HIV binding-entry receptor, expressed in T-helper cells, macrophages/monocytes and dendritic cells |
| CCR5 |
C-C motif-chemokine receptor 5 |
Transmembrane chemokine receptor, important for cytoskeletal reorganisation and cell motility, HIV co-receptor |
| CXCR4 |
CXC motif-chemokine receptor 4 |
Transmembrane chemokine receptor, chemotactic activity for lymphocytes, HIV co-receptor |
| Cyclin CDK9 |
Cyclin dependent kinase 9 |
Cell-cycle regulator, binds Tat and cyclin T1, required for RNA polymerase II transcriptional elongation of HIV |
| |
|
|
|
Mouse strains
|
Definition
|
Description
|
| C.B.-17 SCID/SCID |
Severe combined immunodeficiency (SCID) |
Mutation in the Prkd gene (lack of B and T cells) |
| human-PBL-SCID |
PBL: peripheral blood leucocytes |
Intraperitoneal injection of human PBL into SCID mouse |
| Rag1-/- γc
-/-
Rag2-/- γc
-/-
|
Deficiency in the recombinase activating gene 1 or 2 and the common γ chain of the IL-2 receptor |
Lack of B, T and NK cells |
| NOD |
Non-obese diabetic mouse |
Reduction of NK cell activity |
| NOD/SCID |
NOD and SCID mouse |
Lack of B and T cells and NK cell activity |
| NSG (NOD/SCID/γc-/-) |
NOD/SCID mice with entire knock-out of the common γc chain receptor |
Lack of B, T and NK cells and block of the maturation and activity of these cells |
| NOG (NOD/SCID/γc-/-) |
NOD/SCID mice with knockout of the intracytoplasmatic tail of the common γc receptor |
Lack of B, T and NK cells and block of the maturation and activity of these cells |
| BLT |
Bone marrow, liver, thymus |
NOD/SCID or NOD/SCID/γc-/- mice transplanted with foetal liver, thymus and CD34+ cells |
Humanised mice for studying HIV infections
The hu-PBL SCID and foetal liv/thy SCID hu mouse models have been valuable for the study of HIV infection, including immune responses (e.g., the effect of vaccination with vaccinia gp160 and recombinant gp160 [56]), in vivo drug testing [57–59], anti-HIV effects of CD8+ cytotoxic T-cells [60] and neutralising antibodies [61], virulence of HIV isolates [62], and the significance of distinct HIV accessory proteins on virulence [63, 64], and viral latency [65]. However, these models have several limitations. Most importantly, they lack multilineage haematopoiesis and the capacity to generate an effective human immune response (table 1).
The “new generation” of humanised mice has a number of positive aspects, such as multilineage haematopoiesis, no or very rarely graft-vs-host disease, a longer lifespan of the mice, and the generation of some immune responses (table 1). In the next sections, we will focus exclusively on these new humanised mouse models. Reviews comparing the properties of the various humanised mouse models based on the use of either Rag, NSG or NOG mouse strains have recently been published [66, 67]. In this review, we focused primarily on the overall value of humanised mice for studying HIV infection and specified only the mouse strain used when clear differences were described as related to HIV infection or pathogenesis.
Humanised mice support high-level viraemia
The new humanised mice support high levels of HIV infection with either CCR5- or CXCR4-tropic strains [36, 68–71]. Plasma HIV RNA copy numbers of 104–105/ml in those mice are similar to the levels found in HIV-infected humans (note, that HIV replication can be easily monitored by repetitive sampling of peripheral blood). HIV-infected cells were detected in the spleen, lymph nodes, thymus and lungs, indicating dissemination of the virus. Unlike hu-PBL SCID mice, humanised mice sustain high-level viral replication for more than a year [35]. Depending on the virulence of the HIV strain used, the mice show distinct CD4+ T-cell depletion rates over time. Initial reports noted either very limited or no HIV-specific humoral immune responses [68, 70]. Importantly, expression of the HIV co-receptors CXCR4 and CCR5 on engrafted and differentiated human immune cells was similar to that seen in humans [68, 70, 71]. Co-receptor expression in human CD4+ T cells is the major determinant of HIV tropism in vivo[72]. Indeed, as seen in HIV-infected human, disseminated infection in humanised mice with CCR5-tropic stains leads preferentially to infection and depletion of CD4+ memory T lymphocytes [73]. CCR5 is expressed mainly on memory T lymphocytes and is absent from naive T cells.
Humanised mice for studying sexual transmission and its prevention
A prerequisite for studying HIV sexual transmission in humanised mice is the engraftment of the female reproductive tract and/or the gastro-intestinal tract with virus susceptible human cells. Both humanised Rag1-/-γc-/- mice and Rag2-/-γc -/- mice, as well as BLT mice, are well engrafted with human cells in the vagina [74–77], and vaginal HIV transmission is efficient in all these three new mouse models. Like the human gut, the mouse small intestines include abundant Peyer’s patches and the large intestines are populated with lymphoid follicular aggregates with human T and B lymphocytes, macrophages and DC [43, 74]. Here, memory T cells with prominent expression of CCR5 are permissive to CCR5-tropic strains. BLT mice also show human CD4CD8αα cells, a T-cell subset present only in the gut-associated lymphoid tissue [77]. These mice respond with disseminated HIV infection subsequent to either rectal or vaginal infection with cell-free HIV [43, 74, 75, 77].
However, efficient engraftment of the gastrointestinal tract of Rag2-/-γc-/- mice with human cells appears to depend on the protocol used: mice transplanted with CD34+ cells derived from human foetal liver and cultured overnight with IL-3, IL-6 and stem cell factor showed human cell engraftment in the gut [74]. This is not the case in mice transplanted with uncultured CD34+ cells derived from cord blood [78]. The latter also differed in their susceptibility to rectal HIV challenge [74, 78].
Humanised mice represent a very significant advancement for evaluating novel microbicides for preventing HIV infection and very nicely complement the much more expensive monkey models. Indeed, several recent studies demonstrated the utility of these models for testing oral and topical pre-exposure prophylaxis strategies with different anti-HIV drugs (e.g., Tenofovir, Maraviroc, Raltegravir) currently on the market [77, 79–82] or with compounds in development [83]. In particular, topical application of the CCR5 antagonist Maraviroc formulated as a gel prevented HIV vaginal transmission [81], and the novel CD4 aptamer-siRNA chimeras [83] showed partial protection.
Studies of anti-retroviral treatment strategies
A few reports have noted the utility of the new humanised mice for evaluating antiretroviral therapies [84–89]. We made a major effort for defining a gold standard for ART in humanised mice by first examining the pharmacokinetic of a number of anti-retroviral compounds [90]. In this work we showed efficacious anti-retroviral treatment when the anti-retroviral compounds were added to food pellets or when long-acting drugs were used [90]. We also demonstrated emergence of resistance in insufficiently treated mice, and viral rebound from previously undetectable levels after cART interruption, confirming a latent reservoir as reported recently [85, 86, 88, 91]. Thus, humanised mice represent a highly valuable model for pre-clinical proof-of-concept studies to evaluate novel anti-retroviral compounds and to study latency that closely approximates the status of HIV-infected humans treated with cART.
Several studies also evaluated novel molecules for suppressing HIV in vivo in these new mouse models [89, 92–98]. They involved studies investigating the potential of Tat peptide analogues for inhibiting HIV replication (89) as well as the effects of silencing (si)RNAs directed against viral proteins (e.g., Tat, Rev, Vif) or CCR5 that were delivered either by aptamers binding to the HIV envelope glycoprotein [92], dendrimer nanoparticles [93], single-chain antibodies binding to CD7 [95] or by immunoliposomes targeting the lymphocyte function-associated antigen-1 (LFA-1) [94]. The gp-120-binding aptamers targeted productively infected T cells specifically, and the single-chain antibodies and immunoliposomes targeted the white blood cells independently of HIV infection. In all of these in vivo studies, HIV replication was significantly suppressed. The humanised mice are also useful for studying the protective effect of distinct broadly neutralising antibodies delivered either by antibody-expressing cells administered as “backpacks” [96] or by adeno-associated virus-based vectors [97].
Studying gene therapeutic approaches for HIV/AIDS
Humanised mice represent a unique option to explore haematopoietic stem cell–based gene therapy strategies. Gene manipulation of human CD34+ cells to modulate host factors is also very attractive for HIV pathogenesis studies.
Immunodeficient mice have long been used to assess the ability of gene-transduced human CD34+ cells to differentiate into various cellular subsets. In 1994, retroviral vector–transduced human CD34 cells were shown to differentiate into mature T-cell subsets in SCID-hu grafts [99]. Later, long-term engraftment of human CD34+ cells transduced with an HIV vector was demonstrated in NOD/SCID mice; 4–10% of the human cells were transduced [100]. NOD/SCID and the NSG mice seem to engraft similar numbers of transduced human CD34+ cells [101]. However, when using bone marrow from those mice for secondary reconstitutions in mice on the same background, human tissue from NSG mice engrafted far better than that from NOD/SCID mice, pointing to increased numbers of long-term SCID repopulating cells. These features are favourable for studying long-term transgene expression and the analysis of retroviral-insertion sites in primary and secondary transplanted NSG mice.
These findings, along with the higher levels of human tissue engraftment, suggest that the “novel generation” of humanised mice will be very useful for studying gene therapy approaches and examining distinct genes for their pathogenic effects in various settings. In addition, zinc-finger nuclease–mediated gene engineering is another very promising gene-engineering technology that has been explored in these mouse models [102].
A vast body of literature describes various approaches to control HIV by ex vivo gene therapy [103, 104]. Gene therapy approaches for HIV/AIDS i) target host factors critical for HIV replication or ii) HIV genes mandatory for HIV replication (e.g., Nef) [105], iii) introduce novel gene constructs (e.g., Trim5-cyclophilin fusion protein that inhibits HIV replication) [106], or iv) employ a broadly neutralising anti-HIV antibody [107]. The value of humanised mice for studying genetically altered human CD34+ cells to treat HIV is nicely illustrated by several studies, such as those targeting CCR5 with siRNA- or shRNA-mediated silencing [108, 109] or by zinc finger–mediated excision [102]. Notably, the HIV receptor complex consists of CD4 and either the HIV co-receptor, CCR5 or CXCR4 [72]. CCR5-tropic strains are transmitted and predominate until late-stage disease [110]. CXCR4-tropic strains emerge in advanced stages of HIV disease in about 50% of HIV-infected patients and seem to accelerate the immune deficiency [111]. Silencing CCR5 has been mentioned as a potential “cure” for HIV. A recent report described an HIV-infected patient who suffered from acute myeloid leukaemia and who was transplanted with human CD34+ stem cells lacking CCR5. Strikingly, the patient appears to be fully cured since HIV did not rebound subsequent to interruption of cART [112]. While it involved only a single patient, this report gives credence to genetic approaches targeting CCR5.
Introducing a lentiviral construct silencing CCR5 into human CD34+ cells resulted in a clear reduction of CCR5 on the target cells of HIV in vivo [108]. The engineered progenitor cells showed long-term haematopoietic repopulating capacity by secondary transplantation. Notably, the genetically engineered progeny cells behaved identically as the controls. In vitro the engineered cells were resistant to HIV infection with CCR5-tropic strains. Zinc finger–mediated excision of CCR5 in CD34+ cells resulted in progeny cells lacking CCR5, and the mice showed lower HIV replication and prevention of CD4+ T-cell loss in vivo, as compared to control mice [102]. Very importantly, HIV infection resulted in a selection of cells resistant to HIV over time. This technology demonstrated cells with long-term haematopoietic repopulation capacity. Other genetic approaches have focused on T cells and engineered HIV-resistant CD4+ T cells with CXCR4-specific zinc finger nucleases [113].
These studies clearly indicate that humanised mice are a very promising tool for exploring gene engineering approaches to treat and/or cure HIV. The major hurdles will not be the identification of targets rendering cells resistant to HIV but to achieve sufficiently high numbers of genetically engineered CD34+ cells, the migration of transduced cells to the niches of haematopoietic stem cells, preventing the insertional risk favouring neoplastic transformation or off-target effects (e.g., activation of the innate immune response). Here, too, the humanised mouse model will be a versatile tool for exploring these questions.
Humanised mice for generating an HIV-specific immune response
Inducing a robust HIV-specific immune response was reported in NSG mice reconstituted with human CD34+ cells from newborns [114] and in the BLT mouse model [115]. Both papers reported HIV-specific CD4+ and CD8+ T-cell responses with overlapping HIV peptide pools [114, 115] or ELISPOT assays [115]. The relevance of the CD8+ T cells in constraining HIV replication in humanised mice was illustrated by a significantly higher replication rate when CD8+ T cells were depleted [114]. The humanised mice also developed humoral immune responses against HIV [115]. However, antigen-specific immune responses in humanised mice seem to take longer to develop than in adult humans infected with HIV, possibly reflecting the lack of full maturity of the human immune systems of these mice after reconstitution [115]. In previous work, such solid HIV-specific antibody immune responses were not reported. The analysis might have been performed too soon after HIV infection. In view of limited number of studies, we still do not know if the current generation of humanised mice is suited for studying antigen-specific immune responses and, in particular, vaccine approaches. In mice reconstituted directly with human CD34+ cells the selection of T cells is done by murine thymic stromal cells. The subsequent generation of an antigen-specific immune response, however, is based on the processing of antigens by human antigen-presenting cells in the humanised mouse model and therefore might be suboptimal.
HIV evolution over time
HIV’s diversity is one of its main features. It is also a key element for immune escape and emergence of resistance to ART. HIV’s diversity is due to the inaccuracy of the HIV reverse transcriptase, hyperamination of the nascent DNA strand during reverse transcription by the members of the APOBEC family, and recombination events between distinct HIV strains. Indeed, the genotypic and phenotypic changes in the viral envelope gene in humanised mice infected with a distinct HIV strain, JR-CSF, showed the mean rate of divergence of viral populations over 44 weeks similar to that in humans [116]. They noted a disproportionate number of guanosine-to-adenosine transitions in the HIV envelope, indicating that APOBEC3G is active in this model. Furthermore, a number of substitutions in the envelope gene were identified.
HIV immune activation and dysfunction
Sustained immune activation is the major trigger of HIV-associated immunodeficiency. Various mechanisms, such as disruption of the gastrointestinal tract barrier during acute HIV infection [117] or various HIV accessory gene products, may contribute to the HIV-associated immune activation [18]. Immune activation is also observed on the CD4+ and CD8+ T cells in HIV-infected humanised mice [115, 118]. We used the humanised mice to study the role of macrophages in immune activation [118]. We found that HIV infection results in a disturbed phagocytosis by macrophages. Notably, macrophages are essential for clearing bacterial products. We concluded that disruptions of the gastrointestinal tract barrier, together with the macrophage dysfunction, are a main element of higher blood levels of bacterial products and thus in HIV-associated immune activation.
Immune activation affects also the PDL1-PD1 axis (PDL = programmed death ligand). The inhibitory receptor PD-1, which indicates exhaustion of T cells, was increased on the T cells in HIV-infected humanised mice, reminiscent of the findings in humans [115]. Ongoing studies are examining the benefits of blocking the PD-1 pathway.
The presence of various immune cells, such as plasmacytoid dendritic cells (pDC) and T-regulatory cells, in humanised mice presents a unique opportunity to assess their effects on HIV infection and vice-versa (i.e., HIV’s effect on them). For example, rapid infection and activation of pDCs were seen in HIV-challenged humanised mice [119]. Their activation correlated with activation of CD4+ T cells and their apoptosis. While CD4+ T cells were depleted, pDCs were maintained but functionally impaired. The presence of T-regulatory cells in these mice may help to dissect their role in HIV infection. These cells are preferentially targeted by HIV during acute HIV infection in these mice [120].
CNS invasion by HIV
AIDS-related dementia occurs in about 30% of HIV-infected patients with advanced immunodeficiency [121]. AIDS dementia is characterised by the immigration of macrophages, formation of microglial nodules, and generation of multi-nucleated giant cells, most likely due to viral induced fusion between microglial cells and/or macrophages. HIV-infected humanised mice show pathologic anomalies in the brain reminiscent of those in HIV-infected patients with AIDS dementia. In particular, activated human blood-borne macrophages migrate into the brain. Human cells enter into the brains more quickly in HIV-infected mice than in control mice. Productively infected macrophages and cells of lymphocyte morphology are found in the meninges and perivascular spaces [122]. Strikingly, CD8+ T-cell depletion aggravated the pathological findings, suggesting that CD8+ T cells could subdue HIV infection to some extent. Using advanced neuroimaging and post-mortem examination, HIV-infected mice show a loss of neuronal integrity [123]. These data are encouraging: humanised mice represent a valuable tool for examining mechanistic and therapeutic aspects of HIV-associated dementia. However, as reiterated by the study authors, additional studies are needed for a more detailed characterisation and validation of the neuronal damage associated with HIV infection in this mouse model [124].
Future generations of humanised mice
Despite the advances made in humanised murine models, the reconstituted lymphoid system still lacks a well-elaborated lymphoid architecture. This is partially explained by the lack of human cytokines critical for haematopoiesis and/or by insufficient interactions between cells of the murine stroma and human haematopoietic cells. The less than optimal lymphoid architecture and the education/selection of T-cells on a murine thymic scaffold result in a rather modest adaptive immune response. As outlined above, transplantation of HLA-matched cord blood into a mouse strain transgenic for human HLA gives more robust specific T-cell response. To improve the humanisation of mice, additional human transgenes critical for haematopoiesis are introduced into mice.
The knock-in of human thrombopoietin (TPO) into Rag2-/-γ c-/-, which is essential for the expansion and maintenance of HSC [125], resulted in a higher level of engraftment and an increase in the breadth of multilineage haematopoiesis with higher number of myeloid cells than in control mice expressing the murine TPO [126].
Other knock-in (KI) genes examined for improving the humanisation were human IL-3 in concert with human granulocyte macrophage cytokine stimulating factor (GM-CSF) [127] and colony stimulating factor-1 (CSF-1) [128]. In IL-3/GM-CSF KI mice, transplantation of human pregenitor cells resulted in a more pronounced inflammatory reaction in response to intraperitoneal administration of lipopolysaccharide than in controls. In addition, IL-3/GM-CSF KI mice had improved human myeloid immune reconstitution in the lung as exemplified by the presence of alveolar macrophages; the human alveolar macrophages mounted an innate immune response when challenged with influenza virus but could not control it. This model might be especially good for studying pulmonary diseases [127]. The knock-in of CFS-1 enhanced the differentiation of myeloid cells into monocytes and macrophages [128].
As outlined above, differences in the extent the various mouse backgrounds support human cell engraftment exist. Positional cloning identified alleles of the inhibitory receptor signal regulatory protein alpha (SIRPα) as a reason for the difference of engraftment levels between mouse strains [129]. In NOD mice, which have higher engraftment levels than other mice, SIRPα on murine macrophages showed enhanced binding to the human CD47 ligand. This enhanced binding inhibits phagocytosis of the xenograft and secretion of TNF-α by macrophages. Transgenic expression of SIRPα in Rag2-/-γc-/- increased the engraftment level of HSC to a level similar to NSG mice and improved the functionality of the immune system [130].
These next-generation mice will most likely become important assets for the research community. Furthermore, the step-wise progress made in humanisation methods will continue and will create an array of humanised mouse models appropriately suited to address specific research questions.
References
1 Van Rompay KK. The use of nonhuman primate models of HIV infection for the evaluation of antiviral strategies. AIDS Res Hum Retroviruses. 2012;28(1):16–35.
2 Maddon PJ, Dalgleish AG, McDougal JS, Clapham PR, Weiss RA, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47(3):333–48.
3 Gorry PR, Ancuta P. Coreceptors and HIV-1 pathogenesis. Curr HIV/AIDS Rep. 2011;8(1):45–53.
4 Bieniasz PD, Cullen BR. Multiple blocks to human immunodeficiency virus type 1 replication in rodent cells. J Virol. 2000;74(21):9868–77.
5 Lores P, Boucher V, Mackay C, Pla M, Von Boehmer H, Jami J, et al. Expression of human CD4 in transgenic mice does not confer sensitivity to human immunodeficiency virus infection. AIDS Res Hum Retroviruses. 1992;8(12):2063–71.
6 Browning J, Horner JW, Pettoello-Mantovani M, Raker C, Yurasov S, DePinho RA, et al. Mice transgenic for human CD4 and CCR5 are susceptible to HIV infection. Proc Natl Acad Sci U S A. 1997;94(26):14637–41.
7 Zhang JX, Diehl GE, Littman DR. Relief of preintegration inhibition and characterization of additional blocks for HIV replication in primary mouse T cells. PLoS ONE. 2008;3(4):e2035.
8 Goffinet C, Allespach I, Keppler OT. HIV-susceptible transgenic rats allow rapid preclinical testing of antiviral compounds targeting virus entry or reverse transcription. Proc Natl Acad Sci U S A. 2007;104(3):1015–20.
9 Goffinet C, Michel N, Allespach I, Tervo HM, Hermann V, Krausslich HG, et al. Primary T-cells from human CD4/CCR5-transgenic rats support all early steps of HIV-1 replication including integration, but display impaired viral gene expression. Retrovirology. 2007;4:53.
10 Nomaguchi M, Doi N, Kamada K, Adachi A. Species barrier of HIV-1 and its jumping by virus engineering. Rev Med Virol. 2008;18(4):261–75.
11 Hanna Z, Kay DG, Rebai N, Guimond A, Jothy S, Jolicoeur P. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell. 1998;95(2):163–75.
12 Poudrier J, Weng X, Kay DG, Pare G, Calvo EL, Hanna Z, et al. The AIDS disease of CD4C/HIV transgenic mice shows impaired germinal centers and autoantibodies and develops in the absence of IFN-gamma and IL-6. Immunity. 2001;15(2):173–85.
13 Weng X, Priceputu E, Chrobak P, Poudrier J, Kay DG, Hanna Z, et al. CD4+ T cells from CD4C/HIVNef transgenic mice show enhanced activation in vivo with impaired proliferation in vitro but are dispensable for the development of a severe AIDS-like organ disease. J Virol. 2004;78(10):5244–57.
14 Poudrier J, Weng X, Kay DG, Hanna Z, Jolicoeur P. The AIDS-like disease of CD4C/human immunodeficiency virus transgenic mice is associated with accumulation of immature CD11bHi dendritic cells. J Virol. 2003;77(21):11733–44.
15 Priceputu E, Rodrigue I, Chrobak P, Poudrier J, Mak TW, Hanna Z, et al. The Nef-mediated AIDS-like disease of CD4C/human immunodeficiency virus transgenic mice is associated with increased Fas/FasL expression on T cells and T-cell death but is not prevented in Fas-, FasL-, tumor necrosis factor receptor 1-, or interleukin-1beta-converting enzyme-deficient or Bcl2-expressing transgenic mice. J Virol. 2005;79(10):6377–91.
16 Douek DC, Picker LJ, Koup RA. T cell dynamics in HIV-1 infection. Annu Rev Immunol. 2003;21:265–304.
17 Doitsh G, Cavrois M, Lassen KG, Zepeda O, Yang Z, Santiago ML, et al. Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell. 2010;143(5):789–801.
18 d’Ettorre G, Paiardini M, Ceccarelli G, Silvestri G, Vullo V. HIV-associated immune activation: from bench to bedside. AIDS Res Hum Retroviruses. 2011;27(4):355–64.
19 von Wyl V, Yerly S, Boni J, Burgisser P, Klimkait T, Battegay M, et al. Emergence of HIV-1 drug resistance in previously untreated patients initiating combination antiretroviral treatment: a comparison of different regimen types. Arch Intern Med. 2007;167(16):1782–90.
20 Jaggy C, von Overbeck J, Ledergerber B, Schwarz C, Egger M, Rickenbach M, et al. Mortality in the Swiss HIV Cohort Study (SHCS) and the Swiss general population. Lancet. 2003;362(9387):877–8.
21 Palmer S, Josefsson L, Coffin JM. HIV reservoirs and the possibility of a cure for HIV infection. J Intern Med. 2011;270(6):550–60.
22 Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301(5900):527–30.
23 McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241(4873):1632–9.
24 Namikawa R, Kaneshima H, Lieberman M, Weissman IL, McCune JM. Infection of the SCID-hu mouse by HIV-1. Science. 1988;242(4886):1684–6.
25 Mosier DE, Gulizia RJ, Baird SM, Wilson DB. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335(6187):256–9.
26 Mosier DE, Gulizia RJ, Baird SM, Wilson DB, Spector DH, Spector SA. Human immunodeficiency virus infection of human-PBL-SCID mice. Science. 1991;251(4995):791–4.
27 Rizza P, Santini SM, Logozzi MA, Lapenta C, Sestili P, Gherardi G, et al. T-cell dysfunctions in hu-PBL-SCID mice infected with human immunodeficiency virus (HIV) shortly after reconstitution: in vivo effects of HIV on highly activated human immune cells. J Virol. 1996;70(11):7958–64.
28 Fais S, Lapenta C, Santini SM, Spada M, Parlato S, Logozzi M, et al. Human immunodeficiency virus type 1 strains R5 and X4 induce different pathogenic effects in hu-PBL-SCID mice, depending on the state of activation/differentiation of human target cells at the time of primary infection. J Virol. 1999;73(8):6453–9.
29 Nakata H, Maeda K, Miyakawa T, Shibayama S, Matsuo M, Takaoka Y, et al. Potent anti-R5 human immunodeficiency virus type 1 effects of a CCR5 antagonist, AK602/ONO4128/GW873140, in a novel human peripheral blood mononuclear cell nonobese diabetic-SCID, interleukin-2 receptor gamma-chain-knocked-out AIDS mouse model. J Virol. 2005;79(4):2087–96.
30 Tary-Lehmann M, Lehmann PV, Schols D, Roncarolo MG, Saxon A. Anti-SCID mouse reactivity shapes the human CD4+ T cell repertoire in hu-PBL-SCID chimeras. J Exp Med. 1994;180(5):1817–27.
31 Hoffmann-Fezer G, Gall C, Zengerle U, Kranz B, Thierfelder S. Immunohistology and immunocytology of human T-cell chimerism and graft-versus-host disease in SCID mice. Blood. 1993;81(12):3440–8.
32 Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304(5667):104–7.
33 Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174(10):6477–89.
34 Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7(2):118–30.
35 Berges BK, Akkina SR, Remling L, Akkina R. Humanized Rag2(-/-)gammac(-/-) (RAG-hu) mice can sustain long-term chronic HIV-1 infection lasting more than a year. Virology. 2010;397(1):100–3.
36 Gorantla S, Sneller H, Walters L, Sharp JG, Pirruccello SJ, West JT, et al. Human immunodeficiency virus type 1 pathobiology studied in humanized BALB/c-Rag2-/-gammac-/- mice. J Virol. 2007;81(6):2700–12.
37 Choi B, Chun E, Kim M, Kim ST, Yoon K, Lee KY, et al. Human B cell development and antibody production in humanized NOD/SCID/IL-2Rgamma(null) (NSG) mice conditioned by busulfan. J Clin Immunol. 2011;31(2):253–64.
38 Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100(9):3175–82.
39 Brehm MA, Cuthbert A, Yang C, Miller DM, DiIorio P, Laning J, et al. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation. Clin Immunol. 2010;135(1):84–98.
40 Watanabe S, Ohta S, Yajima M, Terashima K, Ito M, Mugishima H, et al. Humanized NOD/SCID/IL2Rgamma(null) mice transplanted with hematopoietic stem cells under nonmyeloablative conditions show prolonged life spans and allow detailed analysis of human immunodeficiency virus type 1 pathogenesis. J Virol. 2007;81(23):13259–64.
41 Lepus CM, Gibson TF, Gerber SA, Kawikova I, Szczepanik M, Hossain J, et al. Comparison of human fetal liver, umbilical cord blood, and adult blood hematopoietic stem cell engraftment in NOD-scid/gammac-/-, Balb/c-Rag1-/-gammac-/-, and C.B-17-scid/bg immunodeficient mice. Hum Immunol. 2009;70(10):790–802.
42 Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12(11):1316–22.
43 Sun Z, Denton PW, Estes JD, Othieno FA, Wei BL, Wege AK, et al. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J Exp Med. 2007;204(4):705–14.
44 Shultz LD, Saito Y, Najima Y, Tanaka S, Ochi T, Tomizawa M, et al. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proc Natl Acad Sci U S A. 2010;107(29):13022–7.
45 Danner R, Chaudhari SN, Rosenberger J, Surls J, Richie TL, Brumeanu TD, et al. Expression of HLA class II molecules in humanized NOD.Rag1KO.IL2RgcKO mice is critical for development and function of human T and B cells. PLoS ONE. 2011;6(5):e19826.
46 Cocco M, Bellan C, Tussiwand R, Corti D, Traggiai E, Lazzi S, et al. CD34+ cord blood cell-transplanted Rag2-/- gamma(c)-/- mice as a model for Epstein-Barr virus infection. Am J Pathol. 2008;173(5):1369–78.
47 Kwant-Mitchell A, Ashkar AA, Rosenthal KL. Mucosal innate and adaptive immune responses against herpes simplex virus type 2 in a humanized mouse model. J Virol. 2009;83(20):10664–76.
48 Kuruvilla JG, Troyer RM, Devi S, Akkina R. Dengue virus infection and immune response in humanized RAG2(-/-)gamma(c)(-/-) (RAG-hu) mice. Virology. 2007;369(1):143–52.
49 Jaiswal S, Pearson T, Friberg H, Shultz LD, Greiner DL, Rothman AL, et al. Dengue virus infection and virus-specific HLA-A2 restricted immune responses in humanized NOD-scid IL2rgammanull mice. PLoS ONE. 2009;4(10):e7251.
50 Tu W, Zheng J, Liu Y, Sia SF, Liu M, Qin G, et al. The aminobisphosphonate pamidronate controls influenza pathogenesis by expanding a gammadelta T cell population in humanized mice. J Exp Med. 2011;208(7):1511–22.
51 Libby SJ, Brehm MA, Greiner DL, Shultz LD, McClelland M, Smith KD, et al. Humanized nonobese diabetic-scid IL2rgammanull mice are susceptible to lethal Salmonella Typhi infection. Proc Natl Acad Sci U S A. 2010;107(35):15589–94.
52 Song J, Willinger T, Rongvaux A, Eynon EE, Stevens S, Manz MG, et al. A mouse model for the human pathogen Salmonella typhi. Cell Host Microbe. 2010;8(4):369–76.
53 Unsinger J, McDonough JS, Shultz LD, Ferguson TA, Hotchkiss RS. Sepsis-induced human lymphocyte apoptosis and cytokine production in “humanized” mice. J Leukoc Biol. 2009;86(2):219–27.
54 Strowig T, Gurer C, Ploss A, Liu YF, Arrey F, Sashihara J, et al. Priming of protective T cell responses against virus-induced tumors in mice with human immune system components. J Exp Med. 2009;206(6):1423–34.
55 Banerjee P, Tripp A, Lairmore MD, Crawford L, Sieburg M, Ramos JC, et al. Adult T-cell leukemia/lymphoma development in HTLV-1-infected humanized SCID mice. Blood. 2010;115(13):2640–8.
56 Mosier DE, Gulizia RJ, MacIsaac PD, Corey L, Greenberg PD. Resistance to human immunodeficiency virus 1 infection of SCID mice reconstituted with peripheral blood leukocytes from donors vaccinated with vaccinia gp160 and recombinant gp160. Proc Natl Acad Sci U S A. 1993;90(6):2443–7.
57 McCune JM, Namikawa R, Shih CC, Rabin L, Kaneshima H. Suppression of HIV infection in AZT-treated SCID-hu mice. Science. 1990;247(4942):564–6.
58 Pettoello-Mantovani M, Kollmann TR, Katopodis NF, Raker C, Kim A, Yurasov S, et al. thy/liv-SCID-hu mice: a system for investigating the in vivo effects of multidrug therapy on plasma viremia and human immunodeficiency virus replication in lymphoid tissues. J Infect Dis. 1998;177(2):337–46.
59 Stoddart CA, Bales CA, Bare JC, Chkhenkeli G, Galkina SA, Kinkade AN, et al. Validation of the SCID-hu Thy/Liv mouse model with four classes of licensed antiretrovirals. PLoS ONE. 2007;2(7):e655.
60 McKinney DM, Lewinsohn DA, Riddell SR, Greenberg PD, Mosier DE. The antiviral activity of HIV-specific CD8+ CTL clones is limited by elimination due to encounter with HIV-infected targets. J Immunol. 1999;163(2):861–7.
61 Poignard P, Sabbe R, Picchio GR, Wang M, Gulizia RJ, Katinger H, et al. Neutralizing antibodies have limited effects on the control of established HIV-1 infection in vivo. Immunity. 1999;10(4):431–8.
62 Mosier DE, Gulizia RJ, MacIsaac PD, Torbett BE, Levy JA. Rapid loss of CD4+ T cells in human-PBL-SCID mice by noncytopathic HIV isolates. Science. 1993;260(5108):689–92.
63 Aldrovandi GM, Zack JA. Replication and pathogenicity of human immunodeficiency virus type 1 accessory gene mutants in SCID-hu mice. J Virol. 1996;70(3):1505–11.
64 Gulizia RJ, Collman RG, Levy JA, Trono D, Mosier DE. Deletion of nef slows but does not prevent CD4-positive T-cell depletion in human immunodeficiency virus type 1-infected human-PBL-SCID mice. J Virol. 1997;71(5):4161–4.
65 Brooks DG, Hamer DH, Arlen PA, Gao L, Bristol G, Kitchen CM, et al. Molecular characterization, reactivation, and depletion of latent HIV. Immunity. 2003;19(3):413–23.
66 Van Duyne R, Pedati C, Guendel I, Carpio L, Kehn-Hall K, Saifuddin M, et al. The utilization of humanized mouse models for the study of human retroviral infections. Retrovirology. 2009;6:76.
67 Berges BK, Rowan MR. The utility of the new generation of humanized mice to study HIV-1 infection: transmission, prevention, pathogenesis, and treatment. Retrovirology. 2011;8:65.
68 Baenziger S, Tussiwand R, Schlaepfer E, Mazzucchelli L, Heikenwalder M, Kurrer MO, et al. Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2-/-gamma c-/- mice. Proc Natl Acad Sci U S A. 2006;103(43):15951–6.
69 Berges BK, Wheat WH, Palmer BE, Connick E, Akkina R. HIV-1 infection and CD4 T cell depletion in the humanized Rag2-/-gamma c-/- (RAG-hu) mouse model. Retrovirology. 2006;3:76.
70 Watanabe S, Terashima K, Ohta S, Horibata S, Yajima M, Shiozawa Y, et al. Hematopoietic stem cell-engrafted NOD/SCID/IL2Rgamma null mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood. 2007;109(1):212–8.
71 Zhang L, Kovalev GI, Su L. HIV-1 infection and pathogenesis in a novel humanized mouse model. Blood. 2007;109(7):2978–81.
72 Speck RF, Wehrly K, Platt EJ, Atchison RE, Charo IF, Kabat D, et al. Selective employment of chemokine receptors as human immunodeficiency virus type 1 coreceptors determined by individual amino acids within the envelope V3 loop. JVirol. 1997 9/1997;71(9):7136–9.
73 Nie C, Sato K, Misawa N, Kitayama H, Fujino H, Hiramatsu H, et al. Selective infection of CD4+ effector memory T lymphocytes leads to preferential depletion of memory T lymphocytes in R5 HIV-1-infected humanized NOD/SCID/IL-2Rgammanull mice. Virology. 2009;394(1):64–72.
74 Berges BK, Akkina SR, Folkvord JM, Connick E, Akkina R. Mucosal transmission of R5 and X4 tropic HIV-1 via vaginal and rectal routes in humanized Rag2-/- gammac -/- (RAG-hu) mice. Virology. 2008;373(2):342–51.
75 Akkina R, Berges BK, Palmer BE, Remling L, Neff CP, Kuruvilla J, et al. Humanized Rag1-/- gammac-/- mice support multilineage hematopoiesis and are susceptible to HIV-1 infection via systemic and vaginal routes. PLoS ONE. 2011;6(6):e20169.
76 Olesen R, Wahl A, Denton PW, Garcia JV. Immune reconstitution of the female reproductive tract of humanized BLT mice and their susceptibility to human immunodeficiency virus infection. J Reprod Immunol. 2011;88(2):195–203.
77 Denton PW, Estes JD, Sun Z, Othieno FA, Wei BL, Wege AK, et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 2008;5(1):e16.
78 Hofer U, Baenziger S, Heikenwalder M, Schlaepfer E, Gehre N, Regenass S, et al. RAG2-/- gamma(c)-/- mice transplanted with CD34+ cells from human cord blood show low levels of intestinal engraftment and are resistant to rectal transmission of human immunodeficiency virus. J Virol. 2008;82(24):12145–53.
79 Neff CP, Ndolo T, Tandon A, Habu Y, Akkina R. Oral pre-exposure prophylaxis by anti-retrovirals raltegravir and maraviroc protects against HIV-1 vaginal transmission in a humanized mouse model. PLoS ONE. 2010;5(12):e15257.
80 Denton PW, Krisko JF, Powell DA, Mathias M, Kwak YT, Martinez-Torres F, et al. Systemic administration of antiretrovirals prior to exposure prevents rectal and intravenous HIV-1 transmission in humanized BLT mice. PLoS ONE. 2010;5(1):e8829.
81 Neff CP, Kurisu T, Ndolo T, Fox K, Akkina R. A topical microbicide gel formulation of CCR5 antagonist maraviroc prevents HIV-1 vaginal transmission in humanized RAG-hu mice. PLoS ONE. 2011;6(6):e20209.
82 Denton PW, Othieno F, Martinez-Torres F, Zou W, Krisko JF, Fleming E, et al. One percent tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J Virol. 2011;85(15):7582–93.
83 Wheeler LA, Trifonova R, Vrbanac V, Basar E, McKernan S, Xu Z, et al. Inhibition of HIV transmission in human cervicovaginal explants and humanized mice using CD4 aptamer-siRNA chimeras. J Clin Invest. 2011;121(6):2401–12.
84 Sango K, Joseph A, Patel M, Osiecki K, Dutta M, Goldstein H. Highly active antiretroviral therapy potently suppresses HIV infection in humanized Rag2-/-gammac-/- mice. AIDS Res Hum Retroviruses. 2010;26(7):735–46.
85 Choudhary SK, Rezk NL, Ince WL, Cheema M, Zhang L, Su L, et al. Suppression of human immunodeficiency virus type 1 (HIV-1) viremia with reverse transcriptase and integrase inhibitors, CD4+ T-cell recovery, and viral rebound upon interruption of therapy in a new model for HIV treatment in the humanized Rag2-/-{gamma}c-/- mouse. J Virol. 2009;83(16):8254–8.
86 Denton PW, Olesen R, Choudhary SK, Archin NM, Wahl A, Swanson MD, et al. Generation of HIV latency in BLT humanized mice. J Virol. 2011 Oct 19.
87 Choudhary SK, Margolis DM. Curing HIV: Pharmacologic approaches to target HIV-1 latency. Annu Rev Pharmacol Toxicol. 2011;51:397–418.
88 Marsden MD, Kovochich M, Suree N, Shimizu S, Mehta R, Cortado R, et al. HIV latency in the humanized BLT mouse. J Virol. 2012;86(1):339–47.
89 Van Duyne R, Cardenas J, Easley R, Wu W, Kehn-Hall K, Klase Z, et al. Effect of transcription peptide inhibitors on HIV-1 replication. Virology. 2008;376(2):308–22.
90 Nischang M, Sutmuller R, Gers-Huber G, Audige A, Li D, Rochat MA, et al. Humanized mice recapitulate key features of HIV-1 infection: a novel concept using long-acting anti-retroviral drugs for treating HIV-1. PLoS ONE. 2012;7(6):e38853.
91 Choudhary SK, Archin NM, Cheema M, Dahl NP, Garcia JV, Margolis DM. Latent HIV-1 infection of resting CD4 T cells in the humanized Rag2/ gammac/ mouse. J Virol. 2012;86(1):114–20.
92 Neff CP, Zhou J, Remling L, Kuruvilla J, Zhang J, Li H, et al. An aptamer-siRNA chimera suppresses HIV-1 viral loads and protects from helper CD4(+) T cell decline in humanized mice. Sci Transl Med. 2011;3(66):66ra6.
93 Zhou J, Neff CP, Liu X, Zhang J, Li H, Smith DD, et al. Systemic administration of combinatorial dsiRNAs via nanoparticles efficiently suppresses HIV-1 infection in humanized mice. Mol Ther. 2011;19(12):2228–38.
94 Kim SS, Peer D, Kumar P, Subramanya S, Wu H, Asthana D, et al. RNAi-mediated CCR5 silencing by LFA-1-targeted nanoparticles prevents HIV infection in BLT mice. Mol Ther. 2010;18(2):370–6.
95 Kumar P, Ban HS, Kim SS, Wu H, Pearson T, Greiner DL, et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell. 2008;134(4):577–86.
96 Luo XM, Lei MY, Feidi RA, West AP, Jr., Balazs AB, Bjorkman PJ, et al. Dimeric 2G12 as a potent protection against HIV-1. PLoS Pathog. 2010;6(12):e1001225.
97 Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2012;481(7379):81–4.
98 Mukherjee R, Plesa G, Sherrill-Mix S, Richardson MW, Riley JL, Bushman FD. HIV sequence variation associated with env antisense adoptive T-cell therapy in the hNSG mouse model. Mol Ther. 2010;18(4):803–11.
99 Akkina RK, Rosenblatt JD, Campbell AG, Chen IS, Zack JA. Modeling human lymphoid precursor cell gene therapy in the SCID-hu mouse. Blood. 1994;84(5):1393–8.
100 Miyoshi H, Smith KA, Mosier DE, Verma IM, Torbett BE. Transduction of human CD34+ cells that mediate long-term engraftment of NOD/SCID mice by HIV vectors. Science. 1999;283(5402):682–6.
101 Cai S, Wang H, Bailey B, Hartwell JR, Silver JM, Juliar BE, et al. Differential secondary reconstitution of in vivo-selected human SCID-repopulating cells in NOD/SCID versus NOD/SCID/gamma chain mice. Bone Marrow Res. 2011;2011:252953.
102 Holt N, Wang J, Kim K, Friedman G, Wang X, Taupin V, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol. 2010;28(8):839–47.
103 Scherer LJ, Rossi JJ. Ex vivo gene therapy for HIV-1 treatment. Hum Mol Genet. 2011;20(R1):R100-7.
104 Kiem HP, Jerome KR, Deeks SG, McCune JM. Hematopoietic-stem-cell-based gene therapy for HIV disease. Cell Stem Cell. 2012;10(2):137–47.
105 ter Brake O, Legrand N, von Eije KJ, Centlivre M, Spits H, Weijer K, et al. Evaluation of safety and efficacy of RNAi against HIV-1 in the human immune system (Rag-2(-/-)gammac(-/-)) mouse model. Gene Ther. 2009;16(1):148–53.
106 Neagu MR, Ziegler P, Pertel T, Strambio-De-Castillia C, Grutter C, Martinetti G, et al. Potent inhibition of HIV-1 by TRIM5-cyclophilin fusion proteins engineered from human components. J Clin Invest. 2009;119(10):3035–47.
107 Joseph A, Zheng JH, Chen K, Dutta M, Chen C, Stiegler G, et al. Inhibition of in vivo HIV infection in humanized mice by gene therapy of human hematopoietic stem cells with a lentiviral vector encoding a broadly neutralizing anti-HIV antibody. J Virol. 2010;84(13):6645–53.
108 Shimizu S, Hong P, Arumugam B, Pokomo L, Boyer J, Koizumi N, et al. A highly efficient short hairpin RNA potently down-regulates CCR5 expression in systemic lymphoid organs in the hu-BLT mouse model. Blood. 2010;115(8):1534–44.
109 Anderson J, Akkina R. Complete knockdown of CCR5 by lentiviral vector-expressed siRNAs and protection of transgenic macrophages against HIV-1 infection. Gene Ther. 2007;14(17):1287–97.
110 Philpott SM. HIV-1 coreceptor usage, transmission, and disease progression. Curr HIV Res. 2003;1(2):217–27.
111 Arrildt KT, Joseph SB, Swanstrom R. The HIV-1 env protein: a coat of many colors. Curr HIV/AIDS Rep. 2012;9(1):52–63.
112 Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360(7):692–8.
113 Wilen CB, Wang J, Tilton JC, Miller JC, Kim KA, Rebar EJ, et al. Engineering HIV-resistant human CD4+ T cells with CXCR4-specific zinc-finger nucleases. PLoS Pathog. 2011;7(4):e1002020.
114 Gorantla S, Makarov E, Finke-Dwyer J, Gebhart CL, Domm W, Dewhurst S, et al. CD8+ cell depletion accelerates HIV-1 immunopathology in humanized mice. J Immunol. 2010;184(12):7082–91.
115 Brainard DM, Seung E, Frahm N, Cariappa A, Bailey CC, Hart WK, et al. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J Virol. 2009;83(14):7305–21.
116 Ince WL, Zhang L, Jiang Q, Arrildt K, Su L, Swanstrom R. Evolution of the HIV-1 env gene in the Rag2-/- gammaC-/- humanized mouse model. J Virol. Mar;84(6):2740–52.
117 Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–71.
118 Hofer U, Schlaepfer E, Baenziger S, Nischang M, Regenass S, Schwendener R, et al. Inadequate clearance of translocated bacterial products in HIV-infected humanized mice. PLoS Pathog. 2010;6(4):e1000867.
119 Zhang L, Jiang Q, Li G, Jeffrey J, Kovalev GI, Su L. Efficient infection, activation, and impairment of pDCs in the BM and peripheral lymphoid organs during early HIV-1 infection in humanized rag2/gamma C/ mice in vivo. Blood. 2011;117(23):6184–92.
120 Jiang Q, Zhang L, Wang R, Jeffrey J, Washburn ML, Brouwer D, et al. FoxP3+CD4+ regulatory T cells play an important role in acute HIV-1 infection in humanized Rag2-/-gammaC-/- mice in vivo. Blood. 2008;112(7):2858–68.
121 Ghafouri M, Amini S, Khalili K, Sawaya BE. HIV-1 associated dementia: symptoms and causes. Retrovirology. 2006;3:28.
122 Gorantla S, Makarov E, Finke-Dwyer J, Castanedo A, Holguin A, Gebhart CL, et al. Links between progressive HIV-1 infection of humanized mice and viral neuropathogenesis. Am J Pathol. 2010;177(6):2938–49.
123 Dash PK, Gorantla S, Gendelman HE, Knibbe J, Casale GP, Makarov E, et al. Loss of neuronal integrity during progressive HIV-1 infection of humanized mice. J Neurosci. 2011;31(9):3148–57.
124 Gorantla S, Gendelman HE, Poluektova LY. Can humanized mice reflect the complex pathobiology of HIV-associated neurocognitive disorders? J Neuroimmune Pharmacol. 2012 Jan 7.
125 de Graaf CA, Metcalf D. Thrombopoietin and hematopoietic stem cells. Cell Cycle. 2011;10(10):1582–9.
126 Rongvaux A, Willinger T, Takizawa H, Rathinam C, Auerbach W, Murphy AJ, et al. Human thrombopoietin knockin mice efficiently support human hematopoiesis in vivo. Proc Natl Acad Sci U S A. 2011;108(6):2378–83.
127 Willinger T, Rongvaux A, Takizawa H, Yancopoulos GD, Valenzuela DM, Murphy AJ, et al. Human IL-3/GM-CSF knock-in mice support human alveolar macrophage development and human immune responses in the lung. Proc Natl Acad Sci U S A. 2011;108(6):2390–5.
128 Rathinam C, Poueymirou WT, Rojas J, Murphy AJ, Valenzuela DM, Yancopoulos GD, et al. Efficient differentiation and function of human macrophages in humanized CSF-1 mice. Blood. 2011;118(11):3119–28.
129 Takenaka K, Prasolava TK, Wang JC, Mortin-Toth SM, Khalouei S, Gan OI, et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8(12):1313–23.
130 Strowig T, Rongvaux A, Rathinam C, Takizawa H, Borsotti C, Philbrick W, et al. Transgenic expression of human signal regulatory protein alpha in Rag2-/-gamma(c)-/- mice improves engraftment of human hematopoietic cells in humanized mice. Proc Natl Acad Sci U S A. 2011;108(32):13218–23.
131 Sato K, Nie C, Misawa N, Tanaka Y, Ito M, Koyanagi Y. Dynamics of memory and naive CD8+ T lymphocytes in humanized NOD/SCID/IL-2Rgammanull mice infected with CCR5-tropic HIV-1. Vaccine. 2010;28(Suppl 2):B32-7.
132 Sato K, Izumi T, Misawa N, Kobayashi T, Yamashita Y, Ohmichi M, et al. Remarkable lethal G-to-A mutations in vif-proficient HIV-1 provirus by individual APOBEC3 proteins in humanized mice. J Virol. 2010;84(18):9546–56.
133 Stoddart CA, Maidji E, Galkina SA, Kosikova G, Rivera JM, Moreno ME, et al. Superior human leukocyte reconstitution and susceptibility to vaginal HIV transmission in humanized NOD-scid IL-2Rgamma(-/-) (NSG) BLT mice. Virology. 2011;417(1):154–60.
