Nitric oxide mediates the blood pressure response to mental stress in humans
DOI: https://doi.org/10.4414/smw.2012.13627
Lionel
Trueb, Mattia
Lepori, Hervé
Duplain, Urs
Scherrer, Claudio
Sartori
Summary
OBJECTIVE: Nitric oxide (NO) regulates arterial pressure by modulating peripheral vascular tone and sympathetic vasoconstrictor outflow. NO synthesis is impaired in several major cardiovascular disease states. Loss of NO-induced vasodilator tone and restraint on sympathetic outflow could result in exaggerated pressor responses to mental stress.
METHODS: We, therefore, compared the sympathetic (muscle sympathetic nerve activity) and haemodynamic responses to mental stress performed during saline infusion and systemic inhibition of NO-synthase by NG-monomethyl-L-arginine (L-NMMA) infusion.
RESULTS: The major finding was that mental stress which during saline infusion increased sympathetic nerve activity by ~50 percent and mean arterial pressure by ~15 percent had no detectable sympathoexcitatory and pressor effect during L-NMMA infusion. These findings were not related to a generalised impairment of the haemodynamic and/or sympathetic responsiveness by L-NMMA, since the pressor and sympathetic nerve responses to immersion of the hand in ice water were preserved during L-NMMA infusion.
CONCLUSION: Mental stress causes pressor and sympathoexcitatory effects in humans that are mediated by NO. These findings are consistent with the new concept that, in contrast to what has been generally assumed, under some circumstances, NO has a blood pressure raising action in vivo.
Introduction
Nitric oxide (NO), which is synthesised from the amino acid L-arginine by the enzyme NO-synthase, plays an important role in the regulation of vasomotor tone and arterial pressure in both animals and humans [1–4]. NO regulates arterial pressure by a local action on peripheral vascular tone and by modulating sympathetic vasoconstrictor outflow [5–12]. NO synthesis and/or release is defective in patients with cardiovascular diseases such as hypercholesterolemia, hypertension, and heart failure [13–17] as well as normal aging [17]. It has been hypothesised that the conjunction of impaired NO-induced sympathoinhibition and loss of NO-induced vasodilation could lead to augmented sympathetic and pressor responses to mental stress that could trigger acute cardiovascular events [18]. We, therefore, examined the haemodynamic and sympathetic responses to mental stress during systemic inhibition of NO-synthase by NG-monomethyl-L-arginine (L-NMMA, a competitive stereospecific inhibitor of NO-synthase) infusion in healthy volunteers, and compared these responses with those observed during an equipressor infusion of the NO-independent vasoconstrictor phenylephrine and those observed during vehicle infusion. Finally, to test for the specificity of the findings, we studied the effects of D- and L-arginine infusion after the L-NMMA infusion on the haemodynamic and sympathetic responses to mental stress, and we examined the haemodynamic responses to immersion of the hand in ice water during L-NMMA infusion.
Methods
Subjects
We studied 13 healthy male volunteers (mean [±SD] weight 72 ± 9 kg, height 180 ± 7 cm, body mass index 22.3 ± 2.9 kg/m2, age 28 ± 3 years). All the subjects were normotensive, were taking no medications, and had no evidence of cardiovascular disease. All the studies were performed in the morning after an overnight fast. The experimental protocol was approved by the Institutional Review Board on Human Investigation, and all subjects provided written informed consent.
General procedures
The subjects were studied in the supine position. Heart rate, respiratory excursions (pneumobelt), blood pressure (Finapres), blood flow in the forearm and calf, and efferent muscle sympathetic nerve activity were recorded continuously on an electrostatic recorder and a tape recorder. Respiratory excursions were monitored to detect inadvertent performance of a Valsalva maneuver or prolonged expiration, since these manoeuvres can stimulate sympathetic outflow [19]. Drugs were infused through an intravenous catheter inserted in an antecubital vein.
Mental stress
Mental stress was elicited by 4 minutes of a mental arithmetic task [20]. The arithmetic task consisted of orally subtracting a two digit from a four digit number. The stress was maintained throughout the task by pressing the subjects to speed their performance, and by frequently presenting new arithmetic problems.
Recording of sympathetic-nerve activity
Multiunit recordings of postganglionic sympathetic nerve activity were obtained with unipolar tungsten microelectrodes inserted selectively into muscle nerve fasciculi of the peroneal nerve posterior to the fibular head by the microneurographic technique of Vallbo et al. [21]. The neural signals were amplified 20 000 to 50 000 times, filtered (bandwidth 700 to 2000 Hz), rectified and integrated (time constant 0.1 s) to obtain a mean voltage display of sympathetic activity. A recording of sympathetic activity was considered acceptable when it revealed spontaneous, pulse synchronous bursts of neural activity, with the largest bursts showing a minimal signal to noise ratio of 3:1. In each study, we documented that we were recording sympathetic outflow to skeletal muscle by demonstrating that the neural activity did not respond to arousal stimuli (loud noise) or a pinch of the skin, but showed a characteristic biphasic response to the Valsalva manoeuvre [19].
For analysis, printed filtered and mean voltage neurogrammes were visually inspected to identify bursts of sympathetic nerve discharge. The recordings were all analysed by the same observer who was blinded to the pharmacological intervention assigned to the subject. The intraobserver and interobserver coefficients of variation of the mean in identifying bursts are less than 6 percent and less than 9 percent, respectively [22]. Nerve traffic was expressed as number of bursts per minute, an index of the frequency of the activity.
Measurement of muscle blood flow
While recording sympathetic outflow to calf muscles in one leg, we simultaneously measured blood flow in the contra lateral leg and forearm by venous occlusion plethysmography using mercury-in-Silastic strain gauges [19]. The forearm and calf were elevated 10 to 15 cm above the level of the right atrium to collapse the veins. Circulation to the hand and the foot was arrested by inflating a cuff around the wrist and the ankle during blood flow determinations.
Drugs
Drugs were dissolved in physiological saline immediately before use. L-NMMA, L- and D-arginine were obtained from Clinalfa (Läufelfingen, Switzerland), and phenylephrine from Winthrop Pharmaceuticals.
Experimental protocols
Protocol 1
After instrumentation the subjects rested quietly for 30 min. They then received sequential infusions of normal saline (1 ml/min) for 30 min, and L-NMMA (50 μg/kg/min) for 60 min. In each of the 13 subjects, we measured the haemodynamic and sympathetic responses to two 4 min periods of mental stress; one was performed during saline infusion, and one 45 min after the start of the L-NMMA infusion. At the end of each stress, the subjects were asked to rate their perceived stress on a scale of 6 (minimal stress) to 20 (maximal stress) as a subjective index of stress.
To examine whether the findings were related specifically to NO-synthase inhibition, 5 of the subjects received additional sequential infusions of D-arginine (50 mg/kg over 10 min), and L-arginine (50 mg/kg over 10 min) after the L-NMMA infusion. Two additional bouts of mental stress were performed 5 minutes after the end of each, the D-, and the L-arginine infusion.
Haemodynamic measurements and sympathetic nerve activity were recorded during two 5-minute periods of saline infusion, from minute 35 to 45 after the start of L-NMMA infusion, (and the 5 minutes following the arrest of the D- and L-arginine infusions, n = 5), as well as during the two (four) 4-minute-bouts of mental stress.
On a separate occasion, 8 of the subjects underwent two bouts of mental stress at a 1-hour interval during saline infusion to examine the potential effects of habituation on the haemodynamic responses to mental stress. Haemodynamic responses were comparable during the two tests; during the last minute of the mental stress, mean arterial pressure had increased by 8.5 ± 2.8 and by 8.0 ± 1.9 mm Hg, and heart rate by 13 ± 3 and 12 ± 2 beats/min during the first and the second test, respectively.
Protocol 2
To examine the effects of the augmented baseline arterial pressure during L-NMMA infusion on the haemodynamic responses to mental stress, in 6 subjects, we examined the haemodynamic responses to mental stress performed 45 minutes after the start of phenylephrine infusion (titrated at a rate to match the increase in baseline arterial pressure observed during the L-NMMA studies).
Protocol 3
To examine whether the findings were specific for mental stress, on a separate day, we measured in 6 of the subjects, sympathetic and haemodynamic responses during a 2 min immersion of the hand in ice water; one test was performed during saline infusion (1 ml/min), the other 30 min after the start of L-NMMA infusion (50 μg/kg/min).
Data analysis
Mean arterial pressure was calculated as diastolic pressure plus 1/3 pulse pressure. The measurements of muscle sympathetic nerve activity (MSNA), heart rate and mean arterial pressure that were collected over 5-minute periods were averaged to a single value.
Statistical analysis was performed with paired two-tailed t-tests and an analysis of variance with repeated measures followed by Fisher’s post hoc test. A value of P <0.05 was considered significant. Data are given as mean ± SE unless stated otherwise.
Results
Table 1 shows that the mental stress induced increase in arterial pressure was diminished by L-NMMA infusion. This effect was not related to the augmented baseline arterial pressure during L-NMMA infusion, since the pressor response to mental stress was preserved during an equipressive phenylephrine infusion (mean arterial pressure increased by 12% during saline infusion, by 3% during L-NMMA infusion, and by 13% during phenylephrine infusion, P <0.01, L-NMMA vs. phenylephrine). L-NMMA infusion also attenuated the heart rate response throughout the mental stress; during the first minute of the stress heart rate increased from 62 ± 3 to its peak value of 81 ± 3 beats/min during saline infusion, and from 58 ± 3 to 71 ± 3 beats/min during L-NMMA infusion (P <0.001, L-NMMA vs. saline). L-NMMA did not alter the subjective perception of the mental stress; the rates of perceived stress were 13 ± 1 and 14 ± 1, during saline and L-NMMA infusion, respectively.
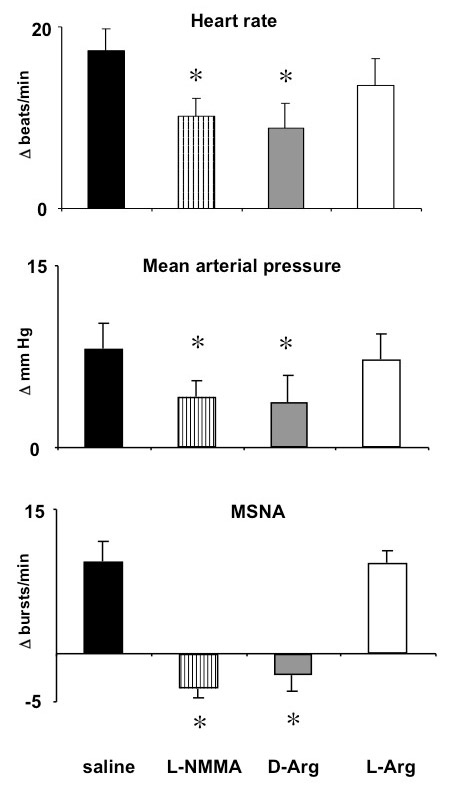
Figure 1
Mean ± SE effects in 5 normal subjects of mental stress on heart rate, mean arterial pressure, and muscle sympathetic nerve activity (MSNA) during saline infusion, and during NG-monomethyl-L-arginine (L-NMMA) infusion followed by D-arginine (D-Arg) and L-arginine (L-Arg) infusion. Bars represent the mean changes from baseline over the 4 minutes of mental stress. L-arginine (but not D-arginine) infusion restored the haemodynamic and sympathetic responses to mental stress. *P <0.05, vs. saline infusion.
To test for potential underlying mechanisms that could be involved in the altered heamodynamic responsiveness to mental stress during L-NMMA infusion, we examined the sympathetic nerve responses in the calf vasculature. As expected, during saline infusion, mental stress increased sympathetic nerve activity and induced a transient reduction of limb vascular resistance during the first 2 minutes of the stress. Table 1 and figure 1 show that L-NMMA diminished the mental stress induced sympathetic activation. The L-NMMA induced suppression of the sympathetic activation in the calf vasculature was associated with the persistence of the vasodilation in the calf during the last two minutes of the mental stress (P <0.02 vs. saline, table 1). The lack of sympathetic activation was not related to a generalised impairment of the sympathetic responsiveness, since L-NMMA infusion did not alter the sympathetic response to the cold pressor test. During the second minute of immersion of the hand in ice water, the rate of sympathetic nerve firing had increased from 19 ± 3 to 25 ± 4 bursts/min during saline infusion, and from 21 ± 3 to 26 ± 3 bursts/min during L-NMMA infusion, mean arterial pressure increased from 78 ± 2 to 86 ± 2 and from 81 ± 3 to 89 ± 2 mm Hg, respectively, and heart rate increased from 61 ± 4 to 68 ± 4 and from 59 ± 3 to 68 ± 4 beats/min, respectively. Finally, we examined whether during L-NMMA infusion, the altered haemodynamic response could be related to augmented stimulation of epinephrine release. We found that mental stress increased (P = 0.04) plasma epinephrine levels from 19 ± 2 pg/ml at baseline to values that were comparable during saline (31 ± 5 pg/ml) and L-NMMA (36 ± 5 pg/ml) infusion.
Figure 1 shows that during L-NMMA infusion, the alterations of the cardiovascular and sympathetic responses to mental stress were related specifically to inhibition of NO synthesis, since they persisted during D-arginine infusion, but were reversed by L-arginine infusion.
|
Table 1: Effects of L-NMMA infusion on cardiovascular and sympathetic responses to mental stress. |
| |
Mental stress plus saline infusion
|
Mental stress plus L-NMMA infusion
|
| |
Baseline |
1' |
2' |
3' |
4' |
Baseline |
L-NMMA |
1' |
2' |
3' |
4' |
| Heart rate (beats/min) |
62 ± 3 |
81 ± 3* |
74 ± 3* |
73 ± 3* |
75 ± 3* |
64 ± 3 |
58 ± 3* |
71 ± 3‡ |
67 ± 3‡ |
67 ± 2‡ |
66 ± 3‡ |
| Mean arterial pressure (mm Hg) |
78 ± 4 |
85 ± 4* |
89 ± 4* |
88 ± 4* |
88 ± 4* |
78 ± 3 |
84 ± 3† |
84 ± 4 |
87 ± 5 |
87 ± 6 |
87 ± 5 |
| Forearm vascular resistance (U) |
32 ± 2 |
22 ± 2* |
28 ± 2† |
31 ± 2 |
30 ± 5 |
33 ± 2 |
35 ± 3 |
26 ± 2‡ |
26 ± 2‡ |
27 ± 2‡ |
26 ± 3‡ |
| Calf vascular resistance (U) |
46 ± 5 |
39 ± 4† |
36 ± 4† |
46 ± 5 |
43 ± 5 |
48 ± 5 |
44 ± 3 |
35 ± 3§ |
38 ± 4§ |
38 ± 3‡ |
37 ± 4§ |
| MSNA (bursts/min) |
16 ± 2 |
23 ± 3† |
24 ± 3* |
24 ± 3* |
28 ± 3* |
18 ± 2 |
19 ± 2 |
15 ± 2 |
15 ± 2 |
17 ± 2 |
15 ± 2 |
| L-NMMA: NG-monomethyl-L-arginine; MSNA: muscle sympathetic nerve activity
Values are mean ± SE for 13 subjects, except for MSNA (n = 10) and calf blood flow and vascular resistance (n = 8)
*P <0.01 vs corresponding baseline; †P <0.05 vs corresponding baseline; ‡P <0.01 vs L-NMMA alone; §P <0.05 vs L-NMMA alone. |
Discussion
We found that mental stress which during saline infusion markedly increased mean arterial pressure and muscle sympathetic nerve activity had no detectable effect on these two variables during L-NMMA infusion. The attenuation of the blood pressure response was not related to the L-NMMA-induced increase of baseline arterial pressure, because an equipressive infusion of the non-endothelium dependent vasoconstrictor phenylephrine did not attenuate the pressor response to mental stress. These findings provide direct evidence for a rise in blood pressure and sympathoexcitatory action of NO in vivo.
The lack of mental stress-induced haemodynamic and sympathetic responses during L-NMMA infusion was not related to habituation, since the haemodynamic responses to two consecutive bouts of stress during saline infusion were comparable, and, most importantly, the haemodynamic and sympathetic responses were restored when the stress was repeated after L-arginine infusion. The attenuated sympathetic and haemodynamic responses also cannot be attributed to a non-specific attenuation of the subjective perception of the psychological stress by L-NMMA, as evidenced by similar rates of perceived stress during saline and L-NMMA infusion. The findings cannot be explained by altered adrenomedullary responsiveness, since, in line with a previous report [23], epinephrine levels were comparable during saline and L-NMMA infusion. Finally, the findings were not related to a non-specific impairment of the haemodynamic and/or sympathetic responsiveness by L-NMMA, since the responses to immersion of the hand in ice water were preserved during L-NMMA infusion.
Sympathetic and vascular responses to mental stress are highly differentiated. For example, whereas in the leg sympathetic activation has been a universal finding [24–26], in the arm, sympathetic activity remains unchanged [24] or may even decrease [27] during mental stress. A previous study reported decreased norepinephrine levels during L-NMMA infusion at baseline and during mental stress that could have been related to altered sympathetic outflow and/or altered norepinephrine release or clearance [23]. Here, we used direct recordings of sympathetic nerve activity to address this issue. While as expected, L-NMMA infusion did not alter sympathetic outflow at rest [9] it suppressed mental stress-induced sympathetic activation in the leg. This suppression of the mental stress-induced sympathetic activation by L-NMMA was associated with persistent vasodilation in the calf that may have contributed to the attenuated pressor response. Moreover, L-NMMA infusion also attenuated the heart rate response to mental stress, even though the smaller increase in arterial pressure presumably resulted in removal of baroreflex restraint on the chronotropic response. Since this response is thought to be, at least in part, sympathetically mediated [28], and sympathetic nerve responses in the leg are correlated with norepinephrine spillover in the heart [29], L-NMMA may also have attenuated the mental stress-induced cardiac sympathetic activation, an effect which may have contributed to the attenuated blood pressure response. In line with this hypothesis, a decreased cardiac output-dependent increase in blood pressure has been suggested to contribute to the attenuated blood pressure response to mental stress during L-NMMA infusion [23]. Alternatively, inhibition of NO synthesis by a 3-min bolus infusion of much larger doses of L-NMMA was found to alter baroreflex control of heart rate during simulated orthostatic stress [30]. We did not assess baroreflex function in the present studies and cannot exclude the possibility that in the present study, prolonged infusion of much lower doses of L-NMMA also altered baroreflex control of heart rate and, in turn may have contributed to altered heart rate responses to mental stress. Finally, it is possible that L-NMMA may also suppress the mental stress-induced sympathetic activation and vasoconstriction in other regions, such as the visceral vascular bed.
In mice, systemic injection of a NO-synthase inhibitor suppresses central neural NO-synthase activity as early as 15 minutes after its administration [31]. Moreover, NO has an excitatory action on neurons of the nucleus tractus solitarius in rats [8], and renal sympathetic preganglionic neurons in rabbits [5]. Finally, NO derived from neuronal NO-synthase has been suggested to increase arterial pressure in intact endothelial NO-synthase knockout mice by stimulating sympathetic activity [31]. Taken together with these findings in experimental animals, our data in conscious humans could be consistent with the hypothesis that, under some conditions, central neural NO increases arterial pressure by stimulating sympathetic nerve activity.
Importantly, the observation that L-NMMA did not alter the sympathoneural and haemodynamic response to the cold pressor test in the present, nor those to exercise in an earlier study [9], suggests that NO has differential effects on sympathetic and cardiovascular responsiveness to different forms of stress in humans.
Previous studies using local intraarterial L-NMMA infusion had suggested that NO, while contributing to the vasodilator response to mental stress in the forearm circulation, plays a modest role in the circulatory adaptation to mental stress in healthy subjects [32–34]. The present findings in conjunction with previously published data [23] challenge this concept and indicate that NO plays an important role in mediating the blood pressure and heart rate adjustments to mental stress which appear to be mediated, at least in part, by NO-induced sympathetic activation. Moreover, our data are consistent with the hypothesis that defective NO synthesis may have differential regional effects on the vascular responsiveness to mental stress, since in the coronary circulation, endothelial dysfunction has been shown to disturb the normal vasodilator response resulting in paradoxical vasoconstriction during mental stress [35], whereas in the present studies, we found that NO-synthase inhibition resulted in persistent limb vasodilation (and lack of a blood pressure response) throughout the entire period of mental stress. Finally, in the clinical setting defective NO synthesis has been consistently shown in pathological conditions characterised by endothelial dysfunction such as hypertension, obesity, dyslipidemia, insulin-resistance, heart failure as well as normal aging [13–17]. The present findings could suggest that in these subjects autonomic and cardiovascular adjustments to mental stress may be altered similarly as those observed during L-NMMA infusion in healthy humans.
References
1 Haynes WG, Noon JP, Walker BR, Webb DJ. Inhibition of nitric oxide synthesis increases blood pressure in healthy humans. J Hypertens. 1993;11(12):1375–80.
2 Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333(6174):664–6.
3 Scherrer U, Randin D, Vollenweider P, Vollenweider L, Nicod P. Nitric oxide release accounts for insulin's vascular effects in humans. J Clin Invest. 1994;94(6):2511–5.
4 Zanzinger J, Czachurski J, Seller H. Inhibition of sympathetic vasoconstriction is a major principle of vasodilation by nitric oxide in vivo. Circ Res. 1994;75(6):1073–7.
5 Hakim MA, Hirooka Y, Coleman MJ, Bennett MR, Dampney RA. Evidence for a critical role of nitric oxide in the tonic excitation of rabbit renal sympathetic preganglionic neurones. J Physiol. 1995;482(Pt 2):401-7.
6 Kumagai K, Suzuki H, Ichikawa M, Jimbo M, Murakami M, Ryuzaki M, et al. Nitric oxide increases renal blood flow by interacting with the sympathetic nervous system. Hypertension. 1994;24(2):220–6.
7 Liu JL, Murakami H, Zucker IH. Effects of NO on baroreflex control of heart rate and renal nerve activity in conscious rabbits. Am J Physiol. 1996;270(6 Pt 2):R1361–70.
8 Ma S, Abboud FM, Felder RB. Effects of L-arginine-derived nitric oxide synthesis on neuronal activity in nucleus tractus solitarius. Am J Physiol. 1995;268(2 Pt 2):R487–91.
9 Owlya R, Vollenweider L, Trueb L, Sartori C, Lepori M, Nicod P, et al. Cardiovascular and sympathetic effects of nitric oxide inhibition at rest and during static exercise in humans. Circulation. 1997;96(11):3897–903.
10 Sakuma I, Togashi H, Yoshioka M, Saito H, Yanagida M, Tamura M, et al. NG-methyl-L-arginine, an inhibitor of L-arginine-derived nitric oxide synthesis, stimulates renal sympathetic nerve activity in vivo. A role for nitric oxide in the central regulation of sympathetic tone? Circ Res. 1992;70(3):607–11.
11 Sander M, Chavoshan B, Victor RG. A large blood pressure-raising effect of nitric oxide synthase inhibition in humans. Hypertension. 1999;33(4):937–42.
12 Zanzinger J, Czachurski J, Seller H. Inhibition of basal and reflex-mediated sympathetic activity in the RVLM by nitric oxide. Am J Physiol. 1995;268(4 Pt 2):R958–62.
13 Creager MA, Cooke JP, Mendelsohn ME, Gallagher SJ, Coleman SM, Loscalzo J, et al. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 1990;86(1):228–34.
14 Linder L, Kiowski W, Buhler FR, Luscher TF. Indirect evidence for release of endothelium-derived relaxing factor in human forearm circulation in vivo. Blunted response in essential hypertension. Circulation. 1990;81(6):1762–7.
15 Panza JA, Casino PR, Kilcoyne CM, Quyyumi AA. Role of endothelium-derived nitric oxide in the abnormal endothelium-dependent vascular relaxation of patients with essential hypertension. Circulation. 1993;87(5):1468–74.
16 Treasure CB, Vita JA, Cox DA, Fish RD, Gordon JB, Mudge GH, et al. Endothelium-dependent dilation of the coronary microvasculature is impaired in dilated cardiomyopathy. Circulation. 1990;81(3):772–9.
17 Zeiher AM, Drexler H, Saurbier B, Just H. Endothelium-mediated coronary blood flow modulation in humans. Effects of age, atherosclerosis, hypercholesterolemia, and hypertension. J Clin Invest. 1993;92(2):652–62.
18 Anggard E. Nitric oxide: mediator, murderer, and medicine. Lancet. 1994;343(8907):1199–206.
19 Randin D, Vollenweider P, Tappy L, Jequier E, Nicod P, Scherrer U. Suppression of alcohol-induced hypertension by dexamethasone. N Engl J Med. 1995;332(26):1733–7.
20 Falkner B, Onesti G, Angelakos ET, Fernandes M, Langman C. Cardiovascular response to mental stress in normal adolescents with hypertensive parents. Hemodynamics and mental stress in adolescents. Hypertension. 1979;1(1):23–30.
21 Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59(4):919–57.
22 Vollenweider P, Tappy L, Randin D, Schneiter P, Jequier E, Nicod P, et al. Differential effects of hyperinsulinemia and carbohydrate metabolism on sympathetic nerve activity and muscle blood flow in humans. J Clin Invest. 1993;92(1):147–54.
23 Lindqvist M, Melcher A, Hjemdahl P. Hemodynamic and sympathoadrenal responses to mental stress during nitric oxide synthesis inhibition. Am J Physiol Heart Circ Physiol. 2004;287(5):H2309–15.
24 Anderson EA, Wallin BG, Mark AL. Dissociation of sympathetic nerve activity in arm and leg muscle during mental stress. Hypertension. 1987;9(6 Pt 2):III114–9.
25 Callister R, Suwarno NO, Seals DR. Sympathetic activity is influenced by task difficulty and stress perception during mental challenge in humans. J Physiol. 1992;454:373–87.
26 Jones PP, Spraul M, Matt KS, Seals DR, Skinner JS, Ravussin E. Gender does not influence sympathetic neural reactivity to stress in healthy humans. Am J Physiol. 1996;270(1 Pt 2):H350–7.
27 Halliwill JR, Lawler LA, Eickhoff TJ, Dietz NM, Nauss LA, Joyner MJ. Forearm sympathetic withdrawal and vasodilatation during mental stress in humans. J Physiol. 1997;504(Pt 1):211–20.
28 Freyschuss U, Hjemdahl P, Juhlin-Dannfelt A, Linde B. Cardiovascular and sympathoadrenal responses to mental stress: influence of beta-blockade. Am J Physiol. 1988;255(6 Pt 2):H1443–51.
29 Kingwell BA, Thompson JM, Kaye DM, McPherson GA, Jennings GL, Esler MD. Heart rate spectral analysis, cardiac norepinephrine spillover, and muscle sympathetic nerve activity during human sympathetic nervous activation and failure. Circulation. 1994;90(1):234–40.
30 Spieker LE, Corti R, Binggeli C, Luscher TF, Noll G. Baroreceptor dysfunction induced by nitric oxide synthase inhibition in humans. J Am Coll Cardiol. 2000;36(1):213–8.
31 Kurihara N, Alfie ME, Sigmon DH, Rhaleb NE, Shesely EG, Carretero OA. Role of nNOS in blood pressure regulation in eNOS null mutant mice. Hypertension. 1998;32(5):856–61.
32 Cardillo C, Kilcoyne CM, Quyyumi AA, Cannon RO, 3rd, Panza JA. Role of nitric oxide in the vasodilator response to mental stress in normal subjects. Am J Cardiol. 1997;80(8):1070–4.
33 Joyner MJ, Dietz NM. Nitric oxide and vasodilation in human limbs. J Appl Physiol. 1997;83(6):1785–96.
34 Seddon MD, Chowienczyk PJ, Brett SE, Casadei B, Shah AM. Neuronal nitric oxide synthase regulates basal microvascular tone in humans in vivo. Circulation. 2008;117(15):1991–6.
35 Yeung AC, Vekshtein VI, Krantz DS, Vita JA, Ryan TJ, Jr., Ganz P, et al. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med. 1991;325(22):1551–6.
