DOI: https://doi.org/10.4414/smw.2012.13603
Mortality in invasive enterococcal (bloodstream) infections has been reported to be as high as 51% [1]. High-level resistance to gentamicin is an independent predictor of mortality in invasive enterococcal infections in addition to underlying disease and resistance to vancomycin [2, 3].

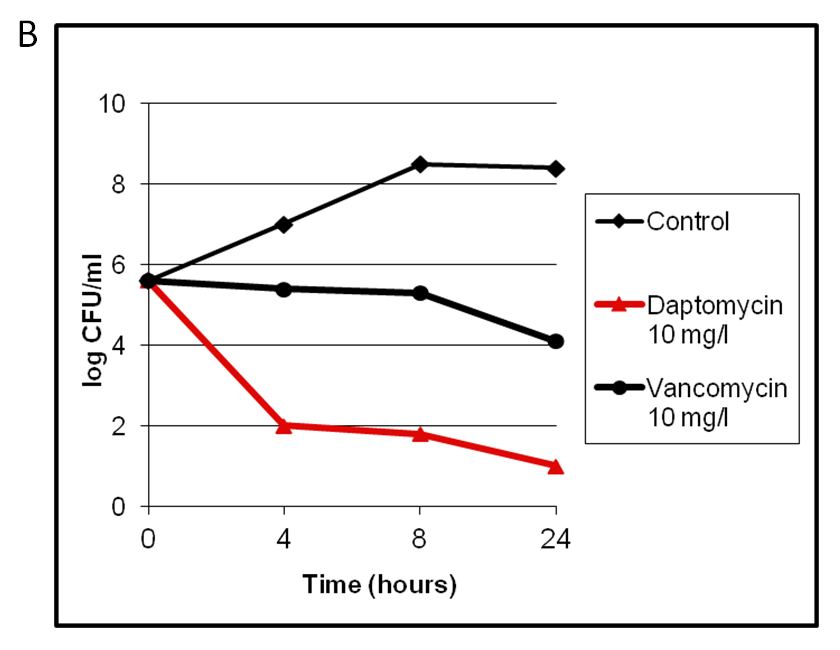
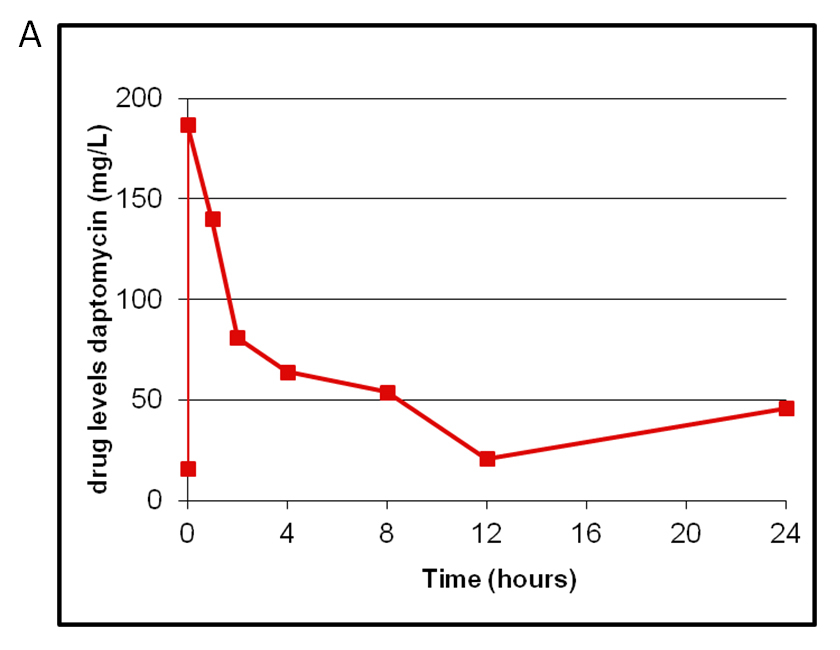
Figure 1
Therapeutic monitoring in one patient with high-dose daptomycin of 10 mg/kg/24 h (A) and in vitro killing curves showing a bactericidal effect of daptomycin at 10 mg/l in contrast to 10 mg/l vancomycin (B).
The treatment of invasive enterococcal infections is a challenge for clinicians due to the reduced susceptibility to penicillins reflected by high minimum inhibitory concentrations (MIC) compared to other streptococci [4, 5]. Treatment recommendations for invasive enterococcal infections advocate synergistic antibiotic combinations such as an aminopenicillin plus gentamicin. In recent years a shift from ampicillin-sensitive E. faecalis to ampicillin-resistant E. faeciumwas noted in many European centres, a development we also observed in our hospital. In the event of resistance by E. faeciumto ampicillin and/or high level gentamicin, vancomycin therapy has been suggested although it is poorly bactericidal [6]. Whilst treatment options of vancomycin resistant enterococci (VRE) are addressed in the literature, studies on treatment of enterococci with resistance to penicillin and/or high-level gentamicin are scarce and no information from controlled studies is available [7, 8]. In expert opinions linezolid, quinupristin-dalfopristin, tigecyclin, daptomycin, or the new antibiotics dalbavancin, telavancin, ceftobiprole and ceftaroline are under discussion as alternative treatments [6, 9–11]. Linezolid, a bacteriostatic agent which can be given orally due to its high oral bioavailability, is not approved for the treatment of endocarditis. Quinupristin-dalfopristin, a bactericidal combination of two synergistic working streptogramins, is not available in Switzerland. Furthermore, neither quinupristin-dalfopristin nor tigecyclin are approved for the treatment of endocarditis. For the new glycopeptides dalbavancin and telavancin, and the novel betalactams ceftobiprole and ceftaroline, only limited clinical data are available. Ceftobiprole showed high affinity to PBP5fm in vitro [12], but was withdrawn from the market by the company for further development.
Daptomycin, a cyclic lipopeptide with bactericidal activity against Gram-positive bacteria [13], is approved for complicated skin and soft tissue infections with Gram positive bacteria, as well as for Staphylococcus aureus bloodstream infections. Daptomycin has been shown to be efficient in rat models of experimental endocarditis with E. faecium[14], but clinical data are scarce [15].
Here we report a case series of 11 retrospectively collected cases of severely ill patients with invasive E. faecium infections treated with daptomycin between 2007 and 2009. The study was approved by the local ethics committee. All strains were resistant to ampicillin (MIC >8 mg/l) but susceptible to vancomycin, and 7/11 strains were highly resistant to gentamicin (MIC >500 mg/l).
Table 1 summarises the underlying disease, the infectious focus, the dosage of daptomycin and the patients’ outcome. All patients had severe underlying diseases: five had haematological malignancies, two had repeated episodes of cholangiosepsis, two had severe atherosclerosis after multiple vascular surgical procedures, and one had undergone liver transplantation. The majority of patients (10/11) were treated either on an intensive care unit or bone marrow transplant unit prior to isolation of the E. faecium.
| Table 1: Patient characteristics, antibiotic treatment and outcome. | |||||
| Age, sex, # | Underlying disease | Focus | Reason for daptomycin | Treatment (antibiotics, surgery) | Outcome 3 months after end of treatment |
| 77, f | Acute leukaemia | Anorectal cellulitis (decubitus) associated bacteraemia | Acute renal failure | Daptomycin 6 mg/kg × 13 days + Meronem 2 × 1 g Surgery: no | Recovered |
| 66, f, # | Acute leukaemia HSCT | Intracardial septic thrombosis | Ongoing fever despite vancomycin | Daptomycin 6 mg/kg × 6 weeks + vancomycin Surgery: no | Death (due to underlying disease: cerebral bleed) |
| 54, m | Acute leukaemia HSCT Gall stone disease | Sepsis from cholecystitis with consecutive endocarditis, spondylodiscitis, pulmonary abscess | Ongoing bacteraemia despite vancomycin | Daptomycin 6 mg/kg + vancomycin Kill kurves, TDM and daptomycin dose escalation to 10 mg/kg × 12 weeks Surgery: removal of choledochal stent | Death (due to underlying disease: gram neg sepsis) |
| 61, m, # | Acute leukaemia Neutropenia | Bacteraemia of unknown origin | Renal and hepatic failure Haemofiltration Ongoing bacteraemia despite vancomycin | Daptomycin 6 mg/kg + meropenem Surgery: no | Death due to infection (after only 2 doses): Multiorgan failure |
| 55, f | Chronic GvHD HSCT | Bacteraemia of unknown origin | Daptomycin 6 mg/kg + vancomycin Surgery: no | Recovered | |
| 65, f | Liver transplant | Cholangiosepsis | Relapse despite treatment with vancomycin Renal impairment Outpatient regimen | Vancomycin 7 days, then daptomycin 6 mg/kg × 7 days Surgery: removal of stent | Recovered |
| 56, m, # | Colorectal cancer Neutropenia | Intraabdominal abscess | Once daily outpatient regimen | Daptomycin 6 mg/kg × 7 days + ciprofloxacin × 7 days Surgery: drainage of abscess | Recovered |
| 91, m, # | Gallstone disease | Polymicrobial Bacteraemia from cholangitis (E. coli, K. pneumoniae, E. faecium, Cl. perfringens) | Relapsing cholangiosepsis | Daptomycin 6 mg/kg × 7 days Surgery: no | Recovered |
| 81, m | Gallstone disease | Right sided endocarditis | Endocarditis developed despite vancomycin | Daptomycin 6 mg/kg + vancomycin × 10 days Then daptomycin monotherapy 10 mg/kg according to TDM × 6 weeks Surgery: no | Recovered |
| 48, m | Aortic dissection vascular graft | Graft infection | Renal and hepatic impairment | vancomycin x 7 d until renal insufficiency, then daptomycin 6 mg/kg x 14 d Surgery: no | Death (due to underlying disease: postoperative bleeding) |
| 78, m | Severe atherosclerosis Vascular graft | Vascular graft-associated deep tissue infection | Once daily outpatient regimen | Daptomycin 6 mg/kg × 14 days Surgery: surgical drainage of abscess with removal of foreign bodies | Recovered |
| f = female; m = male; HSCT = haematopoietic stem cell transplantation; GvHD = Graft-versus-host disease; strains susceptible to high level gentamicin are marked as follows: #. | |||||
All patients had been treated with multiple broad-spectrum antibiotics including betalactams, carbapenems and aminoglycosides prior to detection of E. faecium.
Five patients had bloodstream infections with E. faecium(3 of unknown origin, 1 graft infection, 1 septic thrombosis), 4 patients had relapsing cholangiosepsis (2 with consecutive endocarditis, 2 with infection of a biliary stent) and 2 patients had deep abscesses (1 intraabdominal abscess, 1 deep tissue abscess after angioplasty).
The rationales for the use of daptomycin were renal failure (3/11), vancomycin failure (4/11) in spite of vancomycin susceptibility (MIC ≤4 mg/l), outpatient parenteral therapy (2/11) and ongoing septicaemia (2/11).
In the majority of patients daptomycin was started in a dosage of 6 mg/kg/day. Two patients with endocarditis were treated with a higher dosage of 10 mg/kg/d according to therapeutic drug monitoring (TDM). In one patient with persistent bacteraemia, hypoproteinaemia and severe immunosuppression the dosage was increased to 10 mg/kg/d after in vitro kill-curves had shown a bactericidal effect defined by loss of ≥3 log/CFU/24h with a concentration of 10 mg/l daptomycin (fig. 1B). It was noteworthy that vancomycin at a dose of 10 mg/l was bacteriostatic. In this patient TDM was performed (fig. 1A). In a second patient right-sided endocarditis developed whilst the patient was under treatment with vancomycin. Therapy was therefore switched to daptomycin 10 mg/kg/d and therapeutic drug monitoring was performed (data not shown).
Elevation of creatine kinase was noted in one patient with severe sepsis in multi-organ failure. Eosinophilic pneumonia did not occur.
Treatment outcomes are shown in table 1. Seven patients recovered and 4 died. Only in one case was death attributed to E. faecium infection. The patient died in severe sepsis after 2 doses of antibiotics. Death in the other three cases was attributed to the underlying condition.
Our case series illustrates that patients affected by bacteraemia with resistant enterococci suffer from severe underlying diseases. Monotherapy with vancomycin can result in treatment failure and bactericidal treatment is urgently needed.
Failures of daptomycin monotherapy for enterococcal infections have been described in case reports mainly for E. faecalis [16, 17]. The mechanisms of resistance are not yet elucidated and may potentially limit the use of daptomycin in the treatment of E. faecium [18]. Although we observed favourable effects with daptomycin at 6 mg/kg/d for the treatment of infections with E. faecium, the appropriate dosage remains to be elucidated in controlled studies.
Our case series indicates that salvage therapy with daptomycin was likely to be effective in 7/11 patients with refractory invasive infections due to multi-resistant E. faecium. Thus, daptomycin may be a reasonable alternative for E. faecium infections. However, it has not been proven superior in a comparative clinical study and retrospective cohort studies in patients with vancomycin-resistant enterococci show overlapping results [19–21]. Consequently, randomised controlled trials on the optimal dosage, efficacy and safety are urgently needed for these multiresistant bacteria which are difficult to treat [22] .
Acknowledgement:We thank Alois Gratwohl for support with selected patients as reported in this study.
1 Hoge CW, Adams J, Buchanan B, Sears SD. Enterococcal bacteremia: to treat or not to treat, a reappraisal. Rev Infect Dis. 1991;13(4):600–5.
2 Patterson JE, Sweeney AH, Simms M, Carley N, Mangi R, Sabetta J, et al. An analysis of 110 serious enterococcal infections. Epidemiology, antibiotic susceptibility, and outcome. Medicine (Baltimore). 1995;74(4):191–200.
3 Shaked H, Carmeli Y, Schwartz D, Siegman-Igra Y. Enterococcal bacteraemia: epidemiological, microbiological, clinical and prognostic characteristics, and the impact of high level gentamicin resistance. Scand J Infect Dis. 2006;38(11–12):995–1000.
4 Murray BE. The life and times of the Enterococcus. Clin Microbiol Rev. 1990;3(1):46–65.
5 Sader HS, Jones RN. Antimicrobial susceptibility of Gram-positive bacteria isolated from US medical centers: results of the Daptomycin Surveillance Program (2007–2008). Diagn Microbiol Infect Dis. 2009;65(2):158–62.
6 Arias CA, Murray BE. Emergence and management of drug-resistant enterococcal infections. Expert Rev Anti Infect Ther. 2008;6(5):637–55.
7 Mohr JF, Friedrich LV, Yankelev S, Lamp KC. Daptomycin for the treatment of enterococcal bacteraemia: results from the Cubicin Outcomes Registry and Experience (CORE). Int J Antimicrob Agents. 2009;33(6):543–8.
8 Das SS, Anderson JR, Macdonald AA, Somerville KW. Endocarditis due to high level gentamicin resistant Enterococcus faecium. The Journal of infection. 1994;28(2):185–91.
9 Arias CA, Contreras GA, Murray BE. Management of multidrug-resistant enterococcal infections. Clin Microbiol Infect. 2010;16(6):555–62.
10 Canton R, Ruiz-Garbajosa P, Chaves RL, Johnson AP. A potential role for daptomycin in enterococcal infections: what is the evidence? J Antimicrob Chemother. 2010;65(6):1126–36.
11 Landman D, Quale JM. Management of infections due to resistant enterococci: a review of therapeutic options. J Antimicrob Chemother. 1997;40(2):161–70.
12 Henry X, Amoroso A, Coyette J, Joris B. Interaction of ceftobiprole with the low-affinity PBP 5 of Enterococcus faecium. Antimicrob Agents Chemother. 2010;54(2):953–5.
13 Cottagnoud P. Daptomycin: a new treatment for insidious infections due to gram-positive pathogens. Swiss Med Wkly. 2008;138(7-8):93–9.
14 Vouillamoz J, Moreillon P, Giddey M, Entenza JM. Efficacy of daptomycin in the treatment of experimental endocarditis due to susceptible and multidrug-resistant enterococci. J Antimicrob Chemother. 2006;58(6):1208–14.
15 Gonzalez-Ruiz A, Beiras-Fernandez A, Lehmkuhl H, Seaton RA, Loeffler J, Chaves RL. Clinical experience with daptomycin in Europe: the first 2.5 years. J Antimicrob Chemother.;66(4):912–9.
16 Lewis JS, 2nd, Owens A, Cadena J, Sabol K, Patterson JE, Jorgensen JH. Emergence of daptomycin resistance in Enterococcus faecium during daptomycin therapy. Antimicrob Agents Chemother. 2005;49(4):1664–5.
17 Munoz-Price LS, Lolans K, Quinn JP. Emergence of resistance to daptomycin during treatment of vancomycin-resistant Enterococcus faecalis infection. Clin Infect Dis. 2005;41(4):565–6.
18 Montero CI, Stock F, Murray PR. Mechanisms of resistance to daptomycin in Enterococcus faecium. Antimicrob Agents Chemother. 2008;52(3):1167–70.
19 Grim SA, Hong I, Freeman J, Edwards C, Clark NM. Daptomycin for the treatment of vancomycin-resistant enterococcal infections. J Antimicrob Chemother. 2009;63(2):414–6.
20 Crank CW, Scheetz MH, Brielmaier B, Rose WE, Patel GP, Ritchie DJ, et al. Comparison of outcomes from daptomycin or linezolid treatment for vancomycin-resistant enterococcal bloodstream infection: A retrospective, multicenter, cohort study. Clin Ther.;32(10):1713–9.
21 Poutsiaka DD, Skiffington S, Miller KB, Hadley S, Snydman DR. Daptomycin in the treatment of vancomycin-resistant Enterococcus faecium bacteremia in neutropenic patients. J Infect. 2007;54(6):567–71.
22 Figueroa DA, Mangini E, Amodio-Groton M, Vardianos B, Melchert A, Fana C, et al. Safety of high-dose intravenous daptomycin treatment: three-year cumulative experience in a clinical program. Clin Infect Dis. 2009;49(2):177–80.
Funding / potential competing interests: U.F. has received research support from Novartis AG. No other potential conflict of interest relevant to this article was reported.