Figure 1
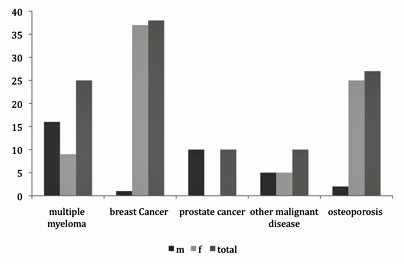
Display of underlying malignant disease and gender distribution of all patients (m = male; f = female).
DOI: https://doi.org/10.4414/smw.2012.13605
A single-centre experience with 110 patients
In 2006, 3 years after the “discovery” of a jaw-affecting disease associated with bisphosphonate therapy, this journal published a report of clinical experiences with bisphosphonate-associated osteonecrosis of the jaw (BRONJ) [1]. BRONJ has been described as a type of osteonecrosis of the jaws that occurs more often in the mandible than the maxilla. The first publication was presented by Robert Marx, who described BRONJ as a rediscovery of a similar disease called “phosphorous necrosis,” which occurred in match-factory workers in the 19th century.
However, the exact pathophysiology of BRONJ is not yet established. Hence, different hypotheses about its pathological mechanism have been offered, e.g., avascular necrosis, suppression of bone remodelling, and direct toxicity of bisphosphonates on bone and soft tissue [2, 3].
Different factors, including the cumulative dosage of bisphosphonate, underlying diseases, and locally invasive dental therapy, have been identified as risk factors for the occurrence of this multifactorial disease. Soon after presentation of BRONJ, treatment with complete remission was determined to be difficult or nearly impossible, and prevention noted as essential.
A definition and staging system of BRONJ was presented in 2007 and updated in 2009 by the American Association of Oral and Maxillofacial Surgeons (AAOMFS) and has been widely used to date [4]. According to this publication, BRONJ is defined by the following three criteria:
1. Current or previous treatment with bisphosphonates (BPs),
2. Exposed bone for more than 8 weeks, and
3. No history of radiation therapy.
The staging system ranges from stage 0: “absence of necrotic bone, but presence of nonspecific clinical findings and symptoms” (which conflicts with criterion 2 of the definition provided by the AAOMFS) to stage 3: “presence of exposed, necrotic bone with signs of infection extending beyond the border of the alveolar bone region” [4].
Eight years after the first publication, a lot of experience has been garnered on this disease. Moreover, information from other clinicians and our own treatment of a large number of patients have led to a clearer clinical picture, better diagnosis, and a change in treatment strategies for BRONJ. Several aspects of the definition and staging system of the AAOMFS have come into question, and the need for revision is obvious, as stated by Colella et al. [5].
One example is the introduction of new drugs to the market that inhibit bone resorption, such as the RANK ligand inhibitors Xgeva® and Prolia® (denosumab; Amgen, Zug, Switzerland). These drugs have led to promising results in various studies, but also to osteopathology of the jaw as a side effect [6–8]. This fact stands in contrast to the first criterion of the AAOMS definition of BRONJ.
The aim of this article is to provide an update on current knowledge regarding jaw-associated side effects of treatment with BPs and other bone resorption inhibitors. An up-to-date overview of epidemiology, risk factors, clinical signs, possible diagnostic methods, and treatment strategies will be presented, based on an analysis of relevant literature and our own clinical experience with 112 patients.
In the last 7 years, 112 patients with osteonecrosis of the jaw (ONJ) associated with bone resorption–inhibiting drugs, such as BPs and receptor activator of nuclear factor kappa-B ligand (RANKL) inhibitors, have been referred to the Department of Craniomaxillofacial Surgery, University Hospital of Zurich, by dentists or different specialists dealing with bone resorption-inhibition therapy. All patients were diagnosed, treated, and followed in an osteonecrosis outpatient clinic. Retrospective data collected included age, gender, and medical history of all patients, as well as type and duration of BP medication.
All patients were examined clinically, and different types of imaging were performed depending on the individual situation of each patient. In most patients, panoramic X-rays were taken to assess the dental situation and necessary preventative measures. Additional diagnostic imaging, such as computed tomography (CT), magnetic resonance imaging (MRI), or cone-beam CT, was performed to assess the extent of the lesion and the involvement of other structures. Additionally, in most patients with a history of malignancy, a bone biopsy was performed to exclude metastases of the underlying disease.
Depending on the individual situation, underlying disease, and general health status, different therapeutic measures were implemented, ranging from conservative treatment in patients with poor general condition to wide resection with subsequent microsurgically revascularised bone reconstruction. Over time, our therapy regimen changed from mainly conservative at the beginning to preferably minimally invasive surgery for complete remission. This change was made with the aim of achieving a higher success rate without limitation on the quality of life. If possible, a drug holiday of about 3 to 6 months was taken from before surgical intervention until complete healing of the oral mucosa about 2 weeks after surgery.
All patients were followed up regularly, with the frequency depending on their individual situation and location.
In 2/112 patients, histological examination detected malignant cells, whereas clinical and imaging findings showed typical signs of BRONJ. These two patients were excluded from statistical analysis.
All patients signed an informed consent form prior to the analysis of their data, which were initially obtained for medical purposes. The study design satisfied the Declaration of Helsinki guidelines and ethical principles for medical research involving human subjects.
Since the discovery of BRONJ in 2003, we have conducted a continuous literature search including the regular screening of current literature databases such as MEDLINE, EMBASE, and the Cochrane Library for key words such as “bisphosphonate”, “osteonecrosis of the jaw”, “osteoporosis”, and “antiresorptive treatment”. Articles in English, German, Italian, and French were evaluated. Additional references of interest identified in the reference lists of relevant articles were also added to the library. The “library” was grouped into different sections such as aetiology, diagnostics, treatment, oral bisphosphonates, guidelines, and prevention, and each section contained different subgroups. Many different kinds of articles, such as case series, animal studies, case reports, original articles, and reviews, were added to the library. Thus, a constant literature update of current knowledge could be ensured.
In total, 110 patients (33 [30%] men, 77 [70%]) women) were referred to the osteonecrosis clinic for BP-induced osteopathology of the jaw during 2003–2010. Eighty-three patients were treated with bone resorption inhibitors due to underlying malignant disease and 27 patients were treated for osteoporosis. The mean age of all patients was 67 years; in patients with osteoporosis, the mean age was 72 years, whereas in patients with malignant disease, the average age was 7 years younger (mean, 65 years).

Figure 1
Display of underlying malignant disease and gender distribution of all patients (m = male; f = female).
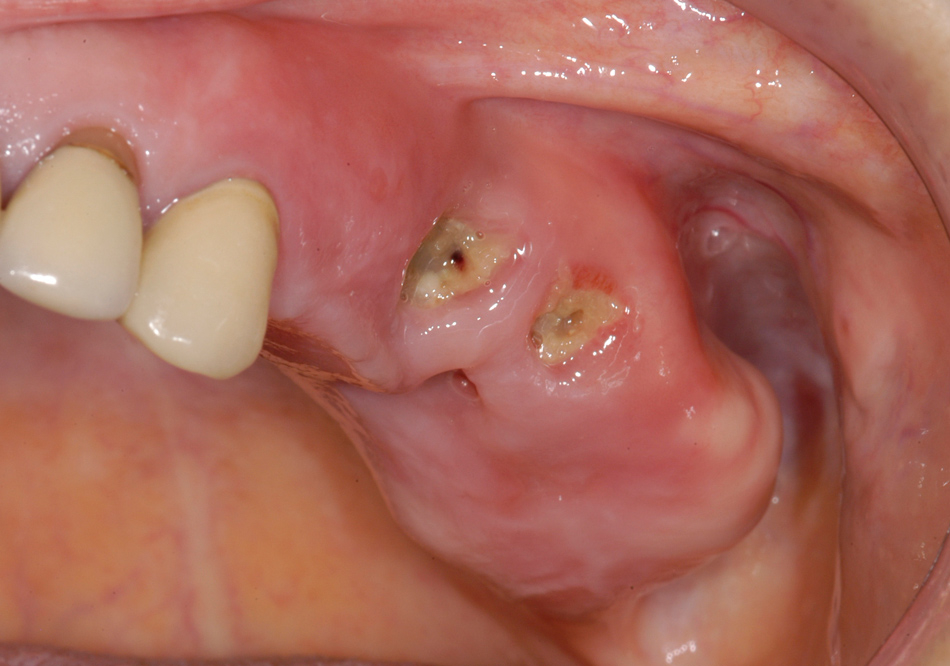
Figure 2
Female patient with osteoporosis and BRONJ of the left posterior maxilla including the maxillary sinus. The clinical picture shows exposed bone with signs of infection of the surrounding and pus discharge.
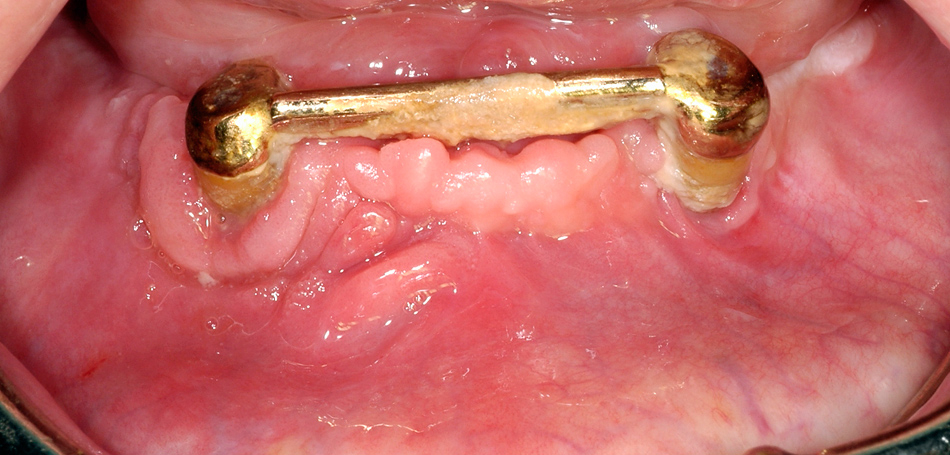
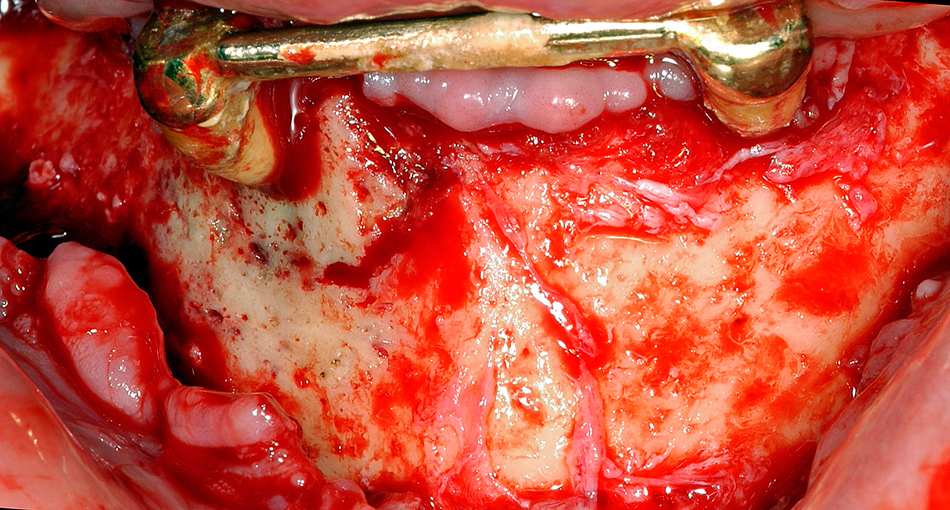
Figure 3
A Female patient with BRONJ of the right anterior mandible associated with periapical infection of the canine. The clinical picture shows no exposed bone, but signs of infection of the surrounding soft tissue and periodontal pus discharge.
B The same patient as figure 3A. Intraoperative view shows clearly the necrotic bone area in contrast to the vital bone in the left mandible. The necrotic area is surrounded from granulation tissue. Bone appears green-greyish.
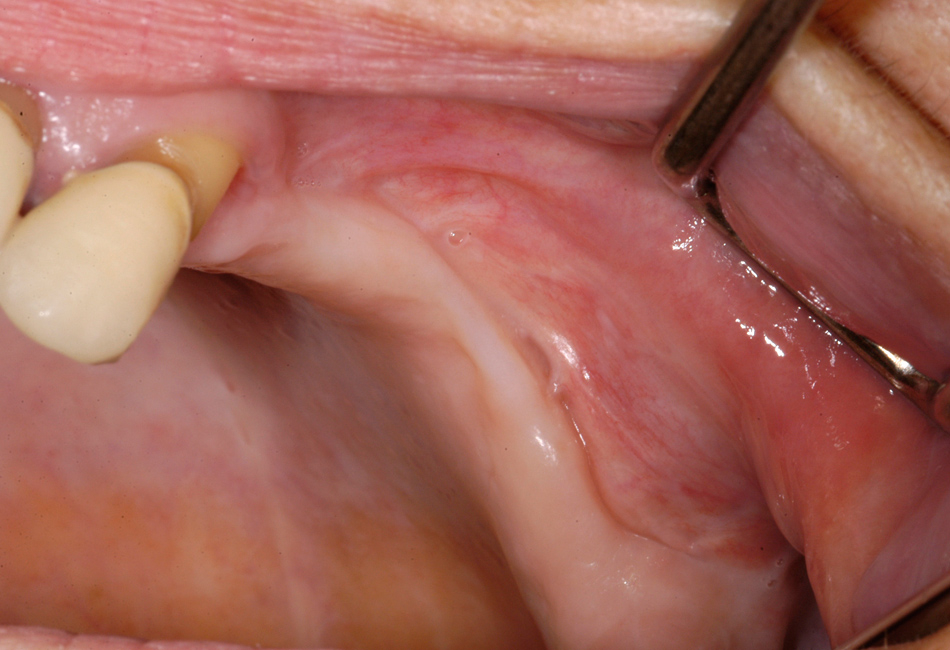
Figure 4
Same patient as figure 2 shows the situation after surgical treatment of BRONJ with complete remission without any remaining dehiscence of the soft tissue.
Of the patients with underlying malignant disease, 46% (n = 38) were treated for metastasised breast cancer, 30% (n = 25) had multiple myeloma, 12% (n = 10) had prostate cancer, and 12% (n = 10) were treated for other malignant diseases, such as lymphoma, carcinoma of the bladder, lung carcinoma, carcinoma of the thyroid, melanoma, and renal cell carcinoma. All patients without underlying malignant disease were treated for osteoporosis (fig. 1).
Most patients with malignancies received zoledronic acid (93%), followed by pamidronate (table 1). Some patients were treated with BPs other than intravenous BPs (ibandronate, n = 5; alendronate, n = 2), and one patient received the RANKL inhibitor denosumab with no previous or subsequent BP therapy. In 15 (18%) patients, the drug regimen was changed during the duration of the underlying disease because of side effects, the onset of osteonecrosis, or the availability of the more potent BP, zoledronic acid. The mean duration of bone resorption-inhibition therapy until the onset of osteopathology was 34 months in patients with underlying malignant disease and 24 months for the patient with denosumab treatment. Most patients with underlying malignant disease were treated with additional chemotherapeutic agents, and in seven patients, specific data were missing. Seven female patients with breast cancer and one male patient with prostate cancer did not receive chemotherapy but only hormone therapy.
Most patients with non-malignant underlying disease were treated with alendronate (44%) or ibandronate (41%). At least six patients received the intravenous agents pamidronate or zoledronic acid. In four patients, therapy was changed to annual doses of zoledronic acid (Aclasta®; Novartis Pharma, Switzerland, Bern). One patient was treated with risedronate. The drug regimens of seven (26%) patients were changed.
The mean duration of BP therapy until the onset of osteopathology was 42 months in patients with osteoporosis.
Of all patients with osteoporosis, five received additional systemic corticosteroids. Of those patients, one received additional immunosuppressive therapy due to previous renal transplantation.
The mandible was affected in 74% of patients and the maxilla was affected in 28%. The molar region was the most common site of osteonecrosis; about 78% of mandibular lesions and 77% of maxillary lesions affected this region. Osteopathology was less common in the frontal areas of the maxilla and mandible; these regions were affected in only 17% (n = 19) of all patients, and most of those lesions were large and originated in the premolar or molar region (table 1).
In all patients, a local incident was correlated with the area of subsequent osteopathology occurrence, meaning that the study sample included no case of spontaneous occurrence. In some patients, more than one factor was correlated with more than one area of subsequent bone necrosis. In most (64%) patients, extractions were performed in the area of bone pathology, mostly due to previous periapical infection or massive loosening due to periodontal disease. Other local incidents were the insertion of screwed implants, periodontal disease, periapical foci, or pressure ulcers due to a poorly fitting prosthesis. Descriptive statistics of patients are displayed in table 1.
The majority (75%) of patients presented with pain as their major concern. All patients showed signs of infection in the area of the affected jaw, which involved pus discharge, abscess formation, or inflamed surrounding soft tissue (fig. 2).
Exposed bone was observed in only 74% of patients; 23% of patients presented with a fistula, but without clinically evident exposed bone (only radiologically evident affected bone) (fig. 3a and b). All of those patients except one were treated surgically; therefore, the diagnosis of osteopathology of the jaw was also affirmed clinically.
Histological examination of bone specimens was performed in 66 patients. In two patients, tumour cells of the underlying disease (breast cancer, n = 1; multiple myeloma, n = 1) were found; thus, their diagnoses were revised and they were excluded from the study. The analysis thus examined histological data from 64 patients.
Dead bone (acellular bone without osteocytes, osteoclasts, or osteoblasts) with signs of acute and chronic inflammation and bacterial colonisation was found in all patients. Plaques of Actinomyceswere specifically described in 46/64 (72%) histological analyses of osteopathology due to bone resorption inhibitors, whereas the remaining cases were not specifically tested for the presence of Actinomyces.
| Table 1: Descriptive Statistics of study cohort, displaying gender, type of bisphosphonate localisation of osteopathology, local risk factor, clinical picture and type of treatment and outcome, respectively for patients with underlying malignant disease and osteoporosis. | ||||
| Underlying malignant disease n (%) | Osteoporosis n (%) | Total n (%) | ||
| Total n (%) | 83 (75) | 27 (25) | 110 | |
| Sex | f | 53 (64) | 24 (89) | 77 (70) |
| m | 30 (36) | 3 (11) | 33 (30) | |
| Mean age | 65 | 72 | 67 | |
| Bisphosphonate1 | Zoledronic acid | 77 (93) | 3* | 80 (73) |
| Pamidronate | 13 | 5 | 18 | |
| Alendronate | 2 | 12 (44) | 14 | |
| Ibandronate | 5 | 11 (41) | 16 | |
| Others** | 1 | 2 | 6 | |
| Localisation1 | Mandible | 62 (75) | 19 (70) | 81 (74) |
| Molars | 48 | 15 | 63 (57) | |
| Premolars | 28 | 9 | 37 | |
| Front | 10 | 4 | 14 | |
| Maxilla | 23 (28) | 8 (30) | 31 (28) | |
| Molars | 18 | 6 | 24 (22) | |
| Premolars | 8 | 5 | 13 | |
| Front | 4 | 1 | 5 | |
| local risk factor1 | Extraction | 55 (66) | 15 (56) | 70 (64) |
| Prosthesis | 11 | 7 | 18 | |
| Implantation | 9 | 6 | 15 | |
| Perioodontal disease | 10 | 1 | 11 | |
| Other *** | 18 | 4 | 22 | |
| Clinical presentation1 n (%) | Pain | 62 | 21 | 83 |
| Pus | 49 | 14 | 63 | |
| Abscess | 23 | 5 | 28 | |
| Paresthesia | 19 | 2 | 21 | |
| Exposed bone | 62 | 19 | 81 (74) | |
| Fistula | 19 | 6 | 25 | |
| Involvmemnt of sinus | 11 | 3 | 14 | |
| Signs of infection | 83 | 27 | 110 | |
| Treatment n (%) | Surgery | 45 (54) | 19 (70) | 64 (58) |
| Complete remission | 32 (71) | 18 (95) | 50 (78) | |
| Partial remission | 7 | 1 | 8 | |
| No improvement | 6 | 6 | ||
| * Three patients received zoledronic acid 1/year and the other monthly. ** Others included: Denosumab, risedronate. *** Other included: periapical infection, tori mandibulares, root canal treatment. 1 Patients may belong to more than one group. | ||||
About ⅔ (n = 19) of patients in the osteoporosis group were treated surgically. In the remaining eight patients, surgery was either unnecessary due to secondary healing after local revision of the affected bone, or refused by the patient.
Forty-five (53%) patients with underlying malignant disease were treated surgically, and the others were treated conservatively due to poor general health status or refusal of surgical treatment.
Of the surgically treated patients with osteoporosis, 95% showed complete remission (fig. 4), and one female patient showed partial remission with a small area of remaining exposed bone, but had no complaint. Complete remission was achieved in 71% of surgically treated patients with underlying malignant disease; partial remission was achieved in seven patients, and six patients showed no improvement at the surgically treated site.
Only nine patients in the conservative treatment group showed some kind of decrease in the size of exposed bone, mostly due to several minor local revisions in the outpatient setting. All of them had improvement of symptoms due to systemic anti-infectious treatment, but complete remission was only achieved in three patients.
ONJ in association with BP therapy is currently a recognised disease, but much about this condition remains unclear. Currently, the definition and classification of the AAOMS, which was introduced in 2007 and updated in 2009, is widely accepted. Exposed bone is one of the three decisive points for the clinical confirmation of the specific diagnosis.
In this study, we analysed data of 112 patients with osteopathology associated with bone resorption–inhibiting drugs. All patients showed local risk factors in addition to therapy with bone resorption inhibitors, and a majority of patients showed impairment of the immune system due to the underlying disease and immunosuppressive therapies. Histological analyses of affected bone showed signs of inflammation in each specimen, with the presence of Actinomyces in a noticeable number of specimens. Surgical therapy showed excellent results, especially in patients with osteoporosis. Interestingly, only 74% of patients actually showed exposed bone, as required for a diagnosis of BRONJ according to the AAOMS criteria. This discrepancy is another example of the need for revision of the current definition and classification of this disease, as proposed by other authors [5]. In the following section, we present a structured overview of our results and updated knowledge about osteopathology of the jaw in association with bone resorption–inhibiting therapy.
It has been shown in the last several years that the incidence of ONJ associated with BP therapy is higher in patients with cancer than in those with a non-malignant underlying disease. Several studies have found the prevalence of BRONJ to be about 0–18.3% in patients with various types of underlying malignant disease [9–11]. The prevalence in patients who received BP therapy for osteoporosis or other non-malignant disease with pathologically high bone resorption seems to be much lower (0.01–0.21%, depending on the duration of BP therapy); post-marketing data suggest an incidence of 1 per 100 000 persons per year [12]. However, few studies have investigated a representative number of such patients [13, 14].
In this study, 75% of patients received BP treatment due to an underlying malignant disease and 25% received these drugs due to osteoporosis, an uncommonly high rate in comparison with other reported studies. In a summary-analysis of 2400 previously published BRONJ cases, Filleul et al. [15] found that 89% of all patients were treated for malignant disease and 11% for underlying osteoporosis, similar to the findings of other studies [16, 17]. The reason for the high rate of patients with BRONJ and osteoporosis in our sample may be an interesting topic for further studies.
Although the patho-aetiological process of BRONJ remains incompletely understood, it seems to be multifactorial. Apart from possibly associated new risk factors, such as genetic polymorphism [18], several major risk factors have been identified and established in the literature. They can be divided into the following categories:
1. Therapy with bone resorption-inhibiting drugs,
2. General factors, and
3. Local factors.
As shown over time, BP type, cumulative dose, and treatment duration are correlated with the risk of BRONJ development [9]. Several authors have shown an increase in the incidence of BRONJ in correlation with the duration of BP treatment or number of doses administered [15, 19, 20], demonstrating the need to revise the duration and schemes of BP therapy in patients with underlying malignant disease and osteoporosis. Nevertheless, verifying a correlation between a specific BP and the risk of BRONJ development is somewhat difficult because the general factors such as immunosuppression are often strongly associated with a high dosage and a high-potency drug regimen and are also believed to be risk factors. In this study, the majority of patients were treated monthly with an intravenous administration of zoledronic acid.
One important new aspect of our developing understanding of BRONJ is the expansion of causative drugs, which include other bone resorption inhibitors. Recently, the RANKL inhibitor Prolia® and Xgeva® (denosumab; Amgen, Zug, Switzerland) were introduced in studies of therapy for skeletal-related tumour events and compared with zoledronic acid therapy [8, 21]. In those studies, BRONJ also occurred in patients receiving denosumab treatment. For example, Saad et al. [22] found a BRONJ incidence of 1.8% in patients receiving denosumab treatment, versus 1.3% in patients receiving zoledronic acid, in three blinded phase III trials. In our patient group, denosumab therapy was the causative agent in one male patient [23, 24]. The appearance of similar osteopathology with denosumab raises the question of whether the actual risk factor might be the degree of osteoclast inhibition, with subsequent decreased remodelling and defence abilities in the bone. Thus, the term BRONJ is, strictly speaking, obsolete and the condition should be renamed (e.g., osteopathology of the jaw associated with bone resorption inhibitors).
A few studies have investigated the influence of drugs other than BPs on the risk of BRONJ development. Most studies found no significant correlation between the incidence of BRONJ and the treatment of underlying malignancies with other drugs, such as tamoxifen, melphalan, and anthracyclines [25]. Further, the role of glucocorticosteroids as a potential additional risk factor remains unclear [10].
Generally, therapy with bone resorption inhibitors is closely correlated with some general factors that have been found to have an influence, such as immunosuppression, regardless of whether it is due to the underlying disease or to medication. Patients with malignant underlying diseases, especially multiple myeloma, are at highest risk of developing ONJ. Usually, the immune defences of those patients are highly impaired by the disease and by various therapies. Some authors, such as Subramaniam et al. [26], have argued that the suppression of osteoblast function, depending on the underlying disease (e.g., in patients with multiple myeloma), is an important general risk factor for the development of ONJ [26]. Another general risk factor under current discussion is the age of the patient [19].
In addition to systemic and general factors, some local events are clearly linked to the occurrence of osteopathology. Similar to our 2006 study [1], each patient in our present sample had a local event in the same area as subsequent bone necrosis. Most (64%) patients had previous dental extractions, but periodontal disease, pressure ulcers, root canal treatment, implant insertion, and periapical infections were also evaluated as local risk factors. Dental extractions were clearly shown to increase the risk of ONJ development. Vahtsevanos et al. [19] reported an 18–33-fold higher risk of ONJ development in patients with a dental extraction history, and other authors have confirmed this finding. In the same study, Vahtsevanos et al. [19] reported a two-fold higher risk in patients wearing dentures, supposedly due to the direct negative influence of BP on mucosal healing. Implant insertion remains under discussion as a local risk factor, especially in patients with osteoporosis. To date, few reports have described patients with BRONJ in association with dental implant placement. Lazarovici et al. [27] for example, presented a case series in which implant insertion was a local triggering factor in 27/145 (18.6%) patients with BRONJ, regardless of whether the patients had a malignant disease or osteoporosis. In this study, dental implant insertion was a local triggering factor for the development of subsequent osteopathology in 15 (14%) patients. According to other studies [28], not only implant insertion, but also peri-implant communication between the implant and the jawbone seems to be a risk factor [29]. Several studies or case series have shown that ONJ in areas of previously inserted implants developed chronologically independently from the insertion procedure [28, 30].
In contrast to most reports, no spontaneous lesion was found in this study. Typical reported rates of potential spontaneous lesions have ranged from 10–25% of cases [15, 16, 31]; however, in our experience, the presence of spontaneous lesions is somewhat questionable and would be an interesting topic for further analysis. Despite the experience gained during the last years, the hypothesised major impact of a potential local factor involving pathogenic invasion of the bone, and the possible infection of a bone with inhibited remodelling potential due to BP therapy, remains unproven [1]. Local injury with pathogenic invasion or primary infection of bone with disturbed remodelling due to a bone resorption inhibitor may be associated with preceding or subsequent bone necrosis in the region. People with restricted systemic immune defences, such as patients with multiple myeloma, are at higher risk than patients with healthy immune system.
In an interesting study, Saia et al. [32] tried to establish predictors for the development of BRONJ after tooth extraction. They tested several variables in 60 patients who received BP therapy and necessary extraction therapy: age, gender, malignancy, and baseline osteomyelitis (histologically detected inflammation of alveolar bone at the time of extraction). Five of the 60 patients developed BRONJ after tooth extraction; bone biopsies of these five patients showed baseline osteomyelitis. Thus, the role of oral pathogens and infection is believed to be an important factor in the pathogenesis of this disease. This hypothesis might be supported by the fact that all patients in the present study showed clinical signs of infection, such as granulation tissue or inflammation of surrounding soft tissue. Pus discharge was found in half of the patients, abscess formation was present in one-quarter of patients, and maxillary sinus infection was clinically evident in 13%. Of all 112 patients, 74% presented with pain. Half of the patients underwent biopsies of the affected bone; all showed signs of infection and Actinomyces was present in 70%. It remains unclear whether the presence of Actinomyces indicates an invasive infection or simple colonisation of the bone [33, 34]. Several study findings have supported the hypothesis of inflammation as a major factor in the aetiological process. For example, Lesclous et al. [35] analysed specimens from 30 patients with BRONJ, and found a correlation between the severity of bone marrow inflammation and the “clinical extent” of BRONJ; they concluded that BRONJ might develop as a sequential event, with bacterial invasion as a major factor. Kassolis et al. [36] presented another interesting finding in 2010. They examined bone from edentulous alveolar ridges histologically and found non-viable bone in 35% and bone with signs of osteomyelitis in 15% of all cases, respectively. With this finding, they suggested that “subclinical infected areas and areas with non-viable bone” might represent high-risk areas for the development of BRONJ.
During the last 7 years, many different diagnostic methods have been evaluated. Still, careful investigation of the patient’s medical history and clinical examination are the most important steps in the decision-making process. The predictive usefulness of specific diagnostic measures, such as the carboxy-terminal collagen crosslink (CTX) level, remains debated; to date, no evidence-based data concerning risk prediction are available [37–39].
Imaging is necessary for the analysis of necrotic lesion size and treatment planning, especially in those patients with non-exposed bone [40, 41], anyhow 26% of patients in this study. Several non-specific radiographic findings have been identified, such as sclerotic changes, periosteal reaction, sequestra, and areas of lucency [42]. CT, cone-beam CT, or MRI is needed to demonstrate the lesions and their extent [42–45]. A plain radiograph is a useful basic tool for the identification of possible current dental infections and necessary dental therapy, but is not sufficient for the identification of the affected jawbone [42]. O’Ryan et al. [46] demonstrated the role of bone scintigraphy as an early indicator, showing tracer uptake in 66% of 59 patients before clinical evidence of jaw lesions. Unfortunately, they did not correlate this information with dental history. Moreover, preventive diagnostic imaging would be too expensive to use in all patients receiving BP therapy and the benefits are questionable. Should a lesion been treated without clinical evidence? [47, 48]
Another important diagnostic measure is the histological analysis of the affected site to exclude tumor invasion. In this study, we saw malignant histological specimens in two patients, although clinical and radiological diagnostic measures clearly suggested ONJ.
ONJ treatment has changed markedly since 2003. Currently, most “therapy-resistant” jaw pathologies can be controlled surgically. Three main pillar strategies remain: (1) treatment of the infection, (2) local rehabilitation of the affected bone and soft tissue, and (3) necessary preventative measures. Early guidelines recommended the most conservative treatment possible [4, 49–51]. Therapy resistance with dehiscence of the surgical incision, progression of the infection, and bone destruction stoked fears of surgical intervention in these patients [52]. As a consequence, many practitioners looked for alternative, noninvasive treatment options for this disease, such as hyperbaric oxygen or low-level laser therapy [53, 54]. However, exclusively conservative treatment of patients with ONJ in our patient population was shown to increase the frequency of acute infectious episodes and therefore the frequency and duration of antibiotic treatment. Additionally, the quality of life in patients with infected exposed bone is often highly restricted in terms of pain, halitosis, antibiotic side effects, and the need for numerous visits to the dentist for local rinsing of the affected jaw. Furthermore, surgical therapy has been shown to be more effective than previously suggested [55]. Several authors have reported successful surgical treatment of ONJ lesions with varying levels of radical surgery and differing protocols for BP therapy (interruption or continuation) [56–58]. Increased experience has revealed that surgical therapy as minor as possible, but with total excision of all necrotic and infected bone areas, after 3–6 months of BP discontinuation has led to a high rate of complete remission in these patients. In this study, 58% of all patients were treated surgically and the frequency of surgical therapy increased over the study period. Complete remission was achieved in 78% of all patients, whereas this success rate was higher (95%) in patients with osteoporosis. Given the previously mentioned association of BRONJ with infection, all patients were additionally given systemic anti-infective therapy with antibiotics; this treatment resolved symptoms, especially pain, in all patients. Apart from surgical treatment, preventative measures, as recommended in earlier studies [1], remain of the utmost importance and have been shown to have beneficial effect [59, 60].
This study shows an ongoing learning effect in all aspects of dealing with osteopathology of the jaws in patients taking bone-resorption inhibitors. After almost 8 years of gathering knowledge in dealing with this disease, we hypothesise that this disease is a kind of osteomyelitis in a specifically pre-treated bone. However, evidence-based data are still lacking; we have only clinical findings. One strength of this study was that all patients were treated and followed up in the special clinic. Therefore, only a limited number of practitioners collected patient data. This article provided an overview about BRONJ. However, outlining all the data in one article was difficult, and not all information could be given, which is the major limitation of this article. Nevertheless, the most important facts for specialists dealing with patients taking bone-resorption inhibitors, and especially the information about the significance of local risk factors and currently successful treatment options, were provided.
The English in this document has been again checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/ayQob5
1 Dannemann C, Zwahlen R, Grätz KW. Clinical experiences with bisphopsphonate induced osteochemonecrosis of the jaws. Swiss Med Wkly. 2006;136(31–32):504–9.
2 Reid IR. Osteonecrosis of the jaw: who gets it, and why? Bone. 2009;44(1):4–10.
3 Reid IR, Bolland MJ, Grey AB. Is bisphosphonate-associated osteonecrosis of the jaw caused by soft tissue toxicity? Bone. 2007;41(3):318–20.
4 Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws AAoOaMS. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2007;65(3):369–76.
5 Colella G, Campisi G, Fusco V. American Association of Oral and Maxillofacial Surgeons position paper: Bisphosphonate-related osteonecrosis of the jaws-2009 update: the need to refine the BRONJ definition. J Oral Maxillofac Surg. 2009;67(12):2698–9.
6 Fusco V, Galassi C, Berruti A, Ciuffreda L, Ortega C, Ciccone G, et al. Osteonecrosis of the jaw after zoledronic acid and denosumab treatment. J Clin Oncol. 2011;29(17):e521–2; author reply e23–4.
7 Yarom N, Elad S, Madrid C, Migliorati CA. Osteonecrosis of the jaws induced by drugs other than bisphosphonates – a call to update terminology in light of new data. Oral Oncol. 2010;46(1):e1.
8 Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–65.
9 Hoff AO, Toth B, Hu M, Hortobagyi GN, Gagel RF. Epidemiology and risk factors for osteonecrosis of the jaw in cancer patients. Ann N Y Acad Sci. 2011;1218(1):47–54.
10 Hoff AO, Toth BB, Altundag K, Johnson MM, Warneke CL, Hu M, et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23(6):826–36.
11 Walter C, Al-Nawas B, Frickhofen N, Gamm H, Beck J, Reinsch L, et al. Prevalence of bisphosphonate associated osteonecrosis of the jaws in multiple myeloma patients. Head & face medicine. 2010;6(1):11.
12 Manfredi M, Merigo E, Guidotti R, Meleti M, Vescovi P. Bisphosphonate-related osteonecrosis of the jaws: a case series of 25 patients affected by osteoporosis. Int J Oral Maxillofac Surg. 2011;40(3):277–84.
13 Lo JC, O’Ryan FS, Gordon NP, Yang J, Hui RL, Martin D, et al. Prevalence of osteonecrosis of the jaw in patients with oral bisphosphonate exposure. J Oral Maxillofac Surg. 2010;68(2):243–53.
14 Rizzoli R, Burlet N, Cahall D, Delmas PD, Eriksen EF, Felsenberg D, et al. Osteonecrosis of the jaw and bisphosphonate treatment for osteoporosis. Bone. 2008;42(5):841–7.
15 Filleul O, Crompot E, Saussez S. Bisphosphonate-induced osteonecrosis of the jaw: a review of 2,400 patient cases. J Cancer Res Clin Oncol. 2010;136(8):1117–24.
16 Thumbigere-Math V, Sabino MaC, Gopalakrishnan R, Huckabay S, Dudek AZ, Basu S, et al. Bisphosphonate-related osteonecrosis of the jaw: clinical features, risk factors, management, and treatment outcomes of 26 patients. J Oral Maxillofac Surg. 2009;67(9):1904–13.
17 Abu-Id MH, Açil Y, Gottschalk J, Kreusch T. Bisphosphonate-associated osteonecrosis of the jaw. Mund-, Kiefer- und Gesichtschirurgie: MKG 2006;10(2):73–81.
18 Katz J, Gong Y, Salmasinia D, Hou W, Burkley B, Ferreira P, et al. Genetic polymorphisms and other risk factors associated with bisphosphonate induced osteonecrosis of the jaw. Int J Oral Maxillofac Surg. 2011;40(6):605–11.
19 Vahtsevanos K, Kyrgidis A, Verrou E, Katodritou E, Triaridis S, Andreadis CG, et al. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J Clin Oncol. 2009;27(32):5356–62.
20 Ruggiero SL. Bisphosphonate-related osteonecrosis of the jaw: an overview. Ann N Y Acad Sci. 2011;1218(1):38–46.
21 Reddy GK, Mughal TI, Roodman GD. Novel approaches in the management of myeloma-related skeletal complications. Support Cancer Ther. 2006;4(1):15–8.
22 Saad F, Brown JE, Van Poznak C, Ibrahim T, Stemmer SM, Stopeck AT, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO 2011.
23 Aghaloo TL, Felsenfeld AL, Tetradis S. Osteonecrosis of the jaw in a patient on denosumab. J Oral Maxillofac Surg. 2010.
24 Taylor KH, Middlefell LS, Mizen KD. Osteonecrosis of the jaws induced by anti-RANK ligand therapy. Br J Oral Maxillofac Surg. 2009:1–3.
25 Durie BG, Katz M, Crowley J. Osteonecrosis of the jaw and bisphosphonates. N Engl J Med. 2005;353(1):99–102; discussion 99–02.
26 Subramanian G, Cohen HV, Quek SYP. A model for the pathogenesis of bisphosphonate-associated osteonecrosis of the jaw and teriparatide’s potential role in its resolution. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics 2011.
27 Lazarovici TS, Yahalom R, Taicher S, Schwartz-Arad D, Peleg O, Yarom N. Bisphosphonate-related osteonecrosis of the jaw associated with dental implants. J Oral Maxillofac Surg. 2010;68(4):790–6.
28 Goss A, Bartold M, Sambrook P, Hawker P. The nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in dental implant patients: a South Australian case series. J Oral Maxillofac Surg. 2010;68(2):337–43.
29 Jacobsen C, Metzler P, Rossle M, Obwegeser J, Zemann W, Gratz KW. Osteopathology induced by bisphosphonates and dental implants: clinical observations. Clin Oral Investig. 2012.
30 Martin DC, O'Ryan FS, Indresano AT, Bogdanos P, Wang B, Hui RL, et al. Characteristics of implant failures in patients with a history of oral bisphosphonate therapy. J Oral Maxillofac Surg. 2010;68(3):508–14.
31 Otto S, Schreyer C, Hafner S, Mast G, Ehrenfeld M, Stürzenbaum S, et al. Bisphosphonate-related osteonecrosis of the jaws – characteristics, risk factors, clinical features, localization and impact on oncological treatment. J Craniomaxillofac Surg. 2012;40(4):303–9. Epub 2011 Jun 14.
32 Saia G, Blandamura S, Bettini G, Tronchet A, Totola A, Bedogni G, et al. Occurrence of bisphosphonate-related osteonecrosis of the jaw after surgical tooth extraction. J Oral Maxillofac Surg. 2010;68(4):797–804.
33 Hansen T, Kunkel M, Springer E, Walter C, Weber A, Siegel E, et al. Actinomycosis of the jaws – histopathological study of 45 patients shows significant involvement in bisphosphonate-associated osteonecrosis and infected osteoradionecrosis. Virchows Arch. 2007;451(6):1009–17.
34 Kaplan I, Anavi K, Anavi Y, Calderon S, Schwartz-Arad D, Teicher S, et al. The clinical spectrum of Actinomyces-associated lesions of the oral mucosa and jawbones: correlations with histomorphometric analysis. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics 2009;108(5):738–46.
35 Lesclous P, Abi Najm S, Carrel JP, Baroukh B, Lombardi T, Willi JP, et al. Bisphosphonate-associated osteonecrosis of the jaw: a key role of inflammation? Bone. 2009;45(5):843–52.
36 Kassolis JD, Scheper M, Jham B, Reynolds MA. Histopathologic findings in bone from edentulous alveolar ridges: a role in osteonecrosis of the jaws? Bone. 2010;47(1):127–30.
37 Kwon YD, Kim DY, Ohe JY, Yoo JY, Walter C. Correlation between serum C-terminal cross-linking telopeptide oftype I collagen and staging of oral bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67(12):2644–8.
38 Lee CYS, Suzuki JB. CTX biochemical marker of bone metabolism. Is it a reliable predictor of bisphosphonate-associated osteonecrosis of the jaws after surgery? Part I: biological concepts with a review of the literature. Implant dentistry. 2009;18(6):492–500.
39 Dodson TB. CTX and its role in managing patients exposed to oral bisphosphonates. J Oral Maxillofac Surg. 2010;68(2):487–8; author reply 88–9.
40 Fedele S, Kumar N, Davies R, Fiske J, Greening S, Porter S. Dental management of patients at risk of osteochemonecrosis of the jaws: a critical review. Oral Dis. 2009;15(8):527–37.
41 Junquera L, Gallego L. Nonexposed bisphosphonate-related osteonecrosis of the jaws: another clinical variant? J Oral Maxillofac Surg. 2008;66(7):1516–7.
42 Morag Y, Morag-Hezroni M, Jamadar DA, Ward BB, Jacobson JA, Zwetchkenbaum SR, et al. Bisphosphonate-related osteonecrosis of the jaw: a pictorial review. Radiographics: a review publication of the Radiological Society of North America, Inc 2009;29(7):1971–84.
43 Gill SB, Valencia MP, Sabino MLC, Heideman GM, Michel MA. Bisphosphonate-related osteonecrosis of the mandible and maxilla: clinical and imaging features. J Comput Assist Tomogr. 2009;33(3):449–54.
44 Arce K, Assael LA, Weissman JL, Markiewicz MR. Imaging findings in bisphosphonate-related osteonecrosis of jaws. J Oral Maxillofac Surg. 2009;67(5 Suppl):75–84.
45 Bedogni A, Blandamura S, Lokmic Z, Palumbo C, Ragazzo M, Ferrari F, et al. Bisphosphonate-associated jawbone osteonecrosis: a correlation between imaging techniques and histopathology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(3):358–64.
46 O’Ryan FS, Khoury S, Liao W, Han MM, Hui RL, Baer D, et al. Intravenous bisphosphonate-related osteonecrosis of the jaw: bone scintigraphy as an early indicator. J Oral Maxillofac Surg. 2009;67(7):1363–72.
47 Fabbricini R, Catalano L, Pace L, Del Vecchio S, Fonti R, Salvatore M, et al. Bone scintigraphy and SPECT/CT in bisphosphonate-induced osteonecrosis of the jaw. J Nucl Med. 2009;50(8):1385; author reply 85.
48 Dore F, Filippi L, Biasotto M, Chiandussi S, Cavalli F, Di Lenarda R. Bone scintigraphy and SPECT/CT of bisphosphonate-induced osteonecrosis of the jaw. J Nucl Med. 2009;50(1):30–5.
49 Van den Wyngaert T, Claeys T, Huizing MT, Vermorken JB, Fossion E. Initial experience with conservative treatment in cancer patients with osteonecrosis of the jaw (ONJ) and predictors of outcome. Ann Oncol. 2009;20(2):331–6.
50 Gevorgyan A, Enepekides DJ. Bisphosphonate-induced necrosis of the jaws: a reconstructive nightmare. Curr Opin Otolaryngol Head Neck Surg. 2008;16(4):325–30.
51 Adamo V, Caristi N, Saccà MM, Ferraro G, Arcanà C, Maisano R, et al. Current knowledge and future directions on bisphosphonate-related osteonecrosis of the jaw in cancer patients. Expert Opin Pharmacother. 2008;9(8):1351–61.
52 Engroff SL, Kim DD. Treating bisphosphonate osteonecrosis of the jaws: is there a role for resection and vascularized reconstruction? J Oral Maxillofac Surg. 2007;65(11):2374–85.
53 Erkan M, Bilgi O, Mutluoğlu M, Uzun G. Bisphosphonate-related osteonecrosis of the jaw in cancer patients and hyperbaric oxygen therapy. JOP. 2009;10(5):579–80; author reply 81–2.
54 Scoletta M, Arduino PG, Reggio L, Dalmasso P, Mozzati M. Effect of low-level laser irradiation on bisphosphonate-induced osteonecrosis of the jaws: preliminary results of a prospective study. Photomed Laser Surg. 2010;28(2):179–84.
55 Wilde F, Heufelder M, Winter K, Hendricks J, Frerich B, Schramm A, et al. The role of surgical therapy in the management of intravenous bisphosphonates-related osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(2):153–63.
56 Williamson RA. Surgical management of bisphosphonate induced osteonecrosis of the jaws. Int J Oral Maxillofac Surg. 2010;39(3):251–5.
57 Carlson ER, Basile JD. The role of surgical resection in the management of bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67(5 Suppl):85–95.
58 Marx RE. Reconstruction of defects caused by bisphosphonate-induced osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67(5 Suppl):107–19.
59 Brock G, Barker K, Butterworth CJ, Rogers S. Practical considerations for treatment of patients taking bisphosphonate medications: an update. Dent Update 2011;38(5):313–4, 17–8, 21–4 passim.
60 Lodi G, Sardella A, Salis A, Demarosi F, Tarozzi M, Carrassi A. Tooth extraction in patients taking intravenous bisphosphonates: a preventive protocol and case series. J Oral Maxillofac Surg. 2010;68(1):107–10.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.