Incidence and time frame of life-threatening arrhythmias in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention
DOI: https://doi.org/10.4414/smw.2012.13604
Patrizia
Cricri, Lukas D.
Trachsel, Peter
Müller, Adrian
Wäckerlin, Walter H.
Reinhart, Piero O.
Bonetti
Summary
BACKGROUND: Life-threatening arrhythmias may complicate the hospital course of patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PPCI). The optimal duration of electrocardiographic monitoring in such patients is not well established. We aimed to determine the incidence and the time of occurrence of life-threatening arrhythmias in STEMI patients undergoing PPCI.
METHODS: Data of 382 consecutive patients with STEMI undergoing PPCI were analysed regarding the occurrence of ventricular fibrillation (VF), sustained ventricular tachycardia (sVT) or bradycardia necessitating temporary or permanent pacing.
RESULTS: Of these patients, 55% had inferior STEMI, 41% anterior and 4% lateral STEMI. The infarct-related arteries were the right in 41%, the left anterior descending in 41%, the left circumflex in 16%, the left main stem in 1% and a vein graft in <1%. During hospitalisation, 27 (7.0%) patients developed 29 life-threatening arrhythmias (incidence 7.6%): 19 episodes occurred during PPCI (VF n = 11, bradycardia n = 8), 9 episodes during the first 24 hours after PPCI (VF n = 7, sVT n = 2), and 1 sVT episode in a hypokalemic patient on the 4th post-procedural day. A total of 17 patients (4.5%) died within the first 30 days, and 3 of these died during the PPCI procedure.
CONCLUSIONS: Life-threatening arrhythmias occur in a considerable proportion of STEMI patients undergoing PPCI during hospitalisation. Most of these arrhythmias occur during the PPCI procedure. Post-procedural life-threatening arrhythmias are virtually limited to the first 24 hours after PPCI. Thus, routine electrocardiographic monitoring beyond the first 24 hours after PPCI might not be required in most patients with uncomplicated STEMI.
Introduction
Primary percutaneous coronary intervention (PPCI) represents the method of choice for coronary revascularisation in patients with ST-segment elevation myocardial infarction (STEMI) [1, 2]. Life-threatening arrhythmias including ventricular tachyarrhythmias, such as ventricular fibrillation and sustained ventricular tachycardia, and bradyarrhythmias due to high-grade atrioventricular block, are well-known complications in patients with STEMI [3]. In order to timely detect and treat such serious arrhythmias, routine electrocardiographic monitoring is performed in STEMI patients during the first 24 to 48 hours after PPCI [1, 3–6]. However, current recommendations for electrocardiographic monitoring of patients with STEMI are mainly based on historical data, which were collected before PPCI was adopted as a superior alternative to thrombolysis for the treatment of such patients. Indeed, despite the widespread use of PPCI worldwide, currently there is a paucity of data regarding the incidence and timing of life-threatening arrhythmias in patients with STEMI undergoing PPCI. Hence, the optimal duration of electrocardiographic monitoring in STEMI patients undergoing PPCI is not well established. Therefore, we performed the present analysis in order to investigate the incidence and the time frame of occurrence of life-threatening arrhythmias in patients with STEMI, who are treated with PPCI, and to further elucidate the optimal duration of electrocardiographic monitoring in such patients.
Materials and methods
Study population
Data of 382 consecutive patients with acute STEMI, who had undergone PPCI within the first 24 hours after the onset of symptoms at the Kantonsspital Graubuenden, Chur, Switzerland were analysed. Death before arrival in the cardiac catheterisation laboratory represented the only exclusion criteria. Acute STEMI was defined as ST-segment elevation of at least 1 mm in two or more contiguous leads, or presumed new left bundle branch block on the presenting 12-lead electrocardiogram (ECG) in the presence of chest pain and/or elevation of cardiac biomarkers [7]. On admission, patients received an intravenous loading dose of aspirin (500 mg), an oral loading dose of clopidogrel (600 mg) and an intravenous bolus of unfractionated heparin (5000 units) before PPCI. In addition, patients were given oral atorvastatin (80 mg) before the procedure. The use of glycoprotein IIb/IIIa inhibitors was left at the discretion of the operator. PPCI was performed according to standard procedures. After PPCI, patients were monitored in our interdisciplinary intensive care unit for at least 24 hours. Post-procedural routine therapy comprised aspirin, clopidogrel and a statin. An additional therapy with an angiotensin converting enzyme inhibitor and a beta-blocker was strongly encouraged but its prescription as well as the timing of its initiation was left at the discretion of the responsible cardiologist.
Data collection
In our study population, the incidence and the time of occurrence of potentially life-threatening arrhythmias, including ventricular tachyarrhythmias such as ventricular fibrillation and sustained ventricular tachycardia, and significant bradyarrhythmias during the index hospitalisation, were studied retrospectively by reviewing the patients’ charts and by analysing various local databases (coronary angiography database, intensive care unit database). In addition, 30-day mortality was assessed routinely as a measure of quality in all patients either by chart review or by phone calls with the patient, his/her relatives or his/her general practitioner. Given the design of the study, approval by an ethical committee as well as patient consent was not obtained.
Definitions
Ventricular fibrillation was defined as irregular undulations of varying contour and amplitude on the ECG, with absent distinct QRS and T waves and haemodynamic compromise requiring direct-current defibrillation. Sustained ventricular tachycardia was defined as a regular wide-complex tachycardia of ventricular origin lasting ≥30 seconds and/or accompanied by haemodynamic compromise requiring electrical cardioversion or anti-arrhythmic therapy. Significant bradycardia was defined as any bradycardia that led to haemodynamic compromise necessitating the application/insertion of a temporary or permanent pacemaker.
Statistical analysis
Data of continuous variables are presented as mean ± SD and categorical data are shown as numbers (percentages). Comparisons between the 2 groups of patients with and without life-threatening arrhythmias were made using Student’s t-test for continuous variables and chi-square test for categorical variables. Statistical analysis was performed using the software IBM SPSS Statistics, version 19 (IBM Corporation, USA). A value of p <0.05 was considered statistically significant.
Results
The characteristics of the 382 patients included in our analysis are shown in table 1. During hospitalisation, 27 (7.0%) of these patients developed a total of 29 life-threatening arrhythmias (incidence 7.6%). Two patients developed 2 episodes of ventricular fibrillation during their hospitalisation course. In both of these patients, the first episode of ventricular fibrillation occurred during the PPCI procedure, while the second occurred within the first 9 hours after the intervention. A total of 19 (65.5%) of the life-threatening arrhythmias occurred during the PPCI procedure, whereas 10 (34.5%) episodes were observed during the post-procedural hospital course (table 2). With 18 (62.1%) of all arrhythmic events, ventricular fibrillation was the most frequent life-threatening arrhythmia observed in our patients. Significant bradycardia was responsible for 8 (27.6%) of life-threatening arrhythmias and occurred exclusively during the PPCI procedure in the cardiac catheterisation laboratory. Sustained ventricular tachycardia occurred in only 3 patients (10.3% of all life-threatening arrhythmias). Notably, except for one episode of sustained ventricular tachycardia occurring in a patient with hypokalemia (serum potassium 3.2 mmol/l) on the 4th day after PPCI, all episodes of post-procedural life-threatening arrhythmias were observed within the first 20 hours after PPCI (table 2 and fig. 1). Only 3 of the 10 patients experiencing post-procedural ventricular tachyarrhythmias were treated with a betablocker at the time of the arrhythmia (including the patient with sustained ventricular tachycardia on the 4th post-procedural day). In 3 patients, arrhythmias occurred within a very short time after the procedure before betablocker therapy could be started. In 4 other patients the reason for withholding betablocker therapy at the time of the arrhythmia was the patient`s disposition to sinusbradycardia and/or hypotension in the first post-procedural hours. A total of 31 (8%) of all patients included in the present analysis experienced cardiac arrest and underwent cardiopulmonary resuscitation before arriving in the cardiac catheterisation laboratory. These patients were more likely to experience life-threatening arrhythmias during or after PPCI than those who were haemodynamically stable before PPCI (30% vs. 6%, p <0.01; table 1). The time between the onset of symptoms and successful PPCI (symptom-to-balloon time) was significantly shorter in individuals experiencing life-threatening arrhythmias than in the group without arrhythmias. This difference was due to a shorter delay between symptom onset and hospital arrival (symptom-to hospital door time, “pre-hospital delay”) in those with arrhythmias, while the time interval between hospital arrival and successful PPCI (hospital door-to-balloon time, “in-hospital delay”) was identical among the 2 groups (table 1).
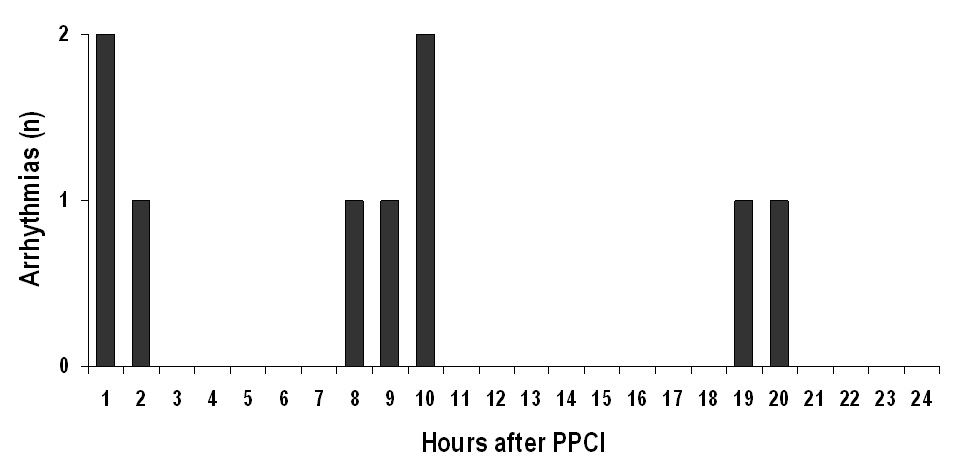
Figure 1
Time of occurrence of post-procedural life-threatening arrhythmias in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention (PPCI). In addition, a single episode of sustained ventricular tachycardia occurred on the 4th post-procedural day in a patient with hypokalemia (not shown).
A total of 17 (4.5%) patients died within 30 days after hospital admission. Of these, 3 died during the PPCI procedure. Causes of death were cardiogenic shock in 8 (47.1%) patients, pulse-less electrical activity in 5 (29.4%) individuals, and hypoxic brain damage or stroke in 4 (23.5%) subjects. Of those who died during the first 30 days after hospital admission, 8 patients had an episode of a life-threatening arrhythmia before the terminal event (table 3). Thus, 30-day mortality was significantly higher in patients who had life-threatening arrhythmias than in those who did not (29.6% vs. 2.5%, p <0.001). Also, patients who had to be resuscitated from cardiac arrest before arriving in the cardiac catheterisation laboratory were more likely to die within 30 days after hospital admission than those who remained haemodynamically stable before admission (19% vs. 3%, p = 0.001).
|
Table 1: Characteristics of the study patients. |
|
|
Patients without life-threatening arrhythmias
(n = 355)
|
Patients with life-threatening arrhythmias
(n = 27)
|
p-value
|
| Mean age (yrs) |
63 ± 12 |
64 ± 13 |
0.80 |
| Males |
273 (77%) |
17 (63%) |
0.11 |
| Medical history |
| Hypertension |
181 (51%) |
15 (56%) |
0.69 |
| Hyperlipidemia |
265 (75%) |
19 (70%) |
0.65 |
| Current smoker |
157 (44%) |
8 (30%) |
0.16 |
| Diabetes |
41 (12%) |
4 (15%) |
0.54 |
| Previous MI |
52 (15%) |
2 (7%) |
0.39 |
| Previous PCI |
52 (15%) |
4 (15%) |
1.00 |
| Previous CABG |
13 (4%) |
1 (4%) |
1.00 |
| Medication before STEMI |
| Aspirin |
86 (24%) |
7 (26%) |
0.81 |
| Thienopyridine |
19 (5%) |
1 (4%) |
1.00 |
| Oral anticoagulation |
7 (2%) |
1 (4%) |
0.44 |
| Statin |
68 (19%) |
4 (15%) |
0.79 |
| Beta-blocker |
70 (20%) |
6 (22%) |
0.80 |
| ACE-inhibitor |
55 (16%) |
3 (11%) |
0.78 |
| Angiotensin receptor-blocker |
44 (12%) |
6 (22%) |
0.14 |
| Calcium-antagonist |
25 (7%) |
2 (7%) |
1.00 |
| Pre-procedural CPR |
23 (6%) |
8 (30%) |
0.01 |
| STEMI localisation |
| Anterior |
147 (41%) |
10 (37%) |
} 0.89 |
| Inferior |
194 (55%) |
16 (59%) |
| Lateral |
14 (4%) |
1 (4%) |
| Infarct-related artery |
| LAD |
149 (42%) |
8 (30%) |
} 0.06 |
| LCX |
57 (16%) |
4 (15%) |
| RCA |
145 (41%) |
13 (48%) |
| Left main |
3 (<1%) |
1 (4%) |
| Saphenous vein graft |
1 (<1%) |
1 (4%) |
| Multi-vessel disease |
212 (60%) |
15 (56%) |
0.68 |
| Symptom-to-balloon time (min) |
337 ± 225 |
233 ± 104 |
0.02 |
| Symptom-to-hospital door time (min) |
274 ± 216 |
169 ± 102 |
0.01 |
| Hospital door-to-balloon time (min) |
64 ± 34 |
64 ± 27 |
0.99 |
| Post-procedural TIMI flow grade |
|
8 (2%) |
2 (7%) |
} 0.33 |
| 1 |
6 (2%) |
0 (0%) |
| 2 |
29 (8%) |
3 (11%) |
| 3 |
312 (88%) |
22 (82%) |
| Peri-/post-procedural IABP |
5 (1%) |
2 (7%) |
0.08 |
| Post-procedural maximum CK (U/l) |
2338 ± 1972 |
2457 ± 2895 |
0.77 |
| Post-procedural LVEF (%) |
54 ± 11 |
50 ± 15 |
0.13 |
| Data are presented as mean ± SD or number (%) of subjects.
MI = myocardial infarction; PCI = percutaneous coronary intervention; CABG = coronary artery bypass grafting; STEMI = ST-segment elevation myocardial infarction; ACE = angiotensin converting enzyme; CPR = cardiopulmonary resuscitation; LAD = left anterior descending coronary artery; LCX = left circumflex coronary artery; RCA = right coronary artery; TIMI = thrombolysis in myocardial infarction; IABP = intra-aortic balloon pump; CK = creatine kinase; LVEF = left ventricular ejection fraction. |
|
Table 2: Number and time frame of all life-threatening arrhythmias observed in the study patients during hospitalisation. |
| |
During PPCI
|
≤24 hours after PPCI
|
>24 hours after PPCI
|
| Ventricular fibrillation |
11 |
7 |
|
| Sustained ventricular tachycardia |
|
2 |
1 |
| Bradycardia |
8 |
|
|
| PPCI = primary percutaneous coronary intervention |
|
Table 3: Characteristics of patients with life-threatening arrhythmias who died within the first 30 days after hospital admission. |
|
Gender
|
Age (years)
|
STEMI localisation
|
IRA
|
Post-procedural
TIMI flow grade
|
Life-threatening arrhythmia
|
Time of death
|
Cause of death
|
| Male |
68 |
Anterior |
LAD |
3 |
Peri-procedural VF |
During PPCI |
Cardiogenic shock |
| Female |
70 |
Inferior |
LCX |
|
Peri-procedural asystole |
During PPCI |
PEA |
| Female |
84 |
Inferior |
LCX |
3 |
Peri-procedural AVB III |
Day 1 |
PEA |
| Male |
70 |
Anterior |
LAD |
2 |
Post-procedural VF |
Day 1 |
Cardiogenic shock |
| Female |
54 |
Inferior |
RCA |
3 |
Post-procedural VT |
Day 1 |
Hypoxic brain damage |
| Male |
45 |
Anterior |
LAD |
3 |
Post-procedural VF |
Day 1 |
Cardiogenic shock |
| Male |
55 |
Anterior |
LM |
3 |
Peri-procedural VF |
Day 2 |
Cardiogenic shock |
| Male |
83 |
Inferior |
LCX |
3 |
Peri-procedural AVB III |
Day 4 |
Cardiogenic shock |
| STEMI = ST-segment elevation myocardial infarction; IRA = infarct-related artery; TIMI = thrombolysis in myocardial infarction; LAD = left anterior descending coronary artery; LCX = left circumflex coronary artery; RCA = right coronary artery; LM = left main; VF = ventricular fibrillation; AVB III = third-degree atrioventricular block; VT = ventricular tachycardia; PPCI = primary percutaneous coronary intervention; PEA = pulse-less electrical activity. |
Discussion
The results of the present study show that in patients with STEMI who are treated with PPCI, the overall incidence of life-threatening arrhythmias during hospitalisation is considerable. The majority of life-threatening arrhythmias that occur in such patients are ventricular tachyarrhythmias, whereas significant bradycardias requiring temporary pacing are less frequent and are limited to the immediate peri-procedural period. In addition, our data indicate that virtually all life-threatening arrhythmias are observed during the first 24 hours after hospital admission, and the majority of these arrhythmias occur in the cardiac catheterisation laboratory during the PPCI procedure itself.
Before the introduction of PPCI as standard therapy for revascularisation of patients with acute STEMI, ventricular arrhythmias were observed in up to 20% of patients undergoing thrombolytic therapy and their occurrence was associated with an adverse prognosis [8]. In the first studies comparing PPCI with thrombolytic therapy in patients with acute myocardial infarction, the in-hospital incidence of sustained ventricular tachyarrhythmias (ventricular fibrillation and ventricular tachycardia) in patients assigned to PPCI was 8.7% and 7.1% [9, 10]. Compared with these early landmark studies, the in-hospital incidence of sustained ventricular tachyarrhythmias in STEMI patients undergoing PPCI was lower in our population (5.5%). These results are in line with those reported in other recent trials, including the APEX AMI trial, in which sustained ventricular tachyarrhythmias occurred in 5.7% of hospitalised patients, and two smaller single centre studies reporting an in-hospital incidence of sustained ventricular arrhythmias of 4.7% or 6.0%, respectively [11–13].
In contrast to ventricular tachyarrhythmias, data on the incidence of significant bradycardia complicating acute STEMI are sparse. In the early PPCI era, atrioventricular block was found to occur in about 6.2% of hospitalised patients with acute myocardial infarction [9]. In contrast to these early data, the results of our study showing a rather low in-hospital incidence of significant bradycardia of 2.1% match well with those of another recently published single centre study, in which complete atrioventricular block complicated the in-hospital course of 3.1% of patients [13].
Our analysis demonstrated that the majority (65.5%) of all life-threatening arrhythmias occurred during the PPCI procedure. For comparison, in the APEX AMI trial, 60.6% of all episodes of sustained ventricular tachyarrhythmias occurred before the end of cardiac catheterisation [11]. An even higher percentage of peri-procedural life-threatening arrhythmias was reported in an Italian study where 84.3% of all life-threatening arrhythmias that were observed during hospitalisation occurred in the cardiac catheterisation laboratory [13]. In summary, these data indicate that the cardiac catheterisation laboratory is not only the site of PPCI but also the place where most life-threatening arrhythmias are observed.
Since the thrombolytic era it is well-known that the risk of major complications in STEMI patients, including that of sustained ventricular arrhythmias, is largest within the first 24 hours after the event and that 95% of all complications occur within the first 3 days after the event [14]. In a recently published single centre observational study, 60% of all sustained ventricular tachyarrhythmias occurred within the first 24 hours and 92% were observed during the first 48 hours after hospital admission [12]. In our population, all except one episode of life-threatening arrhythmias occurring after PPCI were observed within the first 20 hours after the procedure. The only episode of sustained ventricular tachycardia that was observed beyond the first 24 hours after PPCI (notably on the 4th post-procedural day) was probably promoted by hypokalemia, emphasising the importance of appropriate and timely correction of electrolyte imbalances in patients suffering from STEMI. On the other hand, our observations suggest that a post-procedural electrocardiographic monitoring period of 24 hours appears to be sufficient to detect the vast majority of all life-threatening arrhythmias occurring in patients with uncomplicated STEMI undergoing PPCI.
In the absence of contraindications, the early use of betablockers may reduce the frequency of life-threatening ventricular tachyarrhythmias in patients with STEMI undergoing early reperfusion therapy [2, 3]. Given the very early occurrence of post-procedural arrhythmias in some cases or the presence of bradycardia and/or hypotension in others, only 30% of the patients with post-procedural ventricular tachyarrhythmias were treated with a betablocker at the time of the arrhythmia. Thus, it may be speculated that earlier and/or more aggressive initiation of betablocker therapy – if possible – might have decreased the rate of post-procedural arrhythmias in our population.
In the present cohort, the risk of experiencing a life-threatening arrhythmia during or after PPCI, as well as 30-day mortality were significantly higher in patients who had already been resuscitated from cardiac arrest before arrival in the cardiac catheterisation. These findings are in line with very recent data from the Prehospital Myocardial Infarction Registry (PREMIR) that showed a very high rate of adverse events including in-hospital death in patients who needed pre-hospital cardiopulmonary resuscitation before early reperfusion therapy [15].
In our population, average symptom-to-balloon times were significantly shorter in patients who experienced life-threatening arrhythmias than in those who did not. In contrast, in the APEX AMI trial symptom-to-balloon times were similar in patients with and those without sustained ventricular tachyarrhythmias during their hospital course [11]. On the other hand, however, and in line with our findings, the delay between the onset of symptoms and the patients’ arrival in the emergency room was shorter in subjects who experienced sustained ventricular tachyarrhythmias in the catheterisation laboratory than in those who remained arrhythmia-free in the PAMI trial [16]. Moreover, in a retrospective cohort study of patients undergoing PPCI for acute myocardial infarction in New York State, patients presenting within the first 6 hours after symptom onset were more likely to experience early sustained ventricular tachyarrhythmias than those who presented later [17]. In contrast to these and our findings, in a recent study, STEMI patients with a symptom-to-balloon time of less than or equal to 4 hours and a good post-procedural angiographic results were found to have a reduced risk of ventricular tachyarrhythmias in univariate analyses. However, in multivariate analyses, a short symptom-to-balloon time was not an independent factor associated with a reduction of ventricular arrhythmias [12]. In summary, given these controversial data, it remains unclear whether there is an association between symptom-to-balloon time and the risk to experience life-threatening arrhythmias during hospitalisation in patients with STEMI undergoing PPCI.
Data about the impact of life-threatening arrhythmias on clinical outcomes of STEMI patients undergoing PPCI are controversial and largely limited to ventricular tachyarrhythmias. In the PAMI trial population, the occurrence of ventricular fibrillation or ventricular tachycardia in the cardiac catheterisation laboratory during the PPCI procedure had no influence on in-hospital or 1-year clinical outcomes including mortality [16]. In contrast, in the APEX AMI trial, the occurrence of ventricular fibrillation or ventricular tachycardia either during or after PPCI was associated with an increased 90-day mortality in STEMI patients irrespective of their underlying baseline risk [11, 18]. In our study, 30-day mortality was significantly higher in patients who had experienced an episode of life-threatening arrhythmia during hospitalisation than in those who had not. This observation suggests that the occurrence of ventricular tachyarrhythmias or significant bradycardias before or after PPCI is associated with an adverse short-term clinical outcome in patients with STEMI. However, given the small number of events, these results have to be interpreted with caution.
Indeed, the rather small number of patients and events included in the analysis represents a limitation of the present study. Thus, our results must be seen as “hypothesis-generating” and cannot be generalised to all STEMI patients undergoing PPCI. Another limitation is the retrospective nature of the analysis. However, given that the focus of our analysis was put on life-threatening arrhythmias, a major bias is rather unlikely. Finally, we cannot provide any data on long-term follow-up and thus cannot comment on the possible long-term implications of the arrhythmias observed in our patients. Therefore, additional, large-scale, prospective studies with long-term follow-up are needed to confirm our findings and to assess the prognostic implications of life-threatening arrhythmias in STEMI patients undergoing PPCI.
In summary, the results of the present study suggest that life-threatening arrhythmias occur in a considerable proportion of patients with STEMI undergoing PPCI during their hospital course, and that the majority of them occur during the PPCI procedure in the cardiac catheterisation laboratory. Life-threatening arrhythmias that occur later in the hospital course are virtually limited to the first 24 hours after the PPCI procedure. Thus, our findings indicate that routine electrocardiographic monitoring beyond the first 24 hours after PPCI might not be required in the majority of patients with uncomplicated STEMI. However, further studies in larger patient cohorts are needed to define which patient subgroups could benefit from longer electrocardiographic monitoring periods before evidence-based recommendations regarding the optimal duration of electrocardiographic monitoring in STEMI patients undergoing PPCI can be made.
References
1 Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation. Eur Heart J. 2008;29:2909–45.
2 Antman EM, Hand M, Armstrong PW, Bates PW, Green LE, Halasyamani LK, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction. J Am Coll Cardiol. 2008;51:210–47.
3 Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, et al. ACC/AHA Guidelines for the management of patients with ST-elevation myocardial infarction 2004. J Am Coll Cardiol. 2004;44:E1–211.
4 Drew BJ, Califf RM, Funk M, Kaufman ES, Krucoff MW, Laks MM, et al. Practice standards for electrocardiographic monitoring in hospital settings. Circulation. 2004;110:2721–46.
5 Hasin Y, Danchin N, Filippatos GS, Heras M, Janssens U, Leor J, et al. Recommendations for the structure, organization, and operation of intensive cardiac care units. Eur Heart J. 2005;26:1676‒82.
6 Muth K, Senges J, Zeymer U. Monitoring after acute myocardial infarction. Dtsch Med Wochenschr. 2007;132:2021–3.
7 The Joint European Society of Cardiology/American College of Cardiology Committee. Myocardial infarction redefined – a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the Redefinition of Myocardial Infarction. Eur Heart J. 2000;21:1502–13 and J Am Coll Cardiol. 2000;36:959–69.
8 Newby KH, Thompson T, Stebbins A, Topol EJ, Califf RM, Natale A. Sustained ventricular arrhythmias in patients receiving thrombolytic therapy: incidence and outcomes. Circulation. 1998;98:2567–73.
9 Grines CL, Browne KF, Marco J, Rothbaum D, Stone GW, O’Keefe J, et al. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. N Engl J Med. 1993;328:673–9.
10 Zijlstra F, de Boer MJ, Hoorntje JCA, Reifers S, Reiber JHC, Suryapranata H. A comparison of immediate coronary angioplasty with intravenous streptokinase in acute myocardial infarction. N Engl J Med. 1993;328:680–4.
11 Mehta RH, Starr AZ, Lopes RD, Hochman JS, Widimsky P, Pieper KS, et al., for the APEX AMI Investigators. Incidence of an outcomes associated with ventricular tachycardia or fibrillation in patients undergoing primary percutaneous coronary intervention. JAMA. 2009;301:1779–89.
12 Ohlow MA, Geller JC, Richter S, Farah A, Müller S, Fuhrmann JT, Lauer B. Incidence and predictors of ventricular arrhythmias after ST-segment elevation myocardial infarction. Am J Emerg Med. in press, available online 11 May 2011.
13 Giglioli C, Margheri M, Valente S, Comeglio M, Lazzeri C, Chechi T, et al. Timing, setting and incidence of cardiovascular complications in patients with acute myocardial infarction submitted to primary percutaneous intervention. Can J Cardiol. 2006:22:1047–52.
14 Newby LK, Hasselblad V, Armstrong PW, Van de Werf F, Mark DB, White HD, et al. Time-based risk assessment after myocardial infarction. Implications for timing of discharge and applications to medical decision-making. Eur Heart J. 2003;24:182–9.
15 Koeth O, Nibbe L, Arntz HR, Dirks B, Ellinger K, Genzwürker H, et al., for the PREMIR Investigators. Fate of patients with prehospital resuscitation for ST-elevation myocardial infarction and a high rate of early reperfusion therapy. Am J Cardiol. 2012; in press.
16 Mehta RH, Harjai KJ, Grines L, Stone GW, Boura J, Cox D, et al., on behalf of the Primary Angioplasty in Myocardial Infarction (PAMI) Investigators. Sustained ventricular tachycardia or fibrillation in the cardiac catheterization laboratory among patients receiving primary percutaneous coronary intervention. J Am Coll Cardiol. 2004;43:1765–72.
17 Piccini JP, Berger JS, Brown DL. Early sustained ventricular arrhythmias complicating acute myocardial infarction. Am J Med. 2008;121:797–804.
18 Mehta RH, Starr AZ, Lopes RD, Piccini JP, Patel MR, Pieper KS, et al. Relationship of sustained ventricular tachyarrhythmias to outcomes in patients undergoing primary percutaneous coronary intervention with varying underlying baseline risk. Am Heart J. 2011;161:782–9.
