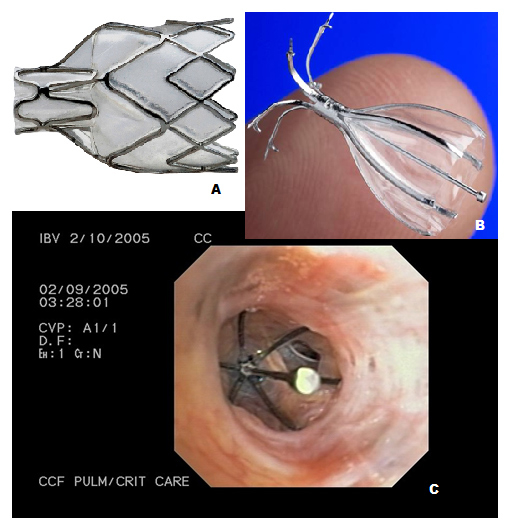
Figure 1
A– Pulmonx intrabronchial valve; B- Spiration valve; C- Bronchoscopic view of the Spiration valve in situ.
DOI: https://doi.org/10.4414/smw.2012.13591
Apart from visualisation of the nasal passages, pharynx, larynx, vocal cords, and tracheo-bronchial tree, the technical developments have led to bronchoscopy being used in complex diagnostic as well as therapeutic procedures. Although the access to the airways in the living was tried by Hippocrates (460–370 BC), it was Gustav Killian who performed the first bronchoscopic extraction of a foreign body in 1897 using a rigid bronchoscope. Instruments were improved over time but rigid bronchoscopes were the sole tools available for diagnostic and therapeutic procedures on the airway for nearly seven decades. With the advent of the flexible bronchoscope after 1966, it became the instrument of choice for nearly all diagnostic airway procedures and many interventions. However, up to this day certain therapeutic procedures can be performed in a safer manner through a rigid bronchoscope and the use of this device is preferred by many interventional pulmonologists. Interventional bronchoscopy has come a long way with exciting new techniques added to the pulmonologist's armamentarium. The term Interventional bronchoscopy encompasses both diagnostic as well as therapeutic procedures, the most important ones are listed in table 1. Though most of the emerging techniques are currently useful for a very limited patient group, their use in the near future is only likely to expand.
Foreign body removal and control of hemoptysis are two of the oldest indications for bronchoscopy. Of all other indications mentioned in table 1, relief of central airway obstruction (CAO) (i.e., trachea, mainstem bronchi, and bronchus intermedius) remains the main indication for therapeutic bronchoscopy [1]. Direct mechanical debridement, use of microdebrider, cryocanalisation, electrocautery and photodynamic therapy are the modalities used to relieve intrinsic airway obstruction caused by a tumour mass, whereas patients with extrinsic airway compression are managed by the placement of silicone stents or self-expanding metallic stents [2]. As an in depth discussion of all techniques mentioned would be way beyond the scope of one article, we have focused on four recent developments with great potential. Bronchoscopic lung volume reduction (BLVR) and bronchial thermoplasty are used in benign lung conditions, whereas, radiofrequency ablation (RFA), and fiducial markers in external beam irradiation (EBI) are useful for malignant lung conditions.
| Table 1: Interventional bronchoscopic techniques. | |
| Diagnostic techniques | Therapeutic indications |
| Bronchoalveolar lavage (BAL) Transbronchial biopsies (TBB) Transbronchial needle aspiration (TBNA) Endobronchial ultrasound (EBUS) Autofluorescence bronchoscopy (AFB) Narrow band imaging (NBI) Electromagnetic navigation (EMN) Optical coherence tomography (OCT) Fibered confocal fluorescence microscopy (FCFM) Alveoloscopy | Airway cleaning (secretions, blood, aspiration) Foreign body removal Treatment of inoperable central airway stenoses Brachytherapy Treatment of early lung cancer Radiofrequency ablation Bronchoscopic lung volume reduction (BLVR) Bronchial thermoplasty Fiducial markers in EBI |
Lung volume reduction surgery (LVRS) is a palliative procedure for selected patients with severe emphysema whose respiratory mechanics are severely impaired owing to hyperinflation of the lungs and thorax. Lung volume reduction therapy reduces gas trapping, improves elastic recoil, increases expiratory flows, and allows the rib cage and diaphragm to function more effectively. Sanchez et al. used the follow-up data of the National Emphysema Treatment Trial (NETT) to determine the long-term outcome of LVRS and stated that dyspnea and health-related quality of life as measured by the St George Respiratory Questionnaire (SGRQ) both showed significant benefit for LVRS [3]. However, LVRS was associated with considerable morbidity and mortality (about 10% in the subgroup of patients with homogenous emphysema). Also, surgery offered no apparent long-term survival benefit, and resulted in less objective and subjective benefit in the latter group [3]. Hence there is a need for safer and better techniques to achieve lung volume reduction. A step in that direction is development of minimally invasive endoscopic techniques which involve either closing or bypassing the airways to achieve lung volume reduction (table 2). The basic principle involves implanting devices endobronchially to achieve segmental or lobar collapse. An ideal endobronchial device should be effective in achieving and maintaining volume reduction comparable to LVRS with reproducible results. The device should be easily deployable with standard bronchoscopes, and it should not migrate while remaining easily removable. Lastly, the device should not interfere with future surgical interventions such as conventional LVRS or lung transplantation [4].

Figure 1
A– Pulmonx intrabronchial valve; B- Spiration valve; C- Bronchoscopic view of the Spiration valve in situ.
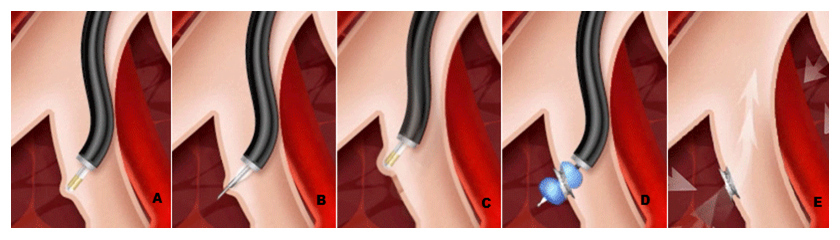
Figure 2
Schematic representations of airway bypass using EXHALE airway stents.
From left to right: A- identification of a blood vessel– free location with a Doppler probe at the level of segmental bronchi; B- fenestration of the bronchial wall using the transbronchial needle; C- confirmation with Doppler; D- using dilating balloon through the fenestration; E- placement of a stent to hold the passage open.

Figure 3
A- Alair Catheter; B- Alair RF Controller; C- Schematic representations of using the catheter.
Intrabronchial valves (IBV) allow secretions and air out of an occluded pulmonary segment during expiration but prevent any distal flow during inspiration [5] leading to atelectasis of the isolated emphysematous segments with subsequent reduction in lung volume. The valves currently studied are Pulmonx® valve, Spiration® valve and newer Miyazawa® valve.
The Pulmonx® implant (fig. 1A) is a silicone-based, one-way valve mounted on a nitinol stent. Toma et al. [6] in their first pilot study of unilateral volume reduction by intrabronchial valve insertion, reported an increase in forced expiratory volume in 1 sec (FEV1) by 34% and an increase in median diffusing capacity for carbon monoxide (DLCO) by 29% at 4 weeks. Though upper lobe collapse was noted in only half of the patients, blocking air entry into the most emphysematous lobes achieved a small but consistent benefit, especially in the DLCO. In a first multicentre experience of using IBVs, Wan et al. [5] reported statistically significant improvement in FEV1 (10.7 ± 26.2%), forced vital capacity (FVC) (9.0 ± 23.9%), RV (–4.9 ± 17.4%), and exercise tolerance i.e. 6-minute walk test (6MWT) (23.0 ± 55.3%) in their 98 patients. They used the IBV system which consists of an implantable one-way, silicone, duckbill valve, a delivery catheter, a loader system, and a guidewire. They noted serious complications of pneumothorax (3.1%), death (1%), and COPD exacerbations (17.3%).
Recently, Sciurba and colleagues [7] compared the safety and efficacy of IBV therapy in 220 patients with heterogeneous emphysema versus standard medical care. At 6 months, there was an increase of 4.3% in FEV1 in the IBV group, as compared with a decrease of 2.5% in the control group with a mean between-group difference of 6.8% in FEV1 (P = 0.005). Roughly similar between-group differences were observed for the 6-minute walk test (6MWT). However, this came at a cost of increased rates of hospitalisations due to COPD exacerbations (7.9% vs. 1.1%, P = 0.03) and hemoptysis (6.1% vs. 0%, P = 0.01) in the IBV group as compared with the control group at 90 days. The rate of pneumonia in the target lobe in the IBV group was 4.2% at 12 months. Although these improvements in the entire IBV group were statistically significant, the clinical relevance is questionable. A more promising result was achieved by subanalysis of those patients with high-heterogeneity and complete lobar fissures (preventing collateral ventilation), who showed greater improvements in both FEV1 and distance on the 6MWT compared with patients with lower heterogeneity and incomplete fissures as noted by quantitative HRCT.
| Table 2: Techniques and devices for BLVR. | |
| Closing airways | Bypassing airways |
| Plugs or blockers Spigots (Novatec) Sealants and biologics AeriSeal (Aeris Therap.) Vapour (UptakeMedical) Valves - IBV (Spiration) - Emphasys/zephyr (Pulmonx) | Internal Exhale drug eluting stent (Broncus) |
This intrabronchial valve (IBV) is an implantable device made of a nitinol framework (fig. 1B and C). The valve has 5 distal anchors and 6 support membrane covered struts in the proximal portion, which expand radially and form an umbrella shape that allows conformation and sealing with the airways with minimal pressure on the bronchial mucosa [8]. Wood and colleagues reported their multicentre experience of the device in 30 patients. A significant improvement in health-related quality of life was demonstrated, the mean change in SGRQ at 6 months was –6.8 ± 14.3 points. Common complications reported were COPD flares, dyspnea, pain, hemoptysis and pneumonia (1–6%) [8]. Recently Springmeyer et al. [9] in their multicentre single-arm, open-label study with the IBV valve and a multi-lobar treatment approach, showed that 56% of subjects had a clinically relevant improvement in health-related quality of life. Quantitative CT analyses of regional lung changes showed lobar volume changes in over 85% of subjects. The authors postulated that a redirection of inspired air resulting in an interlobar shift to healthier lung tissue, was the most common mechanism for a valve treatment response.
Lung volume reduction coils are made from nitinol wire that has been preformed to a shape which results in parenchymal compression after deployment. These coils are implanted bronchoscopically via a catheter and placed over a guidewire under fluoroscopic guidance. Herth at al [10] in a pilot study treated 11 patients with the coils and patients with predominantly heterogeneous disease appeared to show substantial improvements in pulmonary function, lung volumes, 6MWT and quality of life measures. Further trials are ongoing.
Emphysematous lung sealant (ELS) (AeriSeal®) therapy is a novel technique being evaluated for treatment of patients with advanced emphysema. It appears to function by blocking small airways and collateral ventilation pathways, which leads to absorption atelectasis. Herth et al [11], in a pilot study administered ELS therapy bronchoscopically to 25 patients with heterogeneous emphysema in an open-label, noncontrolled study. Therapy was administered with the bronchoscope in a wedge position and ELS foam sealant was delivered through a single lumen catheter. The foam sealant was prepared at the bedside from aqueous polymer solution and cross-linker. Patients received ELS therapy at up to 6 target sites during either a single treatment session or 2 treatment sessions. No treatment-related deaths were reported and the most common serious complication was COPD exacerbation. Acute side effects consisted of an inflammatory mild 'flu-like' reaction that was observed in all patients. Improvements from baseline for the physiological measures at 24 weeks were: FEV1 = +10.0 ± 19.8%, FVC = +15.86 ± 22.2%, RV/TLC = –4.7 ± 9.5%, SGRQ total domain score = –7.5 ± 14.4 units, and 6MWT = + 24.6 ± 58.9 m. Only the improvement in FVC was statistically significant when corrected for multiple comparisons. Physiological improvements following ELS therapy were greater in patients with GOLD stage III disease, than stage IV disease. ELS therapy, in contrast to intrabronchial valves, is not reversible. Whether this is a limiting factor can only be determined by additional studies.
Bronchoscopic introduction of heated water vapor (steam) into segmental airways achieves BLVR by inducing an inflammatory response. This avoids the implantation of foreign-body prostheses, and is independent of the presence of interlobar collateral ventilation. Snell et al. [12] applied this novel technique to 11 patients with severe heterogeneous emphysema who underwent unilateral bronchoscopic application of vapor thermal energy and noted all adverse events during 6 months follow-up (primary end point). They used a nonreusable 2-mm vapor catheter inserted via a flexible bronchoscope through which the desired dose for steam was applied after inflation of an occlusion balloon. Overall, compared with the baseline measurements, improvement was noted only in DLCO (16%). The extent of the improvement in DLCO correlated with the improvement in SGRQ (r = 0.43). The commonest side effects included nausea, cough, mild to moderate hemoptysis, and transient fatigue. Exacerbations of COPD were reported in 4 patients. This study showed that the technique can be used safely and further studies are required to determine its efficacy and safety over long term.
Most of the discussed modalities of BLVR i.e. valves, BTVA, sealants and biologics have been used in heterogeneous emphysema. For the homogenous variant the method of 'airway bypass' is currently studied. There is irreversible destruction of lung parenchyma in emphysema with marked enhancement of collateral ventilation. Airway bypass is the process of creating extra-anatomic passages between the collaterally ventilated pulmonary parenchyma and larger airways, allowing trapped gas to exit from the lung. This decrease in gas trapping reduces hyperinflation, allowing better chest wall and diaphragmatic excursion and therefore improving dyspnea. Cardoso et al. [13] evaluated airway bypass with paclitaxel-eluting stents in 35 patients with severe emphysema. Airway bypass was performed in a single session with a flexible bronchoscope and a minimum of 2 stents in each upper and lower lobe bilaterally. Creation of each stented passage required 3 steps as described in figure 2. A statistically significant reduction in hyperinflation, as reflected by RV, was observed throughout the 6-month follow up without a significant change in FEV1. The major intraoperative risks involved in airway bypass include pneumothorax and airway hemorrhage. One death due to major intraprocedural bleeding occurred but there were no instances of pneumothorax. Recently published data from EASE trial [14], however failed to show sustainable benefit at 6 months with airway bypass. The study recruited 315 patients with severe emphysema, in a randomised, double-blind, sham-controlled, multicentre study with 6-month co-primary efficacy endpoint of 12% or greater improvement in FVC and 1 point or greater decrease in the modified Medical Research Council dyspnoea score from baseline. As with other BLVR techniques more studies are needed to determine the efficacy of airway bypass technique.
Bronchial thermoplasty (BT) is a novel technique (Alair System; Asthmatx; Mountain View, CA) which uses heat generated by radiofrequency energy delivered through the bronchoscope to block bronchial smooth muscle tone [15]. The Alair device got the Food and Drug Administration (FDA) [16] approval for its use in severe refractory asthma in early 2010. In chronic asthma [17], the airway smooth muscles (ASM) undergo hypertrophy as a part of airway remodeling, which contributes to the poor control of symptoms. Bronchial thermoplasty aims at reduction in this muscle mass.
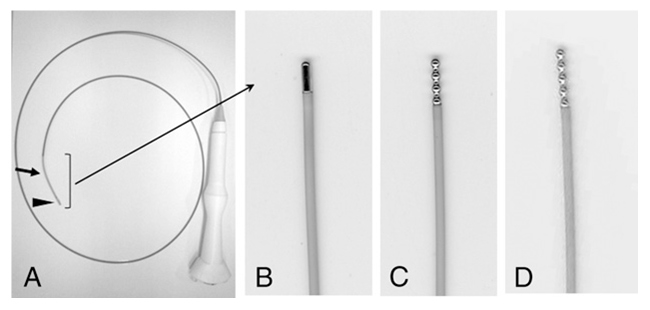
Figure 4
A- Internally cooled radiofrequency ablation (RFA) electrodes; B- 5-mm cylindrical active tip; C- 8-mm active tip with four beads; and D-10-mm active tip with five beads. © Chest, 2010. Reprinted with permission from Tanabe T, Koizumi T, Tsushima K, Ito M, Kanda S, Kobayashi T, et al. Comparative study of three different catheters for CT-bronchoscopy-guided radiofrequency ablation as a potential and novel intervention therapy for lung cancer. Chest. 2010;137(4):890–7 [34].
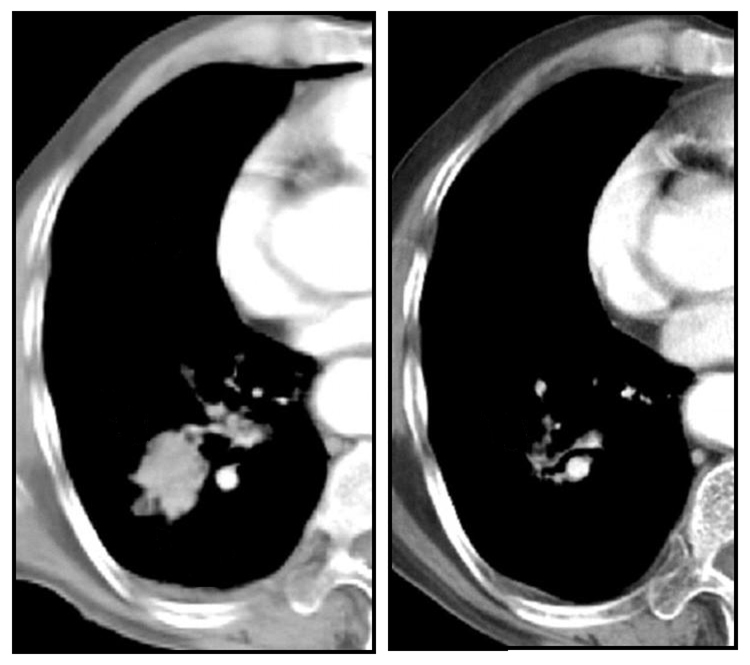
Figure 5
Chest CT of a patient treated with RFA- before and after treatment.
Bronchial thermoplasty is performed using the Alair bronchial thermoplasty system [18] comprising the Alair RF controller (which generates controlled RF energy), and the Alair catheter (with an expandable electrode array attached at one end) (fig. 3). The entire treatment is performed during 3 bronchoscopy sessions, each separated by about 3 weeks. During each session, treatment is delivered from the distal to the proximal airway. The catheter is introduced through the working channel of the bronchoscope and the energy is delivered when 4 electrode wires firmly contact the airway wall. After each activation, the electrode array is repositioned proximally about 5 mm and so on [18].
The earliest evidence of the technique came from Danek et al. [19] who in their animal study demonstrated a reduction in airway responsiveness to methacholine for at least 3 years, by using a temperature-controlled application of radio frequency energy to the airways. They noted a reduction in amount of functional ASM and replacement of smooth muscles by loose connective tissue at the treatment site. All other tissue elements remained intact or returned to normal. Miller et al. [20] carried out the first feasibility studies in humans on patients undergoing lung resection for a neoplasm. They subjected the patients to the procedure 1–3 weeks prior to their scheduled lung surgery and repeated bronchoscopy immediately prior to surgery. The observed changes following thermal injury were airway narrowing, increased mucus, and erythema, but these were milder compared to those from preclinical canine studies. Histologically, there was a reduction in the ASM in sites treated at 65 °C, with variable changes in epithelium, mucus glands and cartilages. The AIR trial group conducted the first randomised, controlled trial [21] to test the efficacy of bronchial thermoplasty in achieving asthma control when long-acting beta agonists (LABA) were discontinued in 112 adult patients with moderate or severe persistent asthma. The patients were randomised into treatment with inhaled corticosteroids (ICS) plus LABA (control group) or to treatment with bronchial thermoplasty (BT) in addition to ICS and LABA (bronchial-thermoplasty group). There was a reduction in the number of mild exacerbations in the BT group (amounting to 10 fewer mild exacerbations per subject per year) at 3 months and at 12 months. Of the secondary outcomes measured, there was improvement in morning peak expiratory flow rate, symptom score, number of symptom free days, Asthma Quality of Life Questionnaire (AQLQ), Asthma Control Questionnaire, and reduction in rescue medication use at 3 and 12 months in the BT treatment group. The BT treatment group had more adverse respiratory events than the control group (407 vs. 106 respectively). The adverse events commonly observed were dyspnea, wheezing, cough, chest discomfort, night awakenings, productive cough and upper respiratory tract infection. In the BT group the majority of the adverse events occurred within 1 day after the procedure and resolved an average of 7 days after the onset of the event [21]. During the long term (5 year) follow up of these patients [22], they noted that more subjects in the BT group required hospitalisations for respiratory symptoms than the control group during year 1 and year 2, but these differences were not statistically significant. The rate of occurrence of respiratory related adverse events remained low and stable between year 2 and year 5 (1.1 to 1.3 events/subject/year) in the BT group and comparable to the control group for year 2 and year 3 (1.2 and 1.3 events/ subject/year, respectively). The rates of emergency room (ER) visits and the proportion of subjects with ER visits in the control group were comparable to the BT group during years 1, 2, and 3. The total lung capacity, residual volumes and the mean post-bronchodilator FEV1 and FVC values remained stable and showed no deterioration over the 5 year period post-BT. There was a statistically significant improvement over baseline in the methacholine PC20 doubling in the BT group compared to the control group in year 2 and year 3 [22]. The AIR group study was limited by the fact that it was non-blinded and a placebo effect is observed in asthma [21]. The follow up study does provide the long term safety data of BT but there may have been underreporting of the adverse events as a result of recall bias [22]. The RISA trial [23] included 34 patients with severe asthma requiring high dose ICS (>750 µg fluticasone propionate per day or equivalent) and LABA (at least 100 µg salmeterol per day or equivalent). Subjects treated with BT had clinically and statistically significant improvements over baseline in rescue medication use, prebronchodilator FEV1 predicted, and AQLQ and ACQ scores. The net improvement in FEV1 of 16% was larger than that seen in the AIR trial. However, these benefits came with an increased short-term risk of procedure-related adverse events similar to that observed in the AIR trial [21]. The AIR2 trial was a large randomised, double-blind, sham-controlled, clinical trial which used a patient-centered subjective endpoint i.e. the Asthma Quality of Life Questionnaire (AQLQ), to assess the added benefit of BT beyond the current standard of care. There were improvements in quality of life and asthma control symptoms; the mean change in AQLQ was 1.35 from baseline and 79% of patients had a clinically meaningful improvement in the AQLQ score of 0.5 or greater. Also there was a 32% reduction in the rate of severe exacerbations and an 84% risk reduction in ER visits for respiratory symptoms in the BT group compared with sham group. The proportion of subjects experiencing severe exacerbations in year 2 after BT was 23.0%, compared with 30.9% in year 1 [24]. There was a reduction of 66% in days lost from work, school and other activities due to asthma. Similar to previous studies [21, 23], subjects in the BT group reported more respiratory adverse events (85% of subjects; 1.0 events/bronchoscopy) than in the sham group (76% of subjects; 0.7 events/bronchoscopy) during the treatment period. However, during the posttreatment period, fewer adverse respiratory events were reported in the BT group (70% of subjects vs. 80% in the sham group).
The RISA and AIR2 trial provide evidence regarding the utility of bronchial thermoplasty as add on therapy for patients with severe refractory asthma, with a reasonably acceptable adverse event profile. However, since the studies have been done in selective patients with positive outcome in only some aspects, the use of this treatment modality should be limited to cases with difficult to control asthma. The current British Thoracic Society (BTS) guideline [25] indicates bronchial thermoplasty as a possible treatment option in selected patients with severe persistent asthma already on maximal therapy, though its place in the routine treatment of asthma remains to be established.
Radiofrequency ablation (RFA) uses an electromagnetic wave with the same frequency band as an electric scalpel used in surgery and a radiofrequency interchange electric current. RFA is a minimally invasive treatment modality and has been used to treat patients with hepatic, renal, and breast cancers [26–28]. The insertion of a radiofrequency electrode into a defined tumour bed and the establishment of an electric field to a reference electrode that oscillates with generated alternating radiofrequency currents ultimately create a conduit for frictional heating. Tissue heating consequently induces coagulative necrosis and cell death, including destruction of centrally located hypoxic tumor that is typically less responsive to chemotherapy and radiation therapy [29]. Dupuy et al. [30] demonstrated successful application of RFA in three patients with lung malignancies via image guided percutaneous approach. Subsequent studies demonstrated usefulness of RFA in localised tumours in patients who are inoperable due to medical reasons. The percutaneous approach, however, has complications [31, 32] similar to those that occur after percutaneous needle biopsy, i.e pneumothorax, pleural effusion, hemorrhage etc. Tsushima and colleagues [33] were the first to demonstrate fibreoptic bronchoscopy guided cooled-RFA as a potential therapeutic tool in their study on sheep lung. The standard non cooled electrode has the disadvantage that the temperature around the tip rises rapidly and the 'pop phenomenon' occurs, causing coagulated necrotic tissue to be formed around the electrode tip, increasing the tissue impedance. The authors developed a new internally cooled electrode (with a 4-mm active tip, diameter: 1.67 mm) suitable for bronchoscopes with a 2.2 mm working channel which was cooled by circulating water in the electrode catheter.
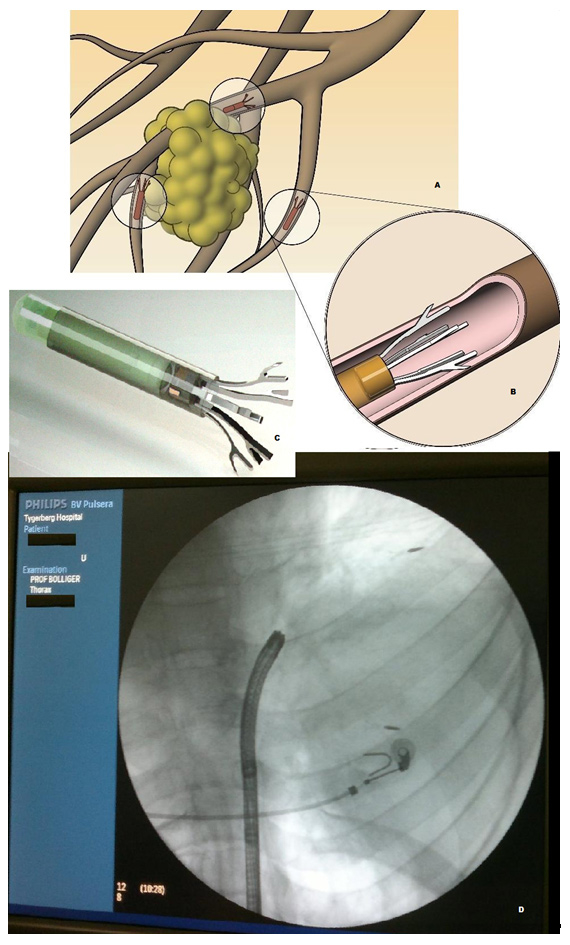
Figure 6
A- Schematic representation of Anchored Beacon® Transponders in situ around the tumor; note: each transponder is deployed in a separate airway; B- Magnified view of the distal end of a transponder showing anchorage in a bronchus; C- The actual Anchored Transponder, approximately the size of a grain of rice; D- Fluoroscopic view of the anchored transponders in situ. In a large right sided lung cancer two out of three transponders have been deployed, one just distal to the ECG electrode, the second one at the distal tumour adjacent to normally aerated lung.
Using this, the tissue temperature just around the electrode tip did not reach excessive levels and the cooled-RFA could reach deeper and wider areas of ablation using the same power output. Coagulation necrosis was achieved over an area of 20 mm with this RFA catheter.
Recently [34], the authors explored its use as a potential therapeutic tool for local tumour control in 10 patients with medically inoperable stage I non small cell lung cancer (NSCLC). They used three types of electrode catheter for RFA (fig. 4): an internal cooled electrode catheter with a 5-mm cylindrical active tip, 8-mm active tip with 4 beads and a 10-mm active tip with five beads (all had a diameter of 1.67 mm).
The probes were passed through the working channel of a video-bronchoscope and attached to a monopolar radiofrequency generator with a power output of 20 W. Low-dose CT imaging guidance was used to determine the location of the electrode catheter tip in the lung tumour. The RFA applications were repeated 3 times in each patient. Improvement of the RFA effect was achieved according to the catheter tip used and prolongation of ablation time. The extent of the maximal ablated area (showing destruction of the alveolar space and coagulation necrosis on histology) obtained was 12 x 10 mm, when using a 10-mm catheter tip with 5 beads. Apart from chest pain in 2 patients, no major complication was noted.
The advantages of bronchoscopic RFA are the non-surgical treatment as well as shorter hospital stay. Even though the size of destroyed tissue is small, CT-bronchoscopic guided RFA has the potency as a therapeutic tool, especially for local control in medically inoperable patients. Surgery ± chemo-radiotherapy is the treatment of choice for stage I and II NSCLC, offering the best chance of cure [35]. For medically inoperable patients, however, stereotactic radiotherapy [36] and percutaneous RFA [31, 32, 37] have been shown to be effective (fig. 5). Whether bronchoscopic RFA can serve as an alternative tool, needs further exploration.
Three dimensional conformal radiotherapy (3D-CRT) is a method of focusing radiation on the tumour, thereby increasing the dose of radiation delivered to the tumour and minimising the volume of irradiated normal lung [38]. However, a major hurdle in effective lung radiation treatment is lung tumour motion during respiration. Current motion management approaches frequently rely on external surrogates and are inaccurate, cumbersome and inefficient. Harada et al. [39] demonstrated feasibility of bronchoscopic implantation of a gold marker into or near peripheral-type lung tumour for real-time tumour-tracking radiation therapy (RTRT). But their accuracy and reliability is limited by low signal-to-noise automatic image recognition algorithms and by non-therapeutic patient radiation from fluoroscopy. An exciting new technique, developed by Calypso Medical Technologies, utilises miniature implanted Beacon® transponders to provide precise, continuous information on the location of the tumour in real time during external beam radiation therapy. The Calypso System, is cleared by the FDA for use in the prostate and post-operative prostatic bed and can potentially be used for real time tumour tracking and localisation in the lungs. Mayse et al. [40] evaluated the feasibility and fixation of electromagnetic transponders bronchoscopically implanted in small airways of canine lungs and compared to results using gold markers. The electromagnetic transponders they used were cylindrical in shape (without anchors) measuring 1.8 mmin diameter and 8.5 mm in length. On excitation by an external source these transponders emit an electromagnetic signal which can then be detected and localised by an electromagnetic array (Calypso 4D Localisation System, Calypso Medical Technologies, Inc.). This localisation information can be used to track the transponder's location in three-dimensional space both before and during radiation therapy beam application. These transponders are bronchoscopically inserted by the use of a polytetra-fluoroethylene (PTFE) implantation catheter.
Mayse et al. [40] demonstrated that bronchoscopic implantation of the electromagnetic transponders into the small peripheral airways was feasible but was limited by the fact that a substantial portion of the successfully implanted transponders and markers dropped out of the lung over the next 60 days. Currently a first in man multicentre study which was started in Cape Town, South Africa and includes among other sites, the Division of Pneumology, University Hospital Basel, Switzerland, is evaluating the use of anchored transponders in lung cancer patients receiving radiation therapy. Bolliger et al. [41] presented early results on clinical experience and safety of implanted anchored Beacon transponders in lung cancer patients. Three anchored transponders were successfully implanted in each patient after identifying suitable implantation sites in small airways in proximity to the lung primary on CT chest. No patient had developed pneumonia, pneumothorax or adverse pulmonary symptoms as a result of implantation of the anchored transponders. All the anchored transponders were subsequently used for tumour localisation and tracking by the Calypso System. Follow-up imaging revealed good positional stability and no migration of the anchored transponders. Further developmental work is under way. Whether these fiducial markers revolutionise the treatment outcomes in patients receiving radiotherapy will be determined only by further studies.
All four techniques described in this article are still in their early phases. Even for BLVR and BT, which have been around for some time, there are hardly any data outside trials; for the practicing pulmonologist both procedures should thus be reserved for patients who have severe COPD and asthma respectively and who are not happy with their quality of life despite optimal pharmaceutical support. Radiofrequency ablation, on the other hand, can certainly be recommended to cancer patients who are deemed inoperable due to insufficient cardio-pulmonary reserves for lung resection. The placing of fiducial markers for more accurate external beam irradiation of lung cancer patients is in its infancy, as no long-term data of the first-in-man study currently taking place have been published.
Finally, a clear recommendation for all these techniques is that they should only be used in very specialised centres with the necessary infrastructure and skills.
1 Ernst A, Feller-Kopman D, Becker HD, Mehta AC. Central airway obstruction. Am J Respir Crit Care Med. 2004;169:1278–97.
2 Gorden J, Ernst A. Endoscopic management of central airway obstruction. Semin Thorac Cardiovasc Surg. 2009;21:263–73.
3 Sanchez PG, Kucharczuk JC, Stacey S, Kaiser LR, Cooper JD. National emphysema treatment trial redux: Accentuating the positive. J Thorac Cardiovasc Surg. 2010;140:564–72.
4 Yim APC, Hwong TM, Lee TW, Li WWL, Lam S, Yeung TK, et al. Early results of endoscopic lung volume reduction for emphysema. J Thorac Cardiovasc Surg. 2004;127:1564–73.
5 Wan IY, Toma TP, Geddes DM, Snell G, Williams T, Venuta F, et al. Bronchoscopic lung volume reduction for endstage emphysema: report on the first 98 patients. Chest. 2006;129:518–26.
6 Toma TP, Hopkinson NS, Hillier J, Hansell DM, Morgan C, Goldstraw PG, et al. Bronchoscopic volume reduction with valve implants in patients with severe emphysema. Lancet. 2003;361:931–3.
7 Sciurba FC, Ernst A, Herth FJF, Strange C, Criner GJ, Marquette CH, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010;363:1233–44.
8 Wood DE, McKenna RJ Jr, Yusen RD, Sterman DH, Ost DE, Springmeyer SC, et al. A multicenter trial of an intrabronchial valve for treatment of severe emphysema. J Thorac Cardiovasc Surg. 2007;133:65–73.
9 Springmeyer SC, Bolliger CT, Waddell TK, et al. IBV Valve Pilot Trials Research Teams: Treatment of heterogeneous emphysema using the spiration IBV valves. Thorac Surg Clin. 2009;19:247–53.
10 Herth FJF, Eberhardt R, Ernst A. Pilot study of an improved lung volume reduction coil for the treatment of emphysema. Am J Respir Crit Care Med. 2009;179:A6160.
11 Herth FJF, Gompelmann D, Stanzel F, Bonnet R, Behr J, Schmidt B, et al. Treatment of advanced emphysema with emphysematous lung sealant (AeriSeal). Respiration. 2011;82:36–45.
12 Snell GI, Hopkins P, Westall G, Holsworth L, Carle A, Williams TJ. A feasibility and safety study of bronchoscopic thermal vapor ablation: a novel emphysema therapy. Ann Thorac Surg. 2009;88(6):1993–8.
13 Cardoso PF, Snell GI, Hopkins P, Sybrecht GW, Stamatis G, Ng AW, et al. Clinical application of airway bypass with paclitaxel-eluting stents: early results. J Thorac Cardiovasc Surg. 2007;134:974–81.
14 Shah PL, Slebos DJ, Cardoso PFG, Cetti E, Voelker K, Levine B, et al. on behalf of the EASE trial study group. Bronchoscopic lung-volume reduction with Exhale airway stents for emphysema (EASE trial): randomised, sham-controlled, multicentre trial. Lancet. 2011;378:997–1005.
15 Cox PG, Miller J, Mitzner W, Leff AR. Radiofrequency ablation of airway smooth muscle for sustained treatment of asthma: preliminary investigations. Eur Respir J. 2004;24:659–63.
16 US Food and Drug Administration (FDA). Approval of Alair bronchial thermoplasty system: Alair catheter and Alair RF controller. 2010. http://www.accessdata.fda.gov/cdrh_docs/pdf8/P080032a.pdf . Accessed November 29, 2011.
17 Carroll N, Elliot J, Morton A, James A. The structure of large and small airways in nonfatal and fatal asthma. Am Rev of Respir Dis. 1993;147:405–10.
18 Mayse ML, Laviolette M, Rubin AS, et al. Clinical pearls for bronchial thermoplasty. J Bronchol. 2007;14:115–23.
19 Danek CJ, Lombard CM, Dungworth DL, et al. Reduction in airway hyperresponsiveness to methacholine by the application of RF energy in dogs. J Appl Physiol. 2004;97:1946–53.
20 Miller JD, Cox G, Vincic L, Lombard CM, Loomas BE, Danek CJ. A prospective feasibility study of bronchial thermoplasty in the human airway. Chest. 2005;127:1999–2006.
21 Cox G, Thomson NC, Rubin AS, Niven RM, Corris PA, Siersted HC, et al, AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356:1327–37.
22 Thomson NC, Rubin AS, Niven RM, et al. The AIR Trial Study Group. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulmonary Medicine. 2011;11:8.
23 Pavord ID, Cox G, Thomson NC, Corris PA, Siersted HC, Olivenstein R, et al; RISA Trial Study Group. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176:1185–91.
24 Castro M, Rubin A, Laviolette M, Hanania NA, Armstrong B, Cox G; AIR2 Trial Study Group. Persistence of effectiveness of bronchial thermoplasty in patients with severe asthma. Ann Allergy Asthma Immunol. 2011;107:65–70.
25 Rand IAD, Barber PV, Goldring J, et al. on behalf of the British Thoracic Society Interventional Bronchoscopy Guideline Group. British Thoracic Society guideline for advanced diagnostic and therapeutic flexible bronchoscopy in adults. Thorax. 2011;66:iii1eiii21.
26 Wood BJ, Ramkaransingh JR, Fojo T, Walther M, Libutti SK. Percutaneous tumor ablation with radiofrequency. Cancer. 2002;2:443–51.
27 Gervais DA, McGovern FJ, Wood BJ, Goldberg SN, McDougal WS, Mueller PR. Renal cell carcinoma: clinical experience and technical success with radiofrequency ablation of 42 tumors. Radiology. 2003;226:417–24.
28 Mirza AN, Fornage BD, Sneige N, et al. Radiofrequency ablation of solid tumors. Cancer J. 2001;7:95–102.
29 Nahum Goldberg S, Dupuy DE. Image-guided radiofrequency tumor ablation: challenges and opportunities. I. J Vasc Interv Radiol. 2001;12:1021–32.
30 Dupuy DE, Zagoria RJ, Akerley W, Mayo-Smith WW, Kavanagh PV, Safran H. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol. 2000;174:57–59.
31 Yasui K, Kanazawa S, Sano Y, Fujiwara T, Kagawa S, Mimura H, et al. Thoracic tumors treated with CT-guided radiofrequency ablation: initial experience. Radiology. 2004;231:850–7.
32 Simon CJ, Dupuy DE, DiPetrillo TA, Safran HP, Grieco CA, Ng T, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243:268–75.
33 Tsushima K, Koizumi T, Tanabe T, Nakagawa R, Yoshikawa S, Yasuo M, et al. Bronchoscopy guided radiofrequency ablation as a potential novel therapeutic tool. Eur Respir J. 2007;29(6):1193–200.
34 Tanabe T, Koizumi T, Tsushima K, Ito M, Kanda S, Kobayashi T, et al. Comparative study of three different catheters for CT-bronchoscopy-guided radiofrequency ablation as a potential and novel intervention therapy for lung cancer. Chest. 2010;137(4):890–7.
35 Ginsberg RJ , Rubinstein LV; Lung Cancer Study Group. Randomized trial of lobectomy versus limited resection for T 1 N 0 non-small cell lung cancer. Ann Thorac Surg. 1995;60(3):615–22, discussion 622–3.
36 Xia T, Li H, Sun Q, Wang Y, Fan N, Yu Y, et al. Promising clinical outcome of stereotactic body radiation therapy for patients with inoperable Stage I/II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;66(1):117–25.
37 Liu B, Liu L, Li Y, Hu M, Qian K, Wang R, et al. Survival after radiofrequency ablation for 100 cases of lung neoplasms. Zhongguo Fei Ai Za Zhi. 2011;14(4):335–9. [Article in Chinese]
38 Bradley J, Graham MV, Winter K, Purdy JA, Komaki R, Roa WH, et al. Toxicity and outcome results of RTOG 9311: A phase I-II dose escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:318–28.
39 Harada T, Shirato H, Ogura S, Oizumi S, Yamazaki K, Shimizu S, et al. Real-time tumor-tracking radiation therapy for lung carcinoma by the aid of insertion of a gold marker using bronchofibroscopy. Cancer. 2002;95:1720–7.
40 Mayse ML, Parikh PJ, Lechleiter KM, Dimmer S, Park M, Chaudhari A, et al. Bronchoscopic implantation of a novel wireless electromagnetic transponder in the canine lung: A feasibility study. Int. J. Radiation Oncology Biol Phys. 2008;72:93–8.
41 Bolliger CT, Koegelenberg CFN, Von Groote-Bidlingmaier F, et al. First report of implantation of anchored electromagnetic fiducials in human lung cancers for real-time tumor localization and tracking during radiation therapy. American Society For Radiation Oncology (ASTRO). 2011; October 2–6.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.