
Figure 1
A. Overview of the IVD. Schematic view of nucleus pulposus and annulus fibrosus. B. Transverse view onto fresh-cut caudal bovine intervertebral disc (animal aged ~1 year). The two tissue types can be clearly distinguished visually.
DOI: https://doi.org/10.4414/smw.2012.13598
The healthy spine is the “backbone” of our society. Healthy discs function as essential load-absorbers between all vertebrae, allowing for bending, flexion and torsion of the spine. As the global population ages, the incidence of intervertebral disc (IVD) degeneration and low back pain (LBP) increases. The occurrence of LBP has been associated in many cases with degenerative disc disease. Given that disc degeneration is a cell-mediated response to progressive structural failure, new treatments are required that normalise disc cell homeostasis and restore full disc function [1]. Here, we review recent studies that aim to regenerate the IVD with biomaterials, molecules and/or cells.

Figure 1
A. Overview of the IVD. Schematic view of nucleus pulposus and annulus fibrosus. B. Transverse view onto fresh-cut caudal bovine intervertebral disc (animal aged ~1 year). The two tissue types can be clearly distinguished visually.
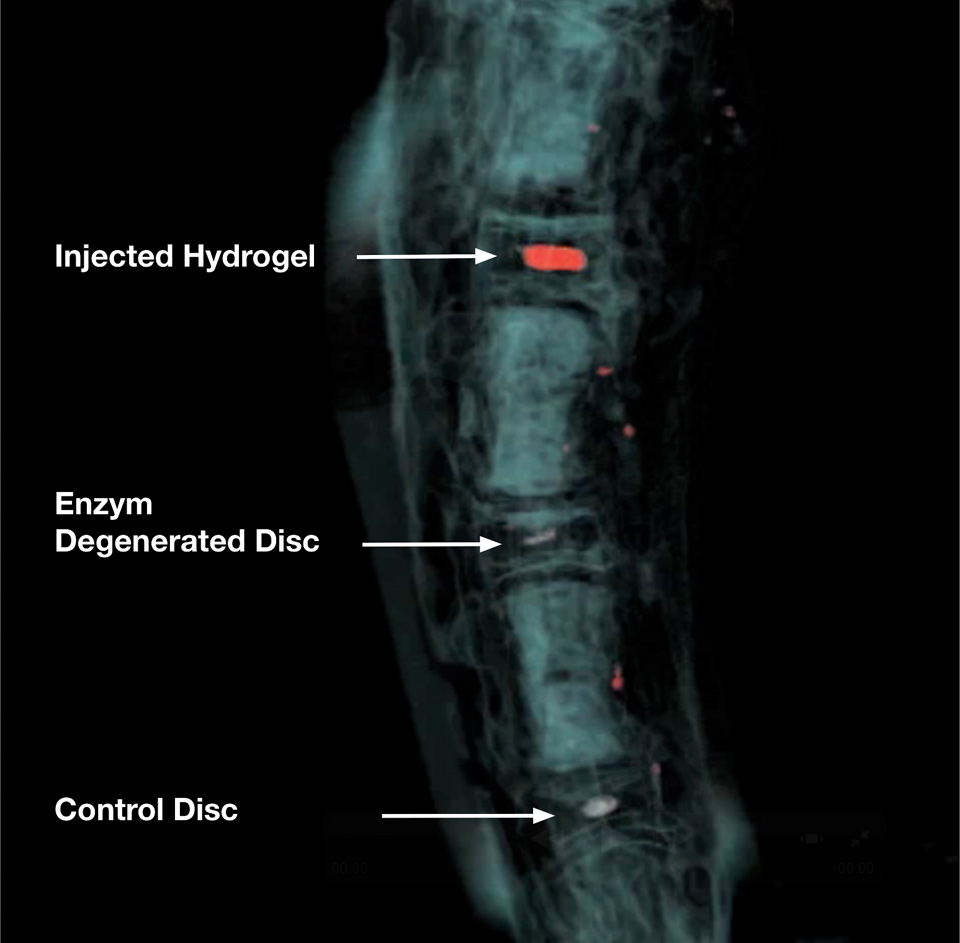
Figure 2
3D reconstruction of Magnetic Resonance Image (MRI) of a bovine tail intervertebral disc animal model. The upper two discs were treated with a certain dose of papain for 7 days to initiate disc degeneration. The papain enzyme was blocked with ebselen after 7 days. The NP region of the uppermost disc was then filled with a thermo-reversible p-NIPAM hydrogel (~200 µL) and imaged after ~24h on a 3 Tesla scanner (Siemens).
The intervertebral discs are embedded between the vertebral bodies connecting them together. A healthy disc acts as a shock-absorbing organ and provides flexibility for the spine. The disc keeps a distance between two adjacent vertebral bodies in a certain tolerance range which altogether makes up about one third of the spinal length. The IVD is composed macroscopically of two different tissues, the annulus fibrosus (AF) and the nucleus pulposus (NP) (fig. 1). The AF anchors to the cartilaginous endplates connecting to the vertebral bodies and keeps the NP in the centre position. Having a closer look there are in fact four different tissue types, from the outside to the inside: 1) the outer AF, a ring of highly oriented densely packed collagen fibril lamellae, 2) the fibro-cartilaginous inner AF, 3) the transition zone, a thin zone between the inner AF and 4) the central NP. The vertebral endplates, initially comprising hyaline cartilage of which the cells resemble chondrocytes found in other hyaline cartilage [2]. The IVD is avascular, thus there are steep gradients in concentration of nutrients and metabolites from the blood supply at the disc’s margins to the centre of the disc [3]. Even in normal healthy animal discs, low oxygen and high lactic acid concentration (therefore, resulting in an acidic pH level) compared to levels in the blood plasma have been recorded [3]. Thus, the “niche” for disc-like cells of the NP is marked by low oxygen concentration, high lactic acid concentration and relatively high hydrostatic pressure [4].
Four out of five people suffer from severe LBP once in their lifetime [5]. It is a high socio-economic burden in modern western countries, since it not only affects the elderly population but also the working population from 25–60 years, who are mainly suffering from disc herniations [6]. Traditionally, the IVD are excised and the vertebral bodies are fused, which is considered a “gold standard”, although adjacent disc degeneration might result due to the altered segmental motion [7]. Even artificial total disc replacement strategies are available to restore the segmental motion of the spine, the surgery is very traumatic and the non-biological prosthesis wears with time. Biological solution using tissue engineering approaches for disc regeneration and repair is the recent focus to restore the disc function by the introduction of functional cells and supporting biomaterials. Regenerative medicine is becoming extremely important in this field to augment or replace the degenerated disc to retain a healthy disc function.
One of the characteristic of disc degeneration is the loss of matrix in the NP, hence, one strategy for disc degeneration is to restore the function of the nucleus pulposus by introducing shock absorption hydrogels, matrix producing cells and molecules which stimulate the endogenous cells to replenish the lost matrix. Treatment strategies may vary depending on the severity of the degeneration.
An ideal hydrogel for NP repair should fulfill the following criteria: 1) allow for implantation by minimally invasive surgery (MIS), 2) solidify after implantation to avoid leaking of cells or gel, 3) possess appropriate mechanical strength and some resistance to degradation, 4) be able to store large amount of water and provide swelling pressure at various loadings, 5) support cell growth and matrix production when cells are embedded, 6) should not provoke adverse biological response during degradation. To facilitate scaffold injection by MIS, the hydrogel should have low viscosity during injection and should be able to solidify after introduction into the body to avoid leakage. Thermo-reversible hydrogel (T-RHG) which possesses a solid to gel (sol/gel) transition range at body temperature and room temperature is a promising candidate. In vitro results showed that these hydrogels, including a chitosan-based hydrogel [8] and a hyaluronan-based hydrogel (HA-pNIPAM) [9], have good biocompatibility for disc cells. We are currently investigating how T-RHG can be delivered in a clinically relevant scenario using our in vitro organ culture model. In order to inject the hydrogel, we developed a papain disc-degeneration model (PDDM), which creates a cavity in the IVD, which then can be filled with such a T-RHG hydrogel (fig. 2). Alternatively, photo-crosslinking of natural polymer, e.g. alginate [10], cellulose [11] with functional groups, e.g. methacrylates or N-vinylpyrrolidone, by exposure to UV light induced a sol/gel transition of the hydrogel can be used. This photo-crosslinked hydrogel provided some manipulation time for injection before it solidified and showed good cytocompactability and increased matrix deposition overtime [10]. Although polymer crosslinking formed by chemical reactions generates considerably amount of heat, a recent study has shown good cytocompatibility and injectability of the polymer in combination with human disc cells for NP repair [12].
Mechanical properties of the hydrogel is one of the crucial criteria to be considered for NP tissue engineering. The mechanical properties and the swelling properties of the polymer (1–4 kPa) can be controlled by varying the macromer and monomer concentration for photo-crosslinking [11] or chemical crosslinking processes. Hyaluronan based polymer enriched with elastin-like polypepetide or fibrin may improve the stiffness of the hydrogel. To provide the swelling properties of the hydrogel, polymers enriched with collagen, hyaluronic acid (HA) and chondroitin sulfate are potential candidates as a injectable system for NP repair [13, 14]. Hyaluronic acid is the most abundant water absorption molecule in the NP, which is able to dehydrate and rehydrate under a range of mechancial loading parameters. Hyaluronan-derived polymers were tested in pigs [15]: Two hyaluronan-derived polymeric substitute materials (HYAFF 120, an ester and HYADD 3, an amide) in combination with homologous mesenchymal stem cells were injected into the NP of the nucleotomized pig lumbar disc. The injection of polymer and stem cells resulted in a less degenerated disc as compared to the nucleotomizesd control. However, hyaluronic acid and collagen are degraded relatively fast in vivo, stability and mechanical properties of the hydrogel may need to be improved by crosslinking with protein cross-linker e.g. carbodiimides [16].
| Table 1: Overview of classical biomaterials and examples used for intervertebral disc engineering. | |
| Class | Examples |
| Synthetic degradable polymers | Polylactides/glycolidesPolycaprolactonePolyhydroxyalkanoatesPoly(propylene fumarates)Polyurethanes |
| Natural biopolymers | ProteinsCollagenElastinFibrin/fibrinogenSilkPolysaccharidesAlginatesChitosanHyaluronic acid |
| Bioactive ceramics | Calcium phosphatesBioactive glasses |
| Composites | Synthetic polymers/ bioactive ceramicsBiopolymers/bioactive ceramics |
| Tissue derived ECM | Small intestine submucosaSkin extracellular matrix |
Owing to the difficulty in obtaining young and healthy nucleus pulposus cells for NP repair, pluripotent and multipotent stem cells have been some of the most favourable candidates for NP repair. Adult stem cells derived from different parts of the body, including the bone marrow [17], adipo-tissue [18] and synovium [19] have been studied and showed encouraging results. A recent clinical study transplanting autologous bone marrow derived mesenchymal stem cells (MSC) has shown promising initial results in terms of improvement of pain and disability [20]. Since the IVD environment is relatively “harsh” there is the problem of low survival of injected MSC in-vivo[21, 22]. It might be possible to improve the survival of the injected MSC by pre-conditioning or using different hydrogel cell carriers [23]. For example, a hyaluronan-based photo-crosslinked polymer with MSC showed positive in vivo results in pigs [15].
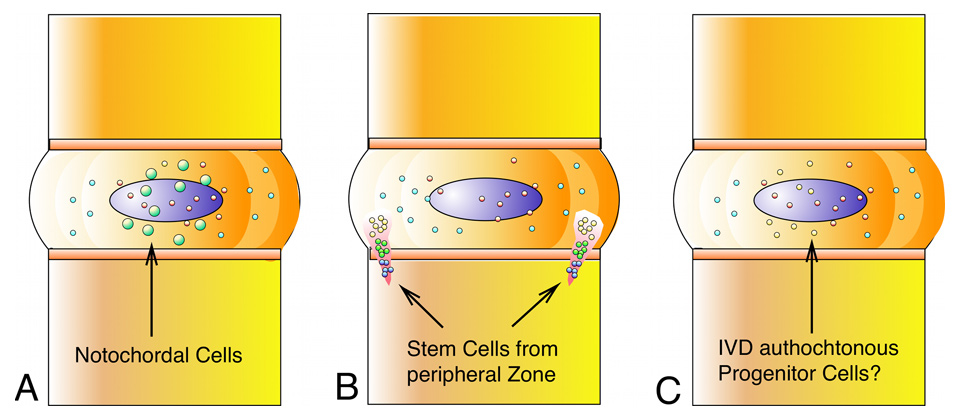
Figure 3
Three niches for stem cell-like progenitor cells (SCLC) in the intervertebral disc (IVD): A. Notochordal cells with regenerative capacity remain persistent in the IVD at different ratios and at different stages in the lifetime (according to [47, 50, 51]). B. The perichondrium region (PR) close to the outer annulus fibrosus acts as a densely packed center where cells migrate out of the PR with 2 zones of interest: perichondrium region of interest 1 (P roi 1) with densely packed cells (red) and perichondrium region of interest 2 (P roi 2) with cells (yellow) that are more dispersed and morphologically mature [41]. C. Intervertebral-disc like progenitor cells could be present in the intervertebral disc itself [38, 39].
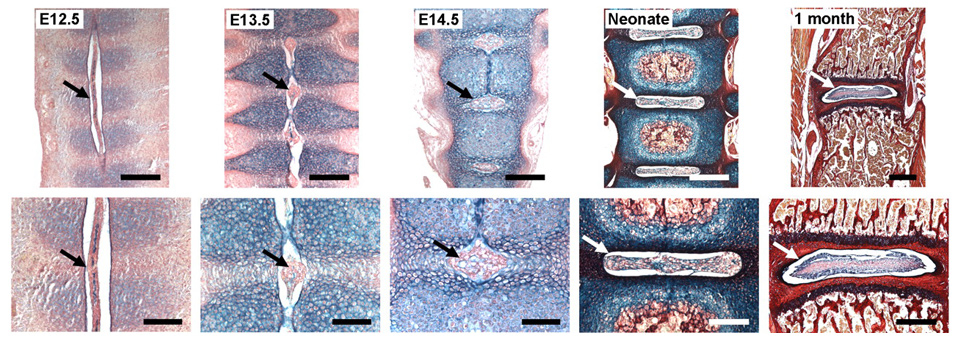
Figure 4
Stages of notochord transformation into the NP in the mouse embryo according to [44] (© Smith LJ, Nerurkar NL, Choi KS, et al. Degeneration and regeneration of the intervertebral disc: lessons from development. Dis Model Mech. 2011;4:31–41. Reprinted with kind permission). Embryos were stained with Alcian Blue (which marks glycosaminoglycans) and Picrosirius Red (which marks collagen). At embryonic day (E)12.5, the notochord (arrow) runs uninterrupted along the length of the vertebral column. Sclerotome cells have condensed perichordially, and metameric patterning of future disc and vertebral body condensations is apparent. At E13.5, the notochord has begun to contract within the vertebral body regions and expand within the disc regions (arrow). At E14.5, the notochord has virtually disappeared from the vertebral bodies, persisting solely in the locations of the future NPs (arrow). In the neonate, lateral expansion of the NP has occurred (arrow) and primary ossification centres are present in the vertebral bodies. At postnatal age 1 month, vertebral bodies are fully ossified and the NP (arrow) contains a glycosaminoglycan-rich extracellular matrix surrounded by the collagenous AF. Scale bars: E12.5, E13.5 and E14.5: 200 mm (top) and 100 mm (bottom); neonate: 400 mm (top) and 200 mm (bottom); 1 month: 500 mm (top) and 400 mm (bottom). Reprinted with permission from the publisher (Dis Model. Mech. 4, 31-41), available under a Creative Commons Attribution Non-Commercial Share-Alike License (http://creativecommons.org/licenses/by-nc-sa/3.0/).
Although the phenotypic character of nucleus pulposus cells (NPC) is still not well defined, numerous works have been done to characterise NPC as compared to annulus fibrosus cells (AFC) and chondrocytes. Some growth factors e.g. insulin-like growth factor (IGF-1), basis fibroblast growth factor (FGF-2), platelet derived growth factor (PDGF) [24] and growth and differentiation factor -5 (GDF 5) [25], could direct differentiation of MSC towards discogensis. Growth factors of the TGF family and BMP family could induce chondrogenic differentiation of MSC [26]. However, these growth factors could also drive MSC towards osteogenesis [27] and hinder discogenic differentiation of stem cells [24].
MSC pre-conditioning by co-culturing of MSC with NPC [28, 29], culturing with notochordal cells conditioned media [30], hypoxia [25, 31], culturing in a 3D environment and mechanical stimulation [32, 33], all have shown to promote chondrogenesis/ discogenesis of MSC. Albeit the promising pilot clinical result, there is also concern about the safety of using MSC due to their multipotency. Cell leakage is one of the problems of injecting cell suspension [34], a study has reported one of the possible downside of mishandling of MSC, where the leaking of MSC in the disc surrounding has led to osteophyte formation [35]. Hence, cells should be embedded in a carrier for injection on the one hand to protect MSC from leaking and on the other hand to provide a framework for supporting growth and differentiation of MSC.
Other stem cell sources, such as embryonic stem cells (ESC) pre-differentiated towards a chondrogenic type [36], neonatal human fibroblast combined with growth factor [31] and umbilical cord blood stem cells or Wharton’s jelly cells [37] are potential sources of cells due to their multipotency and a longer life span as compared to adult stem cells. The avascular structure of the IVD may provide an immune privilege for using allogenic tissue and stem cells [37]. One important unresolved issue is how many cells should be injected and which cell density is the most suitable. One study points to the direction that a minimum of 1 M cells is needed in a canine model [21].
Recently, it has been hypothesised that the IVD might itself contain a population of progenitor cells or so-called “intervertebral-disc stem cells” [38, 39] (fig. 3). Such cells would be, of course, uttermost ideal cell phenotype since they have been perfectly adapted to this niche. On the other hand, clinical studies of autologous disc cell re-implantations have shown encouraging result in pain relief in patients at two years follow up [40]. Furthermore, by immuno staining and BrdU labeling for cell proliferation, a recent study by Henriksson et al. [41] found evidence for cell migration of possible progenitor cell from the adjacent pericellular region of the IVD.
Notochordal cells (NC) are remnant cells originating from the notochord present in all chordates in early embryogenesis and these cells are located in the center of the IVD [42]. With aging, these presumably progenitor-like cells disappear in some species and in other species they persist up to adulthood [43]. In humans they disappear early in childhood [2]. Strikingly, these cells co-exist with nucleus pulposus cells (NPCs) at different ratios among different vertebrate species [43]. Rodents (rats and mice) and lagomorphs (e.g., rabbits) maintain a high number of NC throughout their lifetime whereas in other animals such as bovine, goat and sheep these cells disappear early in life [2] (fig. 4). Figure 4 illustrates the development of the vertebral bodies and the IVDs of a mouse embryo with histological stainings according to Smith et al. [44]. The alcian blue staining emphasises the high amount of proteoglycans present in early stages, which is later a typical feature for the IVD.
Recent investigations on the phenotype of the articular chondrocytes, versus intervertebral disc cells (NPC) and the notochordal cells revealed relatively large differences between chondrocytes and NPC but very little difference between NPC and notochordal cells. Notochordal cells, however, have been shown to possess cell stimulating activity by releasing soluble factors in the “conditioned medium” [30, 45]. It seemed obvious under the microscope that these cells differ in size and in respect to their nutritional demands. However, there has been a dispute on the true identity of intervertebral-disc-like cells unleashed when looking at the recent transcriptomics data: There are only about two dozen genes significantly up-or down-regulated [46–49]. This led to the hypothesis that the notochordal and the nucleus pulposus cells originate from a single progenitor cell population and are not as different as originally suggested [50–52]. In our lab, we have novel in vitro co-culture data between notochordal and nucleus pulposus cells, which points toward progenitor-status of notochordal cells [53]. Due to the possible involvement of notochordal cells with cancer in humans [54] these cells might never be accepted as implantable cells, but the cytokines, which are released from these cells will be a major target for pharmaceuticals [55].
Modulation of the matrix production and the degradation of the IVD can be achieved by direct injection of molecules into the nucleus or by transferring an exogenous gene of certain target molecules to the target cells. There are various molecules that are potentially beneficial to the degeneration of intervertebral disc cells. These molecules include growth factors, inflammatory cytokine antagonists, proteinase inhibitors or intercellular regulators (table 2). Numerous in-vivo and in-vitro studies have obtained positive results of increased matrix production or decreased matrix degradation by using various growth factors, such as bone morphogenetic protein (BMP-2, -7, -14), platelet derived growth factor (PDGF), platelet-rich plasma (PRP), and transforming growth factor beta (TGF-β). Zhang et al. [56] compared over expression of 12 BMPs and SOX-9 on genetically modified bovine NPC and suggested that BMP-2 and 7, and BMP-4 and 14 were the most effective in stimulating proteoglycan and collagen accumulation respectively. However, contradictory results have been reported for basic fibroblast growth factor (bFGF). Recently it has been reported that bFGF is the antagonist of BMP-7, which promoted fibroblast-like cell proliferation, increased degrading enzyme production and inhibited proteoglycans production by disc cells [57]. Meanwhile, the Federal Drug Administration (FDA) has recently approved a first phase clinical trial of injecting osteogenic protein-1 (OP-1 = BMP-7) and growth and differentiation factor-5 (GDF-5 = BMP-14) [58].
One problem of growth factor treatment is the high cost of production. In contrast, peptides such as Link N (DHLSDNYTLDHDRAIH) are relatively cheap to produce [59]. Link N is a glycoprotein that stabilises the proteoglycan aggregates and hyaluronate. A recent study has shown partial recovery of a rabbit disc degeneration model by introducing Link N [59]. Dexamethasone is a synthetic glucocorticoid widely used in the medical practice as an anti-inflammatory drug and has been demonstrated to stimulate chondrogenesis of chondrocytes, increase GAG production and decrease MMP-3 production of NPC. LIM Mineralization Protein (LMP-1) is an intracellular regulatory molecule that induces the secretion of multiple bone morphogenic proteins (BMP) such as BMP-2 & 7 and has been shown to induce GAG production. Although results showed increased matrix production by cells, some growth factors might eventually caused osteogenesis of cells. For example, BMP-2 is used with a collagen sponge for filling of the spinal fusion cage to enhance bone formation [60]. Similar problems have also been identified for TGF-β, LMP-1 and BMP-7. On the other hand, even if these molecules can be introduced by MIS, they often have a short half-life and therefore controlled release of these molecules to maintain the efficiency of the molecules is necessary. Albeit gene therapy could possibly maintain a constant release of the molecules, the expression of the target molecules in the long term is unknown, and the efficacy of transduction and the safety of using virus are other issues.
| Table 2: Overview of potential molecular/growth factor for IVD treatment. | |
| Protein | |
| Intercellular regulator | SOX-9LIM mineralization protein (LMP-1)DexamethasoneTissue inhibitor of matrix metalloproteinase (TIMP)Synthetic peptide (Link-N) |
| Inflammatory cytokines anatogonist | Interleukin-1 receptor antagonist (IL-1 ra)Tumor necrosis factor antagonist (TNF-a) |
| Growth factor | Growth and differentiation factor-5 (GDF-5) or (BMP-14)Insulin-like growth factor (IGF-1)Transforming growth factor β (TGF-β)Epidermal growth factor (EGF)Osteogenic protein-1 (OP-1)/ BMP-7Bone morphogenetic protein (BMP-2)Platelet-rich plasma (PRP)Platelet derived growth factor (PDGF)Basic fibroblast growth factor (bFGF) |
All the above mentioned biological therapies concerned with the degenerative functions of the native disc cells rely on a proper nutrition supply to the disc. Disc degeneration as a result of impaired nutrition due to calcification of the cartilaginous endplates [61], or atherosclerosis of the abdominal aorta and lumbar arteries [62], biological therapy relying on matrix producing cells are implausible. Shortage of nutrient supply will eventually eradicate the endogenous cells and the introduced cells. Glucose deprivation and acidic pH of the disc can hinder matrix production and increase matrix breakdown of the disc [63]. To improve nutrition to the disc, a receptor antagonist 5-hydroxytryptamine (5-HT) has shown to increase blood flow to the nerve roots of patients with lumbar disc herniation [64]. Nimodipine, which acts as a calcium channel antagonist, enhances vascularisation of the cartilage endplates in the disc and can potentially improve the nutrition of the disc [65].
Disc nucleus replacement using an inert load bearing material to restore the natural length of the annulus fibrosus and decompress the painful disc is an alternative treatment therapy. Replacing only the NP instead of the whole disc offers a less invasive option. An injectable non-degradable nucleus replacement material of high compressive resistance can serve to restore disc height and to relieve pain via minimal invasive surgery [66]. A clinical study showed significant pain relief and conserved mobility after implanting a NP replacement, e.g. prosthetic disc nucleus (PDN) composed of polyacrylonitrile and polyacrylamide [67]. On the other hand, injecting a resorbable polyglycolic acid scaffold with serum into a rabbit disc degeneration model has shown to recruit cells deposition in the scaffold and regenerate the disc nucleus [68]. Nucleus replacement materials made of silicone, poly (merthy-methacrylate) (PMMA) – hydroxyethylmethacrylate (pHEMA), polyurethane, poly (vinyl alcohol) (PVA) based polymer, N-vinyl-2-pyrrolidinone copolymerised with 2-(40-iodobenzoyl)-oxo-ethyl methacrylate and photo-crosslinked gellan gum – glycidlmethacrylate have been under research. However, the use of polymers for injectable nucleus replacement involves toxic monomers and radical and therefore may be toxic to the surrounding cells.
Surgical approaches for AF repair aim to stop the NP from leaking out of the AF. At present, there are commercially available implants for closing an injured AF: the Inclose© and the Xclose©, which can be seen as modified sutures with anchors [69]. Barricaid© is another commercially available implant used in adjunction to discectomies that fully bridges the defect in the AF. This product reinforces the complete posterior annulus and could therefore prevent contralateral herniation. However, these approaches cannot maintain the biological AF structure in the long-term or stop AF degeneration. AF closure is also important for nucleus pulposus replacement strategies to prevent the NP replacement from leaking out from the disc. More AF closure methods using biodegradable polymers are under investigation, Hegewald and his colleagues [70] tested a biodegradable polyglycolic acid – hyaluronic acid (PGA-HA) biomaterial in bovine lumbar spinal units with AF opening. The insertion of this PGA-HA implant effectively prevented disc herniation in their mechanical study. Bron et al. [71] lately tested several annulus closure devices (ACDs) in order to contain a collagen nucleus replacement implant. However, most implants were destroyed or displaced after 6 weeks of in-vivo experiment.
Tissue engineering methods aim to close the injured AF to prevent disc herniation as well as to stop AF from degeneration in the long term. Hydrogels have been discussed as good candidates for intervertebral disc replacement for the NP. However, it is known that if AF cells are cultured in hydrogels such as alginate or matrigel that these cells become like NP cells over time of culture [72]. Moreover, hydrogels seem too soft and their elasticity is not ideal to withstand stress and shear in the AF. Nevertheless, Shao et al. [73] constructed a scaffold using wet-spun lyophilised alginate/chitosan forming a scaffold with aligned fibres. They found the AF cells grew in clusters and spread along the alginate/chitosan fibres and these cell expressed collagen 2 and aggrecan. On the other hand, various synthetic and natural polymers have been tested for AF tissue engineering use. Poly-DL-lactide (PDLLA)/Bioglass composite foams were recently investigated to check AF cell attachment and phenotype [74–76]. Their findings provide preliminary evidence for the use of PDLLA/Bioglass composite scaffolds as cell-carrier materials for future treatments of intervertebral discs with damaged AF region. Biodegradable malic acid-based polyester (poly [1,8] octanedial malate) (POM) was tested as a scaffold for AF replacement in rats with good biocompatibility [77]. Later, the same group constructed a biphasic scaffold to structurally and elastically simulate the inner and outer AF [78]. In this study the outer phase of the scaffold was a ring-shaped matrix composed of demineralised bone matrix gelatin (BMG) extracted from cortical bone, which mimics the type I collagen structure and ligamentous properties of outer AF. The inner phase of the scaffold was a polycaprolactone triol malate (PPCLM) matrix oriented in concentric sheets and seeded with chondrocytes to recapitulate the inner layer of the AF, which is rich in type II collagen and proteoglycan.
Silk has been proposed as a major biomaterial to be used as a suture or a tissue construct to close AF lesions and disc herniations [79–81]. The chemical structure of silk is a polypeptide consisting of the amino acids Gly-Ser-Gly-Ala (Glycine-Sericine-Glycine-Alanine). In order to get a high medical grade silk of high biocompatibility and low allergenic reaction the cocoons of the silk worm Bombyx mori are usually boiled for 30 min in low molar Na2CO3 solution and then rinsed with water to extract the Sericin, which has been identified to be a major allergen [82]. Sericin-free silk has been successfully applied in orthopedics for decades. For instance, Chang and his colleagues [79, 83] investigated silk scaffold of different porosity for cell spreading and proliferation of annulus fibrosus cells. They also modified their scaffold by incorporating RGD peptides for better cell attachment. However, they did not find any significant difference in gene expression or in cell spreading using this modification. Recently, Park et al. [84] constructed a lamellar ring structure using silk, and the seeding of porcine AF cells seem to support the native shape of these tissue engineered AF.
The annulus fibrosus structure is highly specialised to sustain its mechanical requirement to withstand stress and strain of different loading direction. It is composed of alternating layers of lamella mainly consisting of collagen fibrils, which are aligned in parallel and tilted at 30˚ of the spinal axis. The direction of the collagen alternates in successive lamellae. Tissue engineering of the highly oriented AF structure is a challenge. The oriented structure of the AF was engineered successfully by electrospinning poly-Σ-caprolactone (PCL) [85]. It was shown that engineered materials with the highly oriented organisation behaved more like native AF tissue and cells seeded in these scaffold orient themselves to the direction of the fibres and deposit matrix as in native tissue [85]. The cultured bovine AF cells elongated and aligned along the predominant fiber direction over time. It was also suggested that a PCL scaffold with round-end nanofibers outperforms the random or aligned PCL scaffold [86]. Although not specially for IVD engineering, silk fibroin alone or combined with hydroxybutyl chitosan have been fabricated by an electrospinning method to create a biomimic extracellular matrix structure [87, 88]. The mechanical properties, especially the tensile strength, of the electrospinning scaffold could be enhanced for a specific tissue for example by using a recombinant silk-elastin-like protein [89]. It is also possible to incorporate growth factors in a electrospinning scaffold, recently, Vadalà et al. [90] produced a bioactive material made of poly-L-lactide scaffold releasing transforming growth factor-b1 (PLLA/TGF) for AF tissue engineering. The continue release of TGF-b from the PLLA scaffold has significantly increased the GAG and collagen synthesis by the seeded AF cells compared to the AF cell in the non- TGFb releasing scaffold.
For a severe disc degeneration, a total disc replacement maybe necessary. A whole IVD tissue engineering construct was produced according to the different properties of the NP and AF. Park et al. constructed a biphasic whole IVD using silk protein for the AF and fibrin/hyaluronic acid gel for the NP using porcine AF cells and chondrocytes respectively [91]. Lazebnik et al. [92] fabricated a bi-phasic IVD by electrospinning using PCL as the AF and agarose as the NP, both parts were seeded with porcine chondrocytes. Bowles et al. [93] constructed a biphasic whole IVD using collagen 1 for AF and alginate for the NP. Recent studies on total disc tissue engineering have attained positive results in small animal models, while the challenge on how to scale this up for preclinical study remains unknown. One of the major challenges, which is left for future tissue engineering of the IVD is not to dedifferentiate the cells in culture after in vitro culture. To construct an artificial IVD with the two major parts, AF and NP, numerous cells are needed. The AF has a cell density of about 9 x 106 cells/cm3 in mature healthy subjects, this is about two times higher than in the NP [69, 94–96]. However, primary cells start to dedifferentiate when they are cultured in vitro over long passaging (p >6) [97]. Strategies are needed to control the state of the cells to ensure these cells are not dedifferentiated over long expansion time and suitable for IVD tissue engineering.
Current approaches for a complete replacement of the intervertebral disc with a complete AF and NP seem difficult but maybe not unachievable. Current in vitro approaches of tissue engineered organs using stiffer materials such as tendon and AF could be successfully mimicked with biphasic biomaterials with high elastic modulus [78, 98]. The emerging 3D bioprinting technologies make it possible to print complex fixable shapes and highly organized tissue structure [99–101]. We might only be footsteps away to print a complete annulus fibrosus and nucleus pulposus tissue engineering construct along with mixed cells such as pre-conditioned mesenchymal stem cells.
There is a “gold standard” in spinal surgery: “no disc – no pain”, which is achieved with spinal fusion. Mechanical IVD prostheses give back some of the motional freedom but can be troublesome and difficult to remove in second surgery. Although there are many approaches currently being investigated in IVD repair, biomechanically stable solutions have not been identified, which are at the same time also highly biocompatible and inductive for progenitor cells. Big assets are believed to come from stem cell therapy investigations. A wide array of polymers and hydrogel-like biomaterials are currently under investigation on the side of NP repair. What we can definitively say is that the IVD will keep puzzling engineers and biologists for decades to come while an increasing demand and pressure for biological solutions is produced by the demographic shift towards an increasing elderly society.
Acknowledgements: We thank Cherry Malonzo and Harald Bonél (Insel University Hospital, Bern) for contributing the MRI image.
1 Leung VY, Chan D, Cheung KM. Regeneration of intervertebral disc by mesenchymal stem cells: potentials, limitations, and future direction. Eur Spine J. 2006;15(Suppl 3):S406–13.
2 Hunter CJ, Matyas JR, Duncan NA. The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng. 2003;9:667–77.
3 Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine. 2004;29:2700–9.
4 Wuertz K, Godburn K, Neidlinger-Wilke C, et al. Behavior of mesenchymal stem cells in the chemical microenvironment of the intervertebral disc. Spine. (Phila Pa 1976) 2008;33:1843–9.
5 Nachemson A. Is there such a thing as degenerative disc disease? In: Gunzburg R, Szpalski M, Andersson GB, ed. Degenerative disc disease. Philadelphia, Lippincott Williams Wilkins, 2004: 1–5.
6 Kara B, Tulum Z, Acar U. Functional results and the risk factors of reoperations after lumbar disc surgery. Eur Spine J. 2005;14:43–8.
7 Etebar S, Cahill DW. Risk factors for adjacent-segment failure following lumbar fixation with rigid instrumentation for degenerative instability. J Neurosurg. 1999;90:163–9.
8 Cheng YH, Yang SH, Su WY, et al. Thermosensitive chitosan-gelatin-glycerol phosphate hydrogels as a cell carrier for nucleus pulposus regeneration: an in vitro study. Tissue Eng Part A. 2010;16:695–703.
9 Peroglio M, Grad S, Mortisen D, et al. Injectable thermoreversible hyaluronan-based hydrogels for nucleus pulposus cell encapsulation. Eur Spine J. 2011; Epub ahead of print.
10 Chou AI, Akintoye SO, Nicoll SB. Photo-crosslinked alginate hydrogels support enhanced matrix accumulation by nucleus pulposus cells in vivo. Osteoarthritis Cartilage. 2009;17:1377–84.
11 Reza AT, Nicoll SB. Characterization of novel photocrosslinked carboxymethylcellulose hydrogels for encapsulation of nucleus pulposus cells. Acta Biomater 2010;6(1):129–86.
12 Moss IL, Gordon L, Woodhouse KA, et al. A novel thiol-modified hyaluronan and elastin-like polypetide composite material for tissue engineering of the nucleus pulposus of the intervertebral disc. Spine. (Phila Pa 1976) 2011;36:1022–9.
13 Collin EC, Grad S, Zeugolis DI, et al. An injectable vehicle for nucleus pulposus cell-based therapy. Biomaterials. 2011;32:2862–70.
14 Huang B, Li CQ, Zhou Y, et al. Collagen II/hyaluronan/chondroitin-6-sulfate tri-copolymer scaffold for nucleus pulposus tissue engineering. J Biomed Mater Res B Appl Biomater. 2010;92:322–31.
15 Revell PA, Damien E, Di Silvio L, et al. Tissue engineered intervertebral disc repair in the pig using injectable polymers. J Mater Sci Mater Med. 2007;18:303–8.
16 Calderon L, Collin E, Velasco-Bayon D, et al. Type II collagen-hyaluronan hydrogel – a step towards a scaffold for intervertebral disc tissue engineering. Eur Cell Mater. 2010;20:134–48.
17 Richardson SM, Hoyland JA, Mobasheri R, et al. Mesenchymal stem cells in regenerative medicine: opportunities and challenges for articular cartilage and intervertebral disc tissue engineering. J Cell Physiol. 2010;222:23–32.
18 Hoogendoorn RJ, Lu ZF, Kroeze RJ, et al. Adipose stem cells for intervertebral disc regeneration: current status and concepts for the future. J Cell Mol Med. 2008;12:2205–16.
19 He F, Pei M. Rejuvenation of nucleus pulposus cells using extracellular matrix deposited by synovium-derived stem cells. Spine. (Phila Pa 1976) 2011;37:459–69.
20 Orozco L, Soler R, Morera C, et al. Intervertebral disc repair by autologous mesenchymal bone marrow cells: A pilot study. Transplantation. 2011;92:822–8.
21 Serigano K, Sakai D, Hiyama A, et al. Effect of cell number on mesenchymal stem cell transplantation in a canine disc degeneration model. J Orthop Res. 2010;28:1267–75.
22 Chan SCW, Gantenbein-Ritter B, Leung VY, et al. Cryopreserved intervertebral disc with injected bone marrow-derived stromal cells: a feasibility study using organ culture. Spine J. 2010;10(6):486–96.
23 Gantenbein-Ritter B, Benneker LM, Alini M, et al. Differential response of human bone marrow stromal cells to either TGF-β(1) or rhGDF-5. Eur Spine J. 2011;20:962–71.
24 Ehlicke F, Freimark D, Heil B, et al. Intervertebral disc regeneration: Influence of growth factors on differentiation of human mesenchymal stem cells (hMSC). Int J Artif Organs. 2010;33:244–52.
25 Stoyanov JV, Gantenbein-Ritter B, Bertolo A, et al. Role of hypoxia and growth and differentiation factor-5 on differentiation of human mesenchymal stem cells towards intervertebral nucleus pulposus-like cells. Eur Cell Mater. 2011;21:533–47.
26 Xu J, Wang W, Ludeman M, et al. Chondrogenic differentiation of human mesenchymal stem cells in three-dimensional alginate gels. Tissue Eng Part A. 2008;14:667–80.
27 Than KD, Rahman SU, Vanaman MJ, Wang AC, Lin CY, Zhang H, et al. Bone morphogenetic proteins and degenerative disc disease. Neurosurgery 2011; Sept. 5. E-pub ahead of print.
28 Strassburg S, Richardson SM, Freemont AJ, et al. Co-culture induces mesenchymal stem cell differentiation and modulation of the degenerate human nucleus pulposus cell phenotype. Regen Med. 2010;5:701–11.
29 Richardson SM, Walker RV, Parker S, et al. Intervertebral disc cell-mediated mesenchymal stem cell differentiation. Stem Cells. 2006;24:707–16.
30 Korecki CL, Taboas JM, Tuan RS, et al. Notochordal cell conditioned medium stimulates mesenchymal stem cell differentiation toward a young nucleus pulposus phenotype. Stem Cell Res Ther. 2010;1:18.
31 Singh M, Pierpoint M, Mikos AG, et al. Chondrogenic differentiation of neonatal human dermal fibroblasts encapsulated in alginate beads with hydrostatic compression under hypoxic conditions in the presence of bone morphogenetic protein-2. J Biomed Mater Res A. 2011;98:412–24.
32 Chan SC, Ferguson SJ, Gantenbein-Ritter B. The effects of dynamic loading on the intervertebral disc. Eur Spine J. 2011;20:1796–812.
33 Potier E, Noailly J, Ito K. Directing bone marrow-derived stromal cell function with mechanics. J Biomech. 2010;43:807–17.
34 Bertram H, Kroeber M, Wang H, et al. Matrix-assisted cell transfer for intervertebral disc cell therapy. Biochem Biophys Res Commun. 2005;331:1185–92.
35 Vadalà G, Sowa G, Hubert M, et al. Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med. 2012;6(5):348–55.
36 Sheikh H, Zakharian K, De La Torre RP, et al. In vivo intervertebral disc regeneration using stem cell-derived chondroprogenitors. J Neurosurg Spine. 2009;10:265–72.
37 Ruan D, Zhang Y, Wang D, et al. Differentiation of human Wharton’s jelly cells toward nucleus pulposus-like cells after coculture with nucleus pulposus cells in vitro. Tissue Eng Part A. 2011;18(1-2):167–75.
38 Sakai D. Endogenous/stem progenitor cell population of the intervertebral disc and its implication on ageing and degeneration, in The symbosium of AO Exploratory Research “Where Science meets clinics” 2011, 2–7 September, Davos.
39 Erwin WM. Intervertebral disc-derived stem cells: Implications for regenerative medicine and neural repair, in Proceedings of ISSLS, June 14–18 2011, Gothenburg
40 Meisel HJ, Siodla V, Ganey T, et al. Clinical experience in cell-based therapeutics: disc chondrocyte transplantation A treatment for degenerated or damaged intervertebral disc. Biomol Eng. 2007;24:5–21.
41 Henriksson H, Thornemo M, Karlsson C, et al. Identification of cell proliferation zones, progenitor cells and a potential stem cell niche in the intervertebral disc region: a study in four species. Spine. (Phila Pa 1976) 2009;34:2278–87.
42 Walmsley R. The development and growth of the intervertebral disc. Edinburgh Med J. 1953;60.
43 Miyazaki T, Kobayashi S, Takeno K, et al. A phenotypic comparison of proteoglycan production of intervertebral disc cells isolated from rats, rabbits, and bovine tails; which animal model is most suitable to study tissue engineering and biological repair of human disc disorders? Tissue Eng Part A. 2009;15:3835–46.
44 Smith LJ, Nerurkar NL, Choi KS, et al. Degeneration and regeneration of the intervertebral disc: lessons from development. Dis Model Mech. 2011;4:31–41.
45 Purmessur D, Schek RM, Abbott RD, et al. Notochordal conditioned media from tissue increases proteoglycan accumulation and promotes a healthy nucleus pulposus phenotype in human mesenchymal stem cells. Arthritis Res Ther. 2011;13:R81.
46 Lee CR, Sakai D, Nakai T, et al. A phenotypic comparison of intervertebral disc and articular cartilage cells in the rat. Eur Spine J. 2007;16:2174–85.
47 Minogue BM, Richardson SM, Zeef LA, et al. Transcriptional profiling of bovine intervertebral disc cells: implications for identification of normal and degenerate human intervertebral disc cell phenotypes. Arthritis Res Ther. 2010;12:R22.
48 Rutges J, Creemers LB, Dhert W, et al. Variations in gene and protein expression in human nucleus pulposus in comparison with annulus fibrosus and cartilage cells: potential associations with aging and degeneration. Osteoarthritis and Cartilage. 2010;18:416–23.
49 Sakai D, Nakai T, Mochida J, et al. Differential phenotype of intervertebral disc cells: microarray and immunohistochemical analysis of canine nucleus pulposus and anulus fibrosus. Spine. (Phila Pa 1976) 2009;34:1448–56.
50 Shapiro IM, Risbud MV. Transcriptional profiling of the nucleus pulposus: say yes to notochord. Arthritis Res Ther. 2010;12:117.
51 Risbud MV, Schaer TP, Shapiro IM. Toward an understanding of the role of notochordal cells in the adult intervertebral disc: From discord to accord. Dev Dyn. 2010;239:2141–8.
52 Weiler C, Nerlich AG, Schaaf R, et al. Immunohistochemical identification of notochordal markers in cells in the aging human lumbar intervertebral disc. Eur Spine J. 2010; 19(10):1761–70.
53 Gantenbein-Ritter B, Chan SC. The evolutionary importance of cell ratio between notochordal and nucleus pulposus cells: an experimental 3-D co-culture study. Eur Spine J. 2011; [epub ahead of print].
54 Vujovic S, Henderson S, Presneau N, et al. Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J Pathol. 2006;209:157–65.
55 Erwin WM, Ashman K, O'Donnel P, et al. Nucleus pulposus notochord cells secrete connective tissue growth factor and up-regulate proteoglycan expression by intervertebral disc chondrocytes. Arthritis Rheum. 2006;54:3859–67.
56 Zhang Y, An HS, Thonar EJ, et al. Comparative effects of bone morphogenetic proteins and sox9 overexpression on extracellular matrix metabolism of bovine nucleus pulposus cells. Spine. (Phila Pa 1976) 2006;31:2173–9.
57 Li X, An HS, Ellman M, et al. Action of fibroblast growth factor-2 on the intervertebral disc. Arthritis Res Ther. 2008;10:R48.
58 Zhang Y, Chee A, Thonar EJ, et al. Intervertebral disk repair by protein, gene, or cell injection: a framework for rehabilitation-focused biologics in the spine. PM R 2011;3:S88–94.
59 Mwale F, Masuda K, Pichika R, et al. The efficacy of Link N as a mediator of repair in a rabbit model of intervertebral disc degeneration. Arthritis Res Ther. 2011;13:R120.
60 Haid RW, Branch CL, Alexander JT, et al. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004; 4:527–38; discussion 538–9.
61 Benneker LM, Heini PF, Alini M, et al. 2004 young investigator award winner: vertebral endplate marrow contact channel occlusions and intervertebral disc degeneration Spine. (Phila Pa 1976) 2005;30:167–73.
62 Kurunlahti M, Kerttula L, Jauhiainen J, et al. Correlation of diffusion in lumbar intervertebral disks with occlusion of lumbar arteries: a study in adult volunteers. Radiology. 2001;221:779–86.
63 Rinkler C, Heuer F, Pedro MT, et al. Influence of low glucose supply on the regulation of gene expression by nucleus pulposus cells and their responsiveness to mechanical loading. J Neurosurg Spine. 2010;13:535–42.
64 Sekiguchi M, Konno S, Kikuchi S. The effects of a 5-HT2A receptor antagonist on blood flow in lumbar disc herniation: application of nucleus pulposus in a canine model. Eur Spine J. 2008;17:307–13.
65 Turgut M, Uysal A, Uslu S, et al. The effects of calcium channel antagonist nimodipine on end-plate vascularity of the degenerated intervertebral disc in rats. J Clin Neurosci. 2003;10:219–23.
66 Bao QB, McCullen GM, Higham PA, et al. The artificial disc: theory, design and materials. Biomaterials 1996;17:1157–67.
67 Selviaridis P, Foroglou N, Tsitlakidis A, et al. Long-term outcome after implantation of prosthetic disc nucleus device (PDN) in lumbar disc disease. Hippokratia. 2010;14:176–84.
68 Endres M, Abbushi A, Thomale UW, et al. Intervertebral disc regeneration after implantation of a cell-free bioresorbable implant in a rabbit disc degeneration model. Biomaterials. 2010;31:5836–41.
69 Bron JL, Helder MN, Meisel HJ, et al. Repair, regenerative and supportive therapies of the annulus fibrosus: achievements and challenges. Eur Spine J. 2009;18:301–13.
70 Hegewald AA, Knecht S, Baumgartner D, et al. Biomechanical testing of a polymer-based biomaterial for the restoration of spinal stability after nucleotomy. J Orthop Surg Res. 2009;4:25.
71 Bron JL, van der Veen AJ, Helder MN, et al. Biomechanical and in vivo evaluation of experimental closure devices of the annulus fibrosus designed for a goat nucleus replacement model. Eur Spine J. 2010;19:1347–55.
72 Alini M, Li W, Markovic P, et al. The potential and limitations of a cell-seeded collagen/hyaluronan scaffold to engineer an intervertebral disc-like matrix. Spine. 2003;28:446–54; discussion 453.
73 Shao X, Hunter CJ. Developing an alginate/chitosan hybrid fiber scaffold for annulus fibrosus cells. J Biomed Mater Res A. 2007;82:701–10.
74 Helen W, Gough JE. Cell viability, proliferation and extracellular matrix production of human annulus fibrosus cells cultured within PDLLA/Bioglass composite foam scaffolds in vitro. Acta Biomater. 2008;4:230–43.
75 Helen W, Merry CL, Blaker JJ, et al. Three-dimensional culture of annulus fibrosus cells within PDLLA/Bioglass composite foam scaffolds: assessment of cell attachment, proliferation and extracellular matrix production. Biomaterials. 2007;28:2010–20.
76 Wilda H, Gough JE. In vitro studies of annulus fibrosus disc cell attachment, differentiation and matrix production on PDLLA/45S5 Bioglass composite films. Biomaterials. 2006;27:5220–9.
77 Wan Y, Feng G, Shen FH, et al. Novel biodegradable poly(1,8-octanediol malate) for annulus fibrosus regeneration. Macromol Biosci. 2007;7:1217–24.
78 Wan Y, Feng G, Shen FH, et al. Biphasic scaffold for annulus fibrosus tissue regeneration. Biomaterials. 2008;29:643–52.
79 Chang G, Kim HJ, Kaplan D, et al. Porous silk scaffolds can be used for tissue engineering annulus fibrosus. Eur Spine J. 2007;16:1848–57.
80 Boyd LM, Carter AJ. Injectable biomaterials and vertebral endplate treatment for repair and regeneration of the intervertebral disc. Eur Spine J. 2006;15(Suppl 3):S414–21.
81 Fielding JW. Complications of anterior cervical disk removal and fusion. Clin Orthop Relat Res. 1992:10–3.
82 Ebner H, Kraft D. Wild silk-induced asthma. A contribution to the knowledge of inhalation allergies caused by wild and tussah silk-filled bed quilts. Wien Klin Wochenschr. 1987;99:542–6.
83 Chang G, Kim HJ, Vunjak-Novakovic G, et al. Enhancing annulus fibrosus tissue formation in porous silk scaffolds. J Biomed Mater Res A. 2010;92:43–51.
84 Park SH, Gil ES, Mandal BB, et al. Annulus fibrosus tissue engineering using lamellar silk scaffolds. J Tissue Eng Regen Med. 2012; [epub ahead of print].
85 Nerurkar NL, Baker BM, Sen S, et al. Nanofibrous biologic laminates replicate the form and function of the annulus fibrosus. Nat Mater. 2009;8:986–92.
86 Koepsell L, Zhang L, Neufeld D, et al. Electrospun nanofibrous polycaprolactone scaffolds for tissue engineering of annulus fibrosus. Macromol Biosci. 2011;11:391–9.
87 Zhang K, Qian Y, Wang H, et al. Electrospun silk fibroin-hydroxybutyl chitosan nanofibrous scaffolds to biomimic extracellular matrix. J Biomater Sci Polym Ed. 2011;22:1069–82.
88 Zhang K, Mo X, Huang C, et al. Electrospun scaffolds from silk fibroin and their cellular compatibility. J Biomed Mater Res A. 2010;93:976–83.
89 Qiu W, Huang Y, Teng W, et al. Complete recombinant silk-elastinlike protein-based tissue scaffold. Biomacromolecules. 2010;11:3219–27.
90 Vadalà G, Mozetic P, Rainer A, et al. Bioactive electrospun scaffold for annulus fibrosus repair and regeneration. Eur Spine J. 2012; [epub ahead of print].
91 Park SH, Gil ES, Cho H, et al. Intervertebral disc tissue engineering using biphasic silk composite scaffolds. Tissue Eng Part A. 2011;18:447–58.
92 Lazebnik M, Singh M, Glatt P, et al. Biomimetic method for combining the nucleus pulposus and annulus fibrosus for intervertebral disc tissue engineering. J Tissue Eng Regen Med. 2011;5:e179–87.
93 Bowles RD, Gebhard HH, Härtl R, et al. Tissue-engineered intervertebral discs produce new matrix, maintain disc height, and restore biomechanical function to the rodent spine. Proc Natl Acad Sci U S A 2011;108:13106–11.
94 Errington RJ, Puustjarvi K, White IR, et al. Characterisation of cytoplasm-filled processes in cells of the intervertebral disc. J Anat. 1998;192(Pt 3):369–78.
95 Cassidy JJ, Hiltner A, Baer E. Hierarchical structure of the intervertebral disc. Connect Tissue Res. 1989;23:75–88.
96 Maroudas A, Stockwell RA, Nachemson A, et al. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro J Anat. 1975;120:113–30.
97 Chou AI, Reza AT, Nicoll SB. Distinct intervertebral disc cell populations adopt similar phenotypes in three-dimensional culture. Tissue Eng Part A. 2008;14:2079–87.
98 Nesti LJ, Li WJ, Shanti RM, et al. Intervertebral disc tissue engineering using a novel hyaluronic acid-nanofibrous scaffold (HANFS) amalgam. Tissue Eng Part A. 2008;14:1527–37.
99 Fedorovich NE, Swennen I, Girones J, et al. Evaluation of photocrosslinked Lutrol hydrogel for tissue printing applications. Biomacromolecules. 2009;10:1689–96.
100 Cui X, Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials. 2009;30:6221–7.
101 Nishiyama Y, Nakamura M, Henmi C, et al. Development of a three-dimensional bioprinter: construction of cell supporting structures using hydrogel and state-of-the-art inkjet technology. J Biomech Eng. 2009;131:035001.
Funding / potential competing interests: The work on the THR-HG is supported by a HansJörg Wyss Start up Award 2011/ Project no. 102646/ AOSpine International. No financial support and no other potential conflict of interest relevant to this article was reported.