Vancomycin-resistantEnterococcus
DOI: https://doi.org/10.4414/smw.2012.13540
Clara
Thierfelder, Peter Michael
Keller, Claudine
Kocher, Roman
Gaudenz, Michael
Hombach, Guido Vincent
Bloemberg, Christian
Ruef
Summary
The reported prevalence of vancomycin-resistant Enterococcus faecium (VRE) in Switzerland for the years 2008–2010 has been low at <5%. At the University Hospital Zurich, 17 cases of VRE were detected between 28 December 2009 and 15 February 2010. Nine cases were diagnosed clinically; eight cases were detected by rectal screening. The centre of the outbreak was the cardiac surgery department. Four patients suffered from VRE-infections; four patients died.
In order to investigate and contain the outbreak, the following measures were taken: prevalence surveys using weekly rectal screening, environmental screening; selective enrichment culturing; pulsed field gel electrophoresis (PFGE) for clonal typing and polymerase chain reaction-analysis (PCR) for resistance determinants and virulence factors detection. Contact isolation in single rooms and enhanced surface-disinfection methods were implemented. Ward nurses were assigned as link nurses. Regular teaching was carried out aiming to improve hand disinfection among healthcare workers.
PFGE revealed two main pulsotypes each including seven patients. Five minor pulsotypes originated from three additional patients and one sample collected from a keyboard. Two of three patients with minor pulsotypes had been treated abroad. PCR-analysis identified vanB resistance-genotypes with exception of one vanA resistance-genotype.
The outbreak was associated with environmental contamination and insufficient compliance with hand-hygiene. Enhanced awareness and infection control measures resulted in termination of the VRE outbreak within eight weeks. The complexity of the outbreak with several clones in parallel suggests a higher baseline prevalence of VRE in Switzerland than previous surveillance data indicate.
A multiple-strain outbreak of eight weeks duration at a Swiss tertiary care hospital
Introduction
Data from Switzerland have shown a sporadic occurrence of vancomycin-resistant Enterococcus faecium(VRE) [1]. Swiss surveillance-data documents a VRE- prevalence of 4.1% among enterococci isolates from hospitalised patients for the year 2010 (http://www.search.ifik.unibe.ch, accessed 10 December 2011), The University Hospital Zurich is an 800-bed tertiary teaching care hospital. Fourteen out of 831 (1.7%) enterococci isolates in 2010/2011 originating from clinical samples tested resistant to vancomycin in the routine microbiological laboratory (outbreak patients excluded). VRE-prevalence, including carriers, cannot exactly be given for the hospital, because no systematic screening for VRE carriage is conducted. This finding is in contrast to surveillance data from the USA. According to data from the National Nosocomial Infections Surveillance System, more than a quarter of all enterococci contributing to nosocomial infections were resistant to vancomycin by the end of 2004 [2]. VRE-prevalence has been increasing substantially in some European countries over the last years reaching 15% in German tertiary care hospitals in 2007 [3].
We describe an outbreak of VRE caused by several clonal strains at a tertiary care hospital in Switzerland with emphasis on molecular characteristics and infection control interventions to combat the outbreak.
Methods
Epidemiological surveillance
After detection of the first two cases of VRE, an epidemiological investigation was started. For this investigation, the following case definition was used: a) VRE-infection required the presence of clinical evidence of infection attributed to VRE detected in samples obtained for clinical reasons b) VRE-colonisation was diagnosed if sampling had been performed in the frame of hospital epidemiology measures. The investigation included 19 weekly prevalence surveys for VRE using rectal screening (total 361 samples): 11 surveys on a cardiac surgery ward, six conducted in the cardiac surgery intensive care unit, two in the medical intensive care unit. In addition, we implemented systematic screening of patients of affected wards before transfer to another hospital on the cardiac surgery intensive care unit and the cardiac surgery ward, three environmental screenings of fomites (72 samples) and one screening of health care workers (10 samples). On-site visits by the infection control team took place weekly. Teaching sessions were held on wards as needed and were used for informal audits of compliance with infection control measures. For environmental screening mainly shared ward items were chosen such as thermometers, blood pressure-cuffs, computer-keyboards, wheel chairs, an echocardiogram, a surveillance-tower, a glove-wagon.
Microbiological and molecular diagnostic methods
For microbiological detection and isolation of potential VRE strains, patient and environmental samples were subjected to enrichment culture in selective broth (EnterococcoselTM, BD, Becton Dickinson, Allschwil, Switzerland) and subsequent culturing on selective agars (VRE/CNA, BioMérieux, Geneva, Switzerland). In addition, patient samples were inoculated onto colistin-nalidixic acid containing agars. Screening cultures for vancomycin resistance were initially performed as stated in the CLSI guidelines [4], using disk diffusion agars containing 30 µg/ml vancomycin. Because two of eight cases (25%) were still negative at 24 hours incubation we prolonged the incubation time to 48 hours. For isolates identified as potential VRE by screening, the minimal inhibitory concentration of teicoplanin and vancomycin (E-test, BioMérieux) was determined. Polymerase chain reaction analysis (PCR) for presence of vanA and vanBvancomycin-resistance determinants and hyl and esp virulence factors was carried out according to established methods [5]. PFGE-analyses were carried out with SmaI digests of chromosomal DNA isolated from representative subtypes of the epidemic VRE-isolates as previously described [6].
Infection control measures
Contact isolation of affected patients in single rooms and daily surface-disinfection of patient rooms using aldehyde containing products (Kohrsolin® FF, Bode) were implemented. Rooms were also disinfected after patient discharge. Ward nurses were assigned to function as link nurses for infection control. Regular teaching of nurses and physicians including feedback on the status of the outbreak and the results of the screenings was carried out with the aim to improve compliance with and quality of hand disinfection among healthcare workers.
Results
With onset in December 2009, a series of patients with VRE was observed. After detection of three cases, active contact tracing and regular screening were implemented and resulted in the detection of 17 cases of VRE carrying patients between 28 December 2009 and 15 February 2010 (table 1). The cardiac surgery department involved 13 patients on one ward and in the intensive care unit. During their stay in the hospital, cardiac surgery patients are routinely transferred between ward, operating room, intensive care unit and finally ward again. Of the remaining four patients, one had been admitted to a medical ward, one to the medical intensive care unit and two to a visceral surgery ward. The clinical characteristics of the patients are shown in table 1. Nine cases were diagnosed clinically by routine antibiotic susceptibility testing, while eight cases were detected by rectal screening.
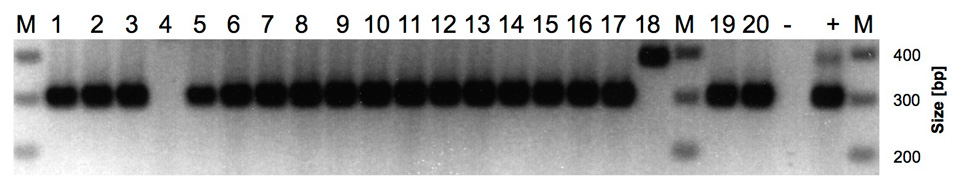
Figure 1
Agarose gel electrophoresis analysis of vanA and vanB PCR screening of VRE isolates. Amplicon sizes: vanA: 377 bp (lane 18); vanB: 298 bp (lanes 1–17, 19, 20). Lanes 1–18: VRE isolates from patient samples (patients 1–17, lanes 4 and 5 are both isolates from patient 4); lanes 19–20: VRE isolates from environmental samples (keyboard and defibrillator); lane –: negative control; lane +: vanA and vanB positive control. Lane M: size marker.
The median duration of hospitalisation until VRE-detection was 23 days (range 8–71 days); the median total duration of hospitalisation was 38 days (range 10–142 days). Four patients (24%) suffered from manifest infections. Four patients (24%) died. Two of them had manifest infections (table 1). However, three deaths could not directly be attributed to an infection with VRE, but one death was the consequence of a complicated sternal wound infection by VRE. Antibiotic treatment options were limited. All VRE strains tested resistant to ampicillin. One of the patients with manifest infection was treated initially with teicoplanin and subsequently linezolide; a second patient was treated with teicoplanin and gentamicin. The third and fourth patient did not receive antibiotic treatment with respect to a palliative situation and transfer out, respectively. Causes of death were multiorgan dysfunction syndrome (MODS), acute respiratory distress syndrome (ARDS), haemorrhagic shock and pneumonia.
Enterococcus spp. were detected from nine environmental samples on wards affected by the outbreak, namely from an echocardiogram machine, a cardiac defibrillator, a wheelchair, a surveillance-tower, a thermometer, a computer-keyboard, two glove-wagons, and one infusion set. One patient colonised with VRE was the index case for another outbreak comprising four patients in a tertiary care hospital of a neighbouring region after being transferred from our hospital.
Pulsed field gel electrophoresis (PFGE) revealed two main pulsotypes. One pulsotype included seven patients and a defibrillator (pattern A); the second (pattern B) included seven patients. Three of four patients of the outbreak in the hospital mentioned above had pattern A; one sample was not typeable. In addition, five minor pulsotypes (patterns C, D, E, F and G) were identified that affected three additional patients (one with two different pulsotypes: patterns C and D), and one environmental sample collected from a keyboard (pattern G). Two out of three patients with minor pulsotypes had been treated in foreign health care systems (Bolivia and Turkey). The patient from Bolivia had a single PFGE-pattern (pattern F); the patient from Turkey had two distinct PFGE-patterns (patterns C and D, table1). VRE-screening PCR-analysis and corresponding resistance phenotypes identified vanB type resistance in most cases with exception of the isolate from Bolivia, which had a vanA resistance genotype and phenotype (fig. 1, lane 18) and one isolate in which neither vanA nor vanBwas detected (fig. 1, lane 4). Presumably the vanA and vanB negative isolate carries a less frequently occurring van resistance locus [3].
Susceptibility to teicoplanin was used to differentiate vanBand vanA phenotypes. MICs for teicoplanin ranged from 0.5 to 3 µg/ml for vanBstrains, whereas MICs of vancomycin in vanB carrying strains ranged from 24 to >256 µg/ml. The vanA strain had a MIC for teicoplanin of 24 µg/ml and a MIC for vancomycin of >256 µg/ml. 15/17 patients (88%) were positive for the pathogenicity marker esp; 6/17 patients (35%) were positive for the virulence factor hyl. Espand hyl were not strictly linked to specific pulsotypes.
The need for contact isolation of all VRE patients resulted in severe bed shortages on the affected ward and compromised the capacity of the cardiac surgery division to admit patients for elective surgery. Frequent visits of the infection control team were necessary to ensure strict enforcement of infection control measures (e.g., conduction and documentation of the weekly prevalence screening; active environmental screening and feedback of results to health care workers on the affected ward). As a consequence of the satellite outbreak in a hospital of a neighbouring region, we made sure that every patient of the cardiac surgery department was screened for VRE shortly before transfer to another hospital. Several teaching sessions resulted in increased awareness of physicians, nurses and physiotherapists regarding the necessary measures to prevent further VRE transmission. During the first weeks of the outbreak the infection control team had to be present on the ward almost daily, whereas towards the end nurses (facilitated by the link nurse) and physicians addressed questions and demands more actively. The outbreak was considered successfully controlled after weekly screenings of patients over a period of 5 weeks failed to detect new cases.
|
Table 1: Demographic, clinical and epidemiological data of affected patients. |
|
Pa-
tient
|
Sex
|
Age
|
Hospital ward
|
Date of onset
|
Case detec-
tion*
|
Underlying disease
|
Preceding surgery
|
Clinical presen-
tation**
|
Type of Infection
|
Hospita-
lisation until
VRE-detection (days)
|
Hospita-
lisation
(total days)
|
Outcome
|
PFGE-pattern
|
Genotype
|
| 1 |
M |
74 |
Cardiac surgery |
28.12.09 |
C |
Myocardial infarction |
Coronary artery bypass graft |
I |
Retrosternal abscess |
22 |
59 |
Discharge |
A |
vanB/esp+/hyl-
|
| 2 |
M |
58 |
Cardiac surgery |
04.01.10 |
C |
Cardiomyopathy |
Heart transplantation |
C |
|
71 |
142 |
Discharge |
A |
vanB/esp+/hyl-
|
| 3 |
M |
43 |
Medical intensive care |
11.01.10 |
C |
Influenza H1N1-ARDS |
Extracorporeal membrane-oxygenation |
C |
|
34 |
104 |
Death |
B |
vanB/esp+/hyl-
|
| 4 |
M |
71 |
Cardiac surgery |
11.01.10 |
C |
A iliaca-aneurysm |
Graft-implantation |
C |
|
37 |
65 |
Discharge |
C, D |
vanB/esp+/hyl-/hyl+
|
| 5 |
M |
45 |
Visceral surgery |
11.01.10 |
C |
Ileum-perforation |
Ileostoma-reimplantation |
C |
|
8 |
87 |
Discharge |
B |
vanB/esp+/hyl+
|
| 6 |
M |
79 |
Cardiac surgery |
11.01.10 |
S |
Myocardial infarction |
Coronary artery bypass graft |
C |
|
23 |
23 |
Discharge |
B |
vanB/esp+/hyl-
|
| 7 |
F |
62 |
Cardiac surgery |
18.01.10 |
S |
Valvular cardiopathy |
Valve-reconstruction |
C |
|
35 |
38 |
Discharge |
A |
vanB/esp+/hyl+
|
| 8 |
F |
39 |
Cardiac surgery |
18.01.10 |
S |
Deep venous thrombosis |
Loop atrio-ventricular-shunt |
C |
|
11 |
13 |
Discharge |
B |
vanB/esp+/hyl-
|
| 9 |
M |
35 |
Cardiac surgery |
25.01.10 |
S |
Valvular cardiopathy |
Valve-reconstruction |
C |
|
10 |
10 |
Discharge |
B |
vanB/esp-/hyl-
|
| 10 |
F |
83 |
Cardiac surgery |
25.01.10 |
C |
Aortal dissection |
Supracoronary graft |
I |
Bacteraemia |
23 |
10 |
Discharge |
A |
vanB/esp+/hyl+
|
| 11 |
M |
72 |
Cardiac surgery |
01.02.10 |
S |
Valvular cardiopathy |
Valve-reconstruction |
C |
|
12 |
14 |
Discharge |
B |
vanB/esp-/hyl-
|
| 12 |
M |
65 |
Cardiac surgery |
08.02.10 |
S |
Valvular cardiopathy |
Valve-reconstruction |
C |
|
12 |
12 |
Discharge |
A |
vanB/esp+/hyl+
|
| 13 |
M |
82 |
Cardiac surgery |
08.02.10 |
S |
Valvular cardiopathy |
Valve-reconstruction |
C |
|
34 |
34 |
Discharge |
A |
vanB/esp+/hyl-
|
| 14 |
M |
48 |
Visceral surgery |
15.02.10 |
C |
Colon-carcinoma |
Colectomy |
I |
Bacteraemia |
63 |
63 |
Death |
E |
vanB/esp+/hyl-
|
| 15 |
M |
81 |
Cardiac surgery |
15.02.10 |
C |
Aortal dissection |
Sternal revision |
I |
Sternal wound infection |
8 |
55 |
Death |
A |
vanB/esp+/hyl-
|
| 16 |
M |
57 |
Cardiac surgery |
15.02.10 |
S |
Coronar cardiopathy |
Coronary artery bypass graft |
C |
|
11 |
32 |
Death |
B |
vanB/esp+/hyl-
|
| 17 |
M |
58 |
Medical ward |
28.12.09 |
C |
Endocarditis |
Valve-reconstruction |
C |
|
24 |
65 |
Discharge |
F |
vanA/esp+/hyl+
|
| *C = case detection on clinical indication, S = case detection by screening
** C = colonisation, I = infection |
Discussion
We describe the characteristics and control of the first published VRE-outbreak in Switzerland. The cardiac surgery department of our hospital was the outbreak’s centre rendering this the first published report with focus on cardiac surgery. The outbreak involved seven VRE clonal strains within a short time period of 14 days, illustrating the complexity of VRE-epidemiology in a single hospital.
We isolated 20 VRE strains from 17 patients (one patient with two different VREs) and two environmental sources. Two main VRE clonal strains affected 12 patients on cardiac surgery (pattern A, seven patients, pattern B, five patients), one patient on the medical intensive care unit and one patient on visceral surgery. Of five additional, unmatched clonal strains, three were likely to have been imported by two patients who were hospitalised abroad, one was a single isolate detected on a defibrillator and another genetically unrelated isolate was cultured from a patient on a visceral surgery ward who had never been in contact with cardiac surgery. These five single, unmatched PFGE-patterns suggest the presence of imported and local sporadic VRE-strains. In the local sporadic cases, plasmid mediated transfer of the vanB resistance cassette potentially caused the appearance of an additional pulsotype. Initially an outbreak strain may have transferred its resistance-plasmid to vancomycin-sensitive enterococci in the intestines of colonised patients[7]. In spite of several strains, the presence of two major pulsotypes indicates substantial horizontal transmission suggestive of two outbreaks within this multiple-strain outbreak. The presence of different pulsotypes in parallel has been previously reported [8].
All four patients with manifest infections due to VRE were esp positive, a result that supports the described association between the presence of esp and the invasiveness of VRE [8]. An association between manifest infection and the presence of the hyl-gene was not found in our study.
This VRE outbreak affected the department of cardiac surgery. In this department, transfer of patients to and from cardiac surgery, insufficient compliance with hand hygiene of staff and subsequent environmental contamination may have facilitated transmission of initially sporadic vanB carrying strains. All patients had undergone surgery (all but two cardiac surgery), which has been described to be a risk factor for VRE-acquisition [9]. The long median duration of hospitalisation prior to detection of VRE reflects the complex, composite morbidities of affected patients. Prolonged hospitalisation is a known risk-factor for VRE acquisition [10]. Eight of 17 patients (47%) received vancomycin during their hospital stay, which could have contributed to the selection of VRE [11]. Therefore, the importance of antibiotic stewardship has once more to be emphasised. Contamination of surfaces of affected wards was documented by the detection of enterocci in samples from several sites of the cardiac surgery intensive care unit and the ward. Environmental contamination is known to play an important role in transmission of VRE in the intensive care unit [10] and on wards [12]. Enterococci can survive on dry surfaces for up to 16 weeks [13]. Patients that are admitted to a room previously occupied by a VRE-colonised patient have an increased risk of VRE acquisition [14]. It was reported that healthcare workers have transmitted VRE after hand-contact with contaminated surfaces without direct contact to a colonised patient [15].
The minimal inhibitory concentration of vanB strains clusters around the breakpoint according to CLSI guidelines making detection more difficult than in vanA carrying strains [16]. A minor error rate of 12% has been described forvanB strains using disk diffusion [16]. Furthermore, the comparison of two commercially available selective agars for diagnosis of vanB revealed a sensitivity in the best performing agar of only 39% at 24 hours [17]. To the best of our knowledge no reports comparing diagnostic sensitivity of the same susceptibility testing method after 24 and ≥48 hours of incubation time have been published to date. If VRE screening during the present outbreak had been performed according to the current CLSI-guidelines that recommend 24 hours of incubation [4], two patients with vanB-mediated VRE would have been missed. Therefore, it was critical to prolong the incubation time on screening media to 48 hours. Being aware of the fact that prolonged reporting time could lead to postponing isolation measures, first reading and reporting of positive results was done at 24 hours. Because implementation of a longer incubation time was already started after identification of the third case, we were able to minimise problems in relation to diagnostic sensitivity.
Probably, a combination of several measures was crucial for containment of the spread of VRE [18]. We started regular prevalence surveys at an early stage of the outbreak. Without this intervention we would have missed 48% of all cases. Those cases missed likely would have contributed to further propagation of the outbreak as contact isolation would not have been implemented. Regular screening has been demonstrated to be successful for the control of an outbreak in France [19]. Because of the important role of hands in transmission of VRE, teaching of hand hygiene measures was an essential part of our teaching and intervention activities. Due to transient colonisation of VRE, there is no evidence for the usefulness of systematic screening of health care worker's hands [15]. Therefore, only a few hand-swabs among staff were done for didactic reasons. In our hospital there is no fixed link-nurse system in place. The appointment of link nurses on affected wards during this outbreak was an important measure to assure continuous communication between our infection control nurses and the cardiac surgery wards. Sufficient staff with an adequate knowledge and awareness-level is of importance: in a successfully contained outbreak in Western Australia, the number of infection control nurses was increased for this reason [18]. After eight weeks of weekly screening, no additional cases were detected during continued weekly screenings for five more weeks and one additional screening three months after the last detected case. The eight weeks duration of this outbreak was short compared to the duration of other published VRE-outbreaks, which lasted four [20], seven [18], 30 [21] or 36 months, respectively [19].
This outbreak does not enable us to infer on national VRE-epidemiology. But its complexity involving seven clonal strains could be an indication that also in Switzerland the incidence of VRE is on the rise. Although Swiss surveillance-data show an increase in VRE incidence in hospitalised patients from 1% in 2007 to 4.1% in 2010 (http://www.search.ifik.unibe.ch, accessed 13 December 2011), the implementation of screening on hospital admission, which has been propagated by some authors from high-prevalence countries [22, 23], is at present not justified at our institution. If screening for VRE is considered, rapid molecular methods for VRE-detection may be useful to reduce reporting time. In low prevalence areas as Zurich molecular methods could be applied to exclude VRE carriage as those methods reportedly show high negative predictive values [24]. However, the positive predictive value of rapid molecular methods remains low and makes cultural confirmation necessary [25]. This situation parallels that for MRSA in low prevalence areas. Using rapid PCR methods to exclude MRSA carriage combined with cultural confirmation in case of positive PCR results was suggested [26]. Such a strategy may also be useful to manage VRE surveillance.
The outbreak was successfully contained in a relatively short period due to early, consequent infection control measures, yet intense efforts were necessary to do so.
References
1 Balzereit-Scheuerlein F, Stephan R. Prevalence of colonisation and resistance patterns of vancomycin-resistant enterococci in healthy, non-hospitalised persons in Switzerland. Swiss Med Wkly. 2001;131:280–2.
2 National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–85.
3 Werner G, Coque TM, Hammerum AM, Hope R, Hryniewicz W, Johnson A, et al. Emergence and spread of vancomycin resistance among enterococci in Europe. Euro Surveill. 2008;13:1–11.
4 Performance standards for antimicrobial susceptibility testing; twentieth informational supplement (June 2010 Update)2010. Report No.: 1-56238-729-4
5 Chang CM, Wang LR, Lee HC, Lee NY, Wu CJ, Ko WC. Characterisation of vancomycin-resistant enterococci from hospitalised patients at a tertiary centre over a seven-year period. J Hosp Infect. 2010;74:377–84.
6 Fleisch F, Oechslin EC, Gujer AR, Ritzler E, Imhof A, Ruef C, et al. Transregional spread of a single clone of methicillin-resistant Staphylococcus aureus between groups of drug users in Switzerland. Infection. 2005;33:273–7.
7 Johnson PD, Ballard SA, Grabsch EA, Stinear TP, Seemann T, Young HL, et al. A sustained hospital outbreak of vancomycin-resistant Enterococcus faecium bacteremia due to emergence of vanB E. faecium sequence type 203. J Infect Dis. 2010;202:1278–86.
8 Ergani_Ozcan A, Naas T, Baysan BO, Ogunc D, Inan D, Colak D, et al. Nosocomial outbreak of vancomycin-resistant Enterococcus faecium in a paediatric unit at a Turkish university hospital. J Antimicrob Chemother. 2008;61:1003–9.
9 Klare I, Konstabel C, Mueller-Bertling S, Werner G, Strommenger B, Kettlitz C, et al. Spread of ampicillin/vancomycin-resistant Enterococcus faecium of the epidemic-virulent clonal complex-17 carrying the genes esp and hyl in German hospitals. Eur J Clin Microbiol Infect Dis. 2005;24:815–25.
10 Byers KE, Anglim AM, Anneski CJ, Germanson TP, Gold HS, Durbin LJ, et al. A hospital epidemic of vancomycin-resistant Enterococcus: risk factors and control. Infect Control Hosp Epidemiol. 2001;22:140–7.
11 Martinez JA, Ruthazer R, Hansjosten K, Barefoot L, Snydman DR. Role of environmental contamination as a risk factor for acquisition of vancomycin-resistant enterococci in patients treated in a medical intensive care unit. Arch Intern Med. 2003;163:1905–12.
12 Kolar M, Vagnerova I, Latal T, Urbanek K, Typovska H, Hubacek J, et al. The occurrence of vancomycin-resistant enterococci in hematological patients in relation to antibiotic use. New Microbiol. 2002;25:205–12.
13 Bonten MJ, Hayden MK, Nathan C, van Voorhis J, Matushek M, Slaughter S, et al. Epidemiology of colonisation of patients and environment with vancomycin-resistant enterococci. Lancet. 1996;348:1615–9.
14 Wendt C, Wiesenthal B, Dietz E, Ruden H. Survival of vancomycin-resistant and vancomycin-susceptible enterococci on dry surfaces. J Clin Microbiol. 1998;36:3734–6.
15 Huang SS, Datta R, Platt R. Risk of acquiring antibiotic-resistant bacteria from prior room occupants. Arch Intern Med. 2006;166:1945–51.
16 Vonberg RP, Chaberny IF, Kola A, Mattner F, Borgmann S, Dettenkofer M, et al. Prävention und Kontrolle der Ausbreitung von Vancomycin-resistenten Enterokokken. Anaesthesist. 2007;56:151–7.
17 Tenover FC, Swenson JM, O’Hara CM, Stocker SA. Ability of commercial and reference antimicrobial susceptibility testing methods to detect vancomycin resistance in enterococci. J Clin Microbiol. 1995;33:1524–7.
18 Grabsch EA, Ghaly-Derias S, Gao W, Howden BP. Comparative study of selective chromogenic (chromID VRE) and bile esculin agars for isolation and identification of vanB-containing vancomycin-resistant enterococci from feces and rectal swabs. J Clin Microbiol. 2008;46:4034–6.
19 Pearman JW. 2004 Lowbury Lecture: the Western Australian experience with vancomycin-resistant enterococci – from disaster to ongoing control. J Hosp Infect. 2006;63:14–26.
20 Aumeran C, Baud O, Lesens O, Delmas J, Souweine B, Traore O. Successful control of a hospital-wide vancomycin-resistant Enterococcus faecium outbreak in France. Eur J Clin Microbiol Infect Dis. 2008;27:1061–4.
21 Ergani-Ozcan A, Naas T, Baysan BO, Ogunc D, Inan D, Colak D, et al. Nosocomial outbreak of vancomycin-resistant Enterococcus faecium in a paediatric unit at a Turkish university hospital. J Antimicrob Chemother. 2008;61:1033–9.
22 Pereira GH, Muller PR, Zanella RC, de Jesus Castro Lima M, Torchio DS, Levin AS. Outbreak of vancomycin-resistant enterococci in a tertiary hospital: the lack of effect of measures directed mainly by surveillance cultures and differences in response between Enterococcus faecium and Enterococcus faecalis. Am J Infect Control. 2010;38:406–9.
23 Furuno JP, McGregor JC, Harris AD, Johnson JA, Johnson JK, Langenberg P, et al. Identifying groups at high risk for carriage of antibiotic-resistant bacteria. Arch Intern Med. 2006;166:580–5.
24 Marner ES, Wolk DM, Carr J, Hewitt C, Dominguez LL, Kovacs T, et al. Diagnostic accuracy of the Cepheid GeneXpert vanA/vanB ver. 1.0 to detect the vanA and van B vancomycin reistance genes in Enterococcus from perianal specimens. Diagn Microbiol Infect Dis. 2011;69:382–0.
25 Gazin M, Lammens C, Goossens H, Malhotra-Kumar S. Evaluation of GeneOhm VanR and Xpert vanA/van B molecular assays for the rapid detection of vancomycin-reistant enterococci. Eur J Clin Microbiol Infect Dis. 2011;Jun 12. [Epub ahead of print].
26 Hombach M, Pfyffer GE, Roos M, Lucke K. Detection of methicillin-resistant Staphylococcus aureus (MRSA) in specimens from various body sites: performance characteristics of the BD GeneOhm MRSA assay, the Xpert MRSA assay, and broth-enriched culture in an area with a low prevalence of MRSA infections. J Clin Microbiol. 2010;48:3882–7.
