Treatment of immune thrombocytopenia with intravenous immunoglobulin and insights for other diseases
DOI: https://doi.org/10.4414/smw.2012.13593
Summary
Since 1946 the development of fractionation and purification methods of human plasma led to biologic immunoglobulin for intravenous use (IVIG). In 1980 a child with refractory immune thrombocytopenia (ITP), bleeding and secondary hypogammaglobulinaemia due to long term immunosuppressive treatment got IVIG. His platelet counts dramatically increased. In 13 consecutive children with ITP, but without hypogammaglobulinaemia, similar rapid platelet increases were observed and confirmed in a controlled, multicentre study.
During the past three decades this biologic treatment modality evoked clinical and laboratory research on the mechanisms of action and in disorders with similar immune pathogenesis. It was recognised that IVIG modulates the disturbed immune response in multiple, synergistic ways between the different components of the immune system.
Beside other immune hematologic disorders other inflammatory and autoimmune diseases, mainly in the field of neurology and dermatology, IVIG showed beneficial effects. The worldwide consumption of IVIG increased from 300 kg per year in 1980 to 100 tonnes per year in 2010.
Due to the heterogeneity of immunopathological mechanisms of autoimmune diseases evidence based indications of IVIG remain rare and off label use high. Registries of large numbers of patients and first endpoints of defining less heterogenous subgroups in immune related disorders are the next steps toward establishment of evidence based IVIG indications.
Abbreviations
IVIG Intravenous immunoglobuline
ITP Immune thrombocytopenia
ISG Immune serum globulin
Introduction
Immune thrombocytopenia ITP is a rare bleeding disorder characterised by antibody or immune complex mediated platelet destruction and disturbed platelet production. The platelet counts are below 100x109/l. The degree of bleeding and the bleeding risk vary individually from none to life-threatening. Spontaneous, severe bleeding occurs in patients with less than 10x109/l. The duration of the disorders may be transient, persistent (3–12 months) or chronic (>12 months).
Until 1980 the classic management mainly consisted of treating bleeding episodes with corticosteroid treatments. In patients with refractory ITP, splenectomy, immunosuppressive or cytostatic treatments on a personalised medical basis were indicated.
The key observation of treatment of ITP with intravenous immunoglobulin G
At the end of the year 1979, at the University Children’s Hospital in Bern, we observed in a child with Wiskott-Aldrich Syndrome, a rare recessive x-linked disease with hypogammablobulinaemia-associated infections and thrombocytopenia, that intravenous immunoglobulin (IVIG) substitution increased his platelet counts. This observation provided the impetus to use IVIG in a 12-year-old boy with severe, refractory ITP who developed secondary hypogammaglobulinaemia and recurrent infections with lymphocytopenia after long term treatment with corticosteroids, vincristine, cyclophosphamide and other immunosuppressive drugs. In 1980 we administered the newly available IVIG. After the first infusion of 0,4 g /kg body weight, his platelet count increased within 24 hours and continued to increase upon administration of four additional, daily IVIG infusions (fig. 1, patient 1) [1].
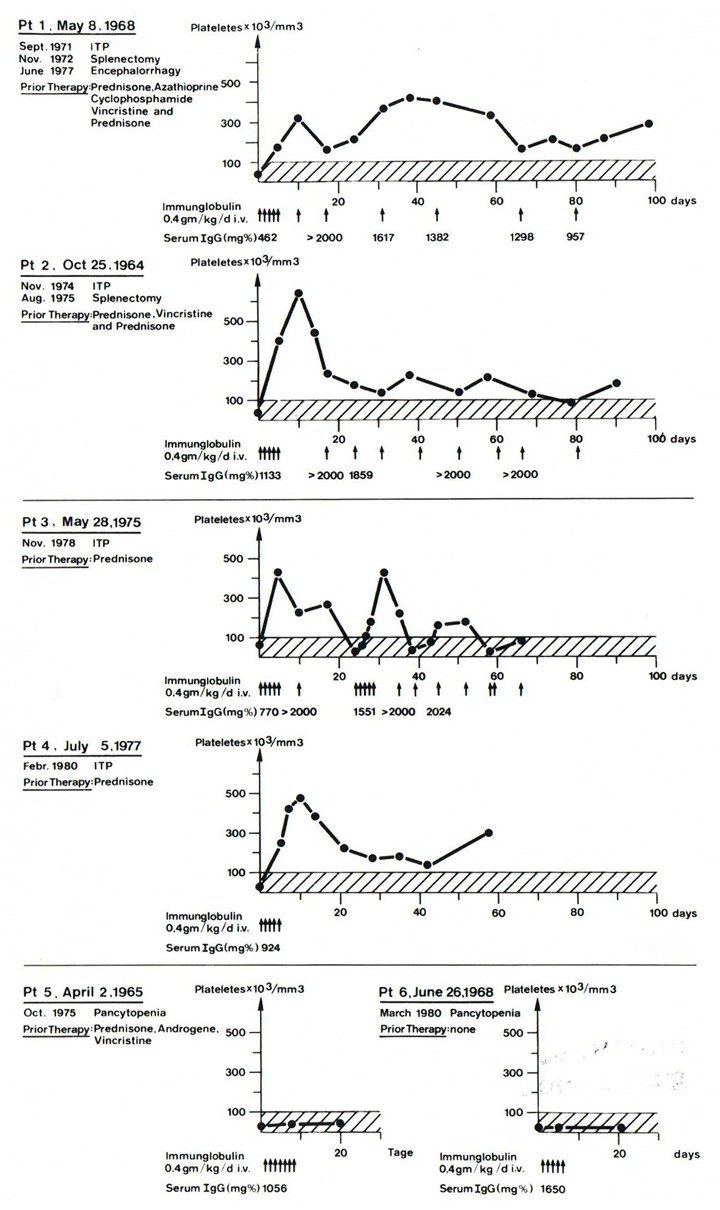
Figure 1
Pilotstudy 1 with controls. Response rate of IVIG during the first 80 days, 20 days respectively, of observation. Patient 1 is the key patient with ITP and secondary hypogammaglobulinaemia. Patients 2–4 with ITP, but with normal serum IgG levels. Patients 5 and 6 with aplastic anaemia and no response to IVIG.
Arrow means 0.4 g IVIG per kilogram body weight/day. Serum IgG in mg% (© Imbach P, Barandun S, Baumgartner C, Hirt A, Hofer F, Wagner HP. High‑dose intravenous immunoglobulin therapy of refractory, in particular idiopathic thrombocytopenia in childhood. Helv Paediat Acta. 1981;46:81–6. Reprinted with kind permission).
With this observation additional 3 children with ITP, but without hypogammaglobulinaemia, and 2 children with aplastic anaemia and low platelet counts received the same doses of IVIG as the first patient. Within 5–10 days the platelet counts of the children with ITP rose to 300–650x109/l and could be maintained at normal levels with one IVIG administration every 1–3 weeks. In the 2 children with aplastic anaemia no platelet count increase was observed whatsoever (fig. 1). Consequently a pilot study in 13 consecutive children with newly diagnosed or chronic ITP showed similar platelet increases (fig. 2) [2]. On this basis the first randomised multicenter study comparing corticosteroid and IVIG administration was initiated in children with ITP. The results confirmed that IVIG is an effective therapy for increasing platelet counts in ITP [3].
Retrospectively we found out, that a similar effect of gammaglobulin was observed before 1980. A child with ITP under corticosteroid treatment became infected with varicella in 1964 and received standard gammaglobulin intravenously as a treatment against varicella and showed an increase in platelet counts [4]. In 1978, two adult patients with agammaglobulinaemia developed hemolytic anaemia and thrombocytopenia – well known complications of agammaglobulinaemia – which subsided under intense substitutive treatment with standard gammaglobulin [5].
In 1982, confiramtion of this new IVIG treatment was published in adults with ITP, by Fehr et al. [6], in 1983, by Newland et al. [7], in 1984 in children and adults with ITP, by Bussel et al. [8]; and by many others, later on.
IVIG treatment of ITP evoked huge numbers of laboratory studies concerning the mechanisms of actions of IVIG and clinical trials in other inflammatory and autoimmune diseases with similar immune pathogenesis (see chapter below). As of today over 34,000 articles on IVIG treatments and its immunomodulatory effects are listed in Pubmed.
History of IVIG
In 1946, at Harvard Medical School, Cohn et al. [9] separated blood plasma or serum by 8% ethyl alcohol into protein and lipoprotein fractions. By further fractionation, in using a status of low pH, gammaglobulin could be extracted beside prothrombin, isoagglutinin, plasminogen and other components [10].
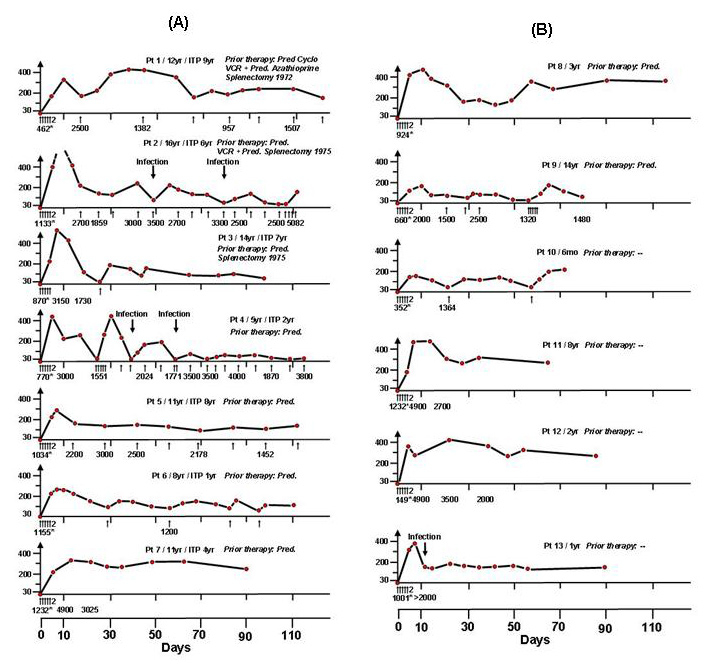
Figure 2
Pilotstudy 2:(A) 7 patients with chronic or intermittent ITP, (B) 6 patients with newly diagnosed ITP. All, consecutive patients with primary ITP demonstrated increases of platelet counts. Arrows indicate IVIG 0,4 g/kg body weight/day (© Imbach P, Barandun S, d’Apuzzo V, Baumgartner C, Hirt A, Morell A, et al. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet. 1981;1:1228–31. Reprinted with kind permission).
When the gammaglobulin, also named “immune serum globulin ISG”, was administered intravenously, aggregated IgG contained therein caused severe adverse reactions, whilst the subcutanteous administration route of the same preparation was well tolerated.
In 1962, Barandun et al. [11] identified the anticomplementary activities of IgG aggregates as the main cause of the side effects of ISG administration. The anticomplementary activitiy of the IgG aggregates could be reduced by enzymatic treatment of the purified immunoglobulin preparations using pepsin, trypsin, or plasmin digestion or chemical modification of gammaglobulin, all of which avoided aggregate formation. As a consequence of such modifications, however, the products were rapidly removed from the circulation by the reticulo endothelial system [12] and their intended complement-activating capacity once bound to the cognate antigen was impaired as were some other important biological functions.
The fortuitous observation of acidification by pH 4 at the Central Laboratory of the Swiss Red Cross, Bern, led to a reduction in anticomplementary effects in ISG without impairing the IgG functions [13, 14].
Other processes of purification of the IgG preparation on the basis of methods by Kistler and Nitschmann [15] led to the unmodified, mono- or dimmer immunoglobulin and was renamed “intravenous immunoglobulin, IVIG”.
Properties of IVIG
Intravenous immunoglobulin is extracted from pooled plasma from 10,000 to 60,000 blood or plasma donations. The final product of antibody concentrate encompasses several millions of antibody specificities, mainly natural antibodies and anti-idiotypic antibodies [16]. Antibody specificities contained in few contributing donations to the pool become diluted, common antibodies become enriched. The characteristics of the different IVIG products are summarised in ref. [17].
The safety of IVIG is controlled by an ongoing and careful selection and deferral of donors, and by testing and validation of donated blood, and plasma. During production of each IVIG lot different viral inactivation methods decrease the risk of transmission of infection [18, 19]. The last outbreak of hepatitis C caused by an IVIG product due to inappropriate virus inactivation dates back to 1993 [20]. An overview on the specific aspects of plasma-derived biologicals and their safety aspects are given in [12].
Adverse effects of IVIG occur in about 5% of individuals [21] and may be reduced by lowering the infusion rate. The symptoms mostly are benign, consisting of chills, headache, myalgia, nausea and fever. Acute aseptic meningitis after IVIG is infrequent and transient [22]. Thrombo-embolic events (i.e. stroke [23]) are associated with high osmolality of the solution and the serum viscosity of the recipient [24]. Each IVIG product has its own characteristics that may affect tolerability and adverse effects [12].
Possible mechanisms of action of IVIG
The mechanisms of action are still incompletely understood. While overviewing the nearly 500 articles listed in Pubmed on the mechanisms of action we may recognise that after IVIG administration synergistic changes of the different immune components (fig. 3) favourably impact on inflammatory and/or immunopathological backgrounds (for details see [25, 26]).
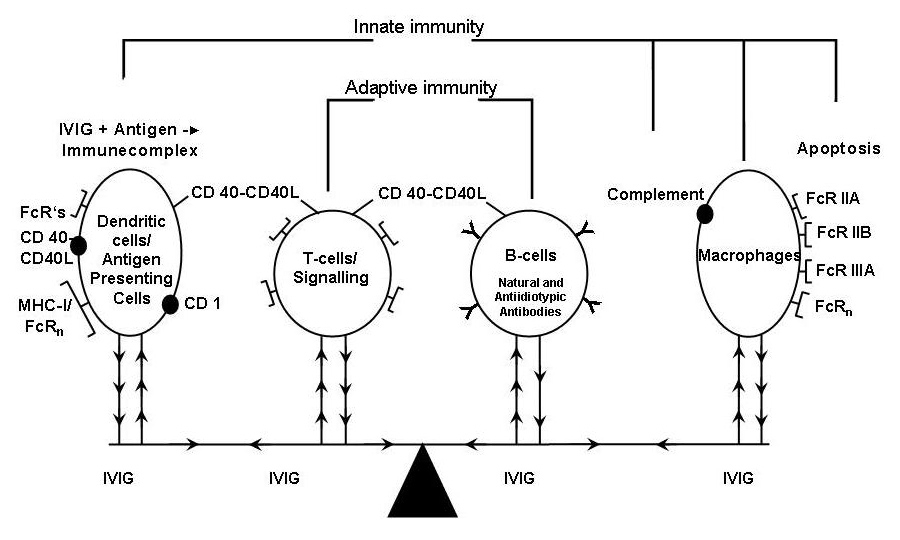
Figure 3
Synergistic (arrows) interactions after IVIG administration.
From left to right hand side: – Immune complex formation, activation of dendritic cells and their Fc receptors; – Antiplatelet T-cell reactivity, suppression of T cells, cytokine release and increase of T regulatory cells; – Downregulation of B-cells and anti-idiotypic antibodies; – Blockade of Fc receptors by IVIG; Complement binding/clearing Apoptosis and Fas inhibition (modified from © Imbach P, Lazarus A, Kühne T. Intravenous immunoglobulins induce potentially synergistic immunomodulations in autoimmune disorders. Vox Sanguinis. 2010;98:385–94. Reprinted with kind permission).
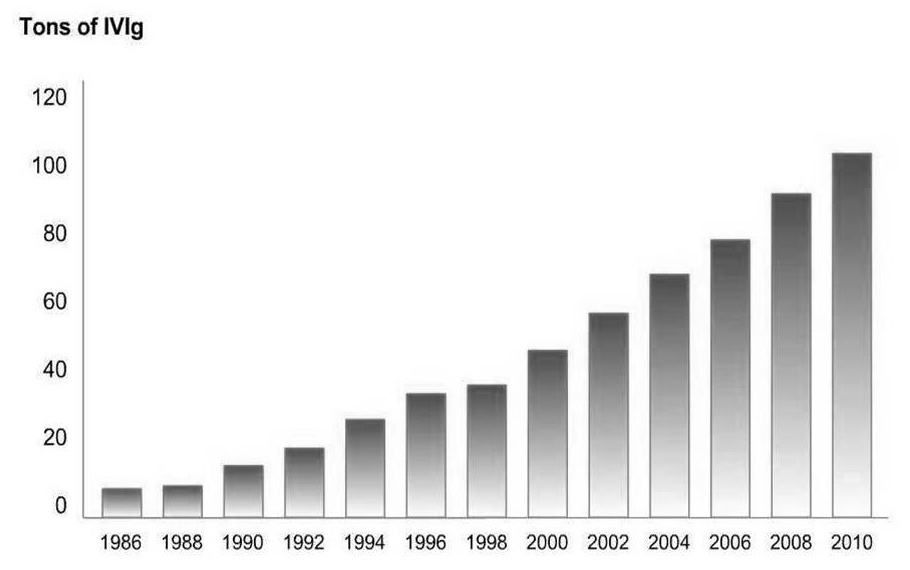
Figure 4
Total world consumption of IVIG: currently the IVIG production exceed 100 tonnes per year (published in International Blood/Plasma News (ibpn), ©The Marketing Research Bureau Inc., Orange CT 40677 (mrb_ibpn@earthlink.net). Reprinted with kind permission).
Blockade of Fc receptors by IVIG
Firstly Fehr et al. reported competitive downregulation of the Fc receptor mediated phagocytosis by IVIG using clearance analysis of autologous 99m labelled platelets and anti-Rhesus D sensitised erythrocytes in adults with ITP, who were treated with IVIG. He observed platelet increase and marked prolongation of clearance time of erythrocytes [6].
The intravenous administration of Fc-g fragments of IgG demonstrated similar increases of platelets counts and an increase of soluble Fc-g receptor III (sCD 16) in a study of eleven children with newly diagnosed ITP supporting the Fc-g blockade and other immunomodulatory effects of IVIG [27]. In an in vitro study IVIG inhibited interferon-g signalling in macrophages which is dependent on Fc-g receptor III – a well known activity receptor [28].
In 2001, Samuelson in Ravetch’s group, New York, demonstrated, that Fcgamma RIIB – the only inhibitory Fc-receptor on macrophages – are upregulated by IVIG, which resulted in downregulation of phagocytosis and no decrease of platelet counts in mice treated with antibodies against platelets [29].
Chang observed reversal of vaso-occlusive crisis in sickle cell mice after inhibition of neutrophil adhesion via FcR blocking by IVIG [30].
Anti-idiotypic antibodies and downregulation of B-cells
A patient with aquired FVIII autoantibody and severe bleeding and successful IVIG treatment from our hospital [31] has been discussed by Nydegger, working in the hematologic department for adults of our hospital, with Kazachkine and his group in Paris. They could demonstrate anti-idiotypic antibodies in IVIG against FVIII which may neutralise the specific autoantibody [32, 33]. The group could also detect other anti-idiotypic antibodies in IVIG (against thyroglobulin, DNA, peripheral nerve, acetylcholine receptor, endothelial cells and others [33]). Later on, Berchtold et al also could demonstrate anti-idiotypic antibodies against GPIIB/IIIA in ITP [34].
In 1991 Kondo et al described suppression of immunoglobulin production including pathologic autoantibodies by B cells as well as downregulation of antigen presenting cells via their Fc receptors after IVIG administration [35].
In addition IVIG impinge on the cytokine network, the anti-B cell activating factors of the TNF-family (BAFF) and the antiproliferation-inducing-ligand (APRIL). For example anti-BAFF antibodies in IVIG prevents the apoptotic effects on B-cells and the overproduction of BAFF and APRIL, which is over-expressed in autoimmune diseases and lymphoid malignancies [36].
Antiplatelet T-cell reactivity, suppression of T cells, cytokine release and increase of T regulatory cells
In 1991 Semple et al. in Toronto observed T-cell differences [37, 38] and in 1996 differences of cytokine levels between patients with acute and chronic ITP [39]. Kessel et al. recently discovered that IVIG increases T regulatory cells and their suppressive functions [40]. Ephrem et al. demonstrated prevention of experimental autoimmune encephalitis by IVIG on the basis of expansion of T regulatory cells [41].
Immune complex formation, activation of dendritic cells and their Fc receptors
In recent years Crow and Lazarus et al focused their research of the mechanisms of action of IVIG on the early stages of the immune response. IVIG forms dimers, multimers and possibly immune complexes with proteins, microorganisms, virus infected cells, pathogenic antigens and other foreign particles [42, 43], which activates dendritic cells (Nobel Prize in Medicine 2011 to the dendritic cell discoverer, Ralph Steinmann). In ITP such bindings directly prime dendritic cells [44]. They also play an integral role with T-cells and anti-inflammatory cytokines [45].
Neonatal Fc receptors FcRn – responsible for maintaining IgG levels in the fetus [46] – are competitively saturated by IVIG causing clearance of antibodies including pathogenic antibodies [47, 48].
In contrast IgG glycoforms may play a role in modulation antibody effector function in vivo. Glycosyliation by binding of glycan on IgG of its fragment with peptide-N-glycosidase or neuramidase results in blocking FcRn binding, unchanged serum half life of IgG and no anti-inflammatory activity [49, 50].
On the other hand suppression of autoantibodies induced inflammation by IVIG may be triggered by sialic acid Fc treated dendritic cells or macrophages, which induces IL-33 production (TH-2 cytokine) and IL-4 producing basophiles. IL-33 and IL-4 producing basophiles increase the expression of the inhibitory Fc-g receptor IIB. Thus, sialic Fc particles may stabilise the immune homeostasis [51].
Complement binding/clearing
Complement attenuation by IVIG have been reported in complement dependent autoimmune diseases by Lutz et al. [52] and by Basta et al. [53]. Platelets express an intrinsic capacity to interact with and trigger both classical and alternative pathways of complement [54].
Apoptosis and Fas inhibition
Viard showed that IVIG contains Fas antibodies which block Fas-Fas ligands and inhibits epidermal necrolysis [55].
ITP as model of IVIG treatment in other immune related disorders, in inflammatory and autoimmune disorders
On the basis of the synergistic mechanisms of action various immune related disorders with similar pathogenesis as ITP have clinically been studied using IVIG (table 1; for detailed information see ref. [56–59]).
As in ITP the heterogeneity of autoimmune disorders are based on variations from patient to patient, the age, the disease stage, the duration of the disease and from other factors. Therefore, not many controlled studies exist and the off-label uses of IVIG remain frequent.
The total world consumption of IVIG is still increasing (fig. 4). Before 1980 the indications of gammaglobulin/IVIG were primary and secondary immune deficiencies and severe infections only and the worldwide production was 300 kg – currently, the IVIG production exceeds 100 tonnes per year (fig. 4).
|
Table 1: IVIG use in other cytopenias, other inflammatory or autoimmune disorders, in neurologic and dermatologic, inflammatory and autoimmune disorders. Recommendations according to the various guidelines [59]. |
|
IVIG use in other cytopenias
|
Recommendation
|
| Fetal alloimmune thrombocytopenia |
First line |
| Neonatal alloimmune thrombocytopenia |
First or 2nd line |
| Childhood ITP with bleeding symptoms |
First or 2nd line |
| Adulthood ITP with bleeding symptoms |
First or 2nd line |
| Evans syndrome |
First or 2nd line |
| Autoimmune neutropenia |
First or 2nd line |
| HIV thrombocytopenia with bleeding |
Parallel to HIV medication |
| Posttransfusion purpura PTP |
First or 2nd line |
| Neonatal hemochromatosis in pregnant women |
First line |
| |
|
|
IVIG use in other disorders
|
|
| Kawasaki disease |
First line |
| Stem cells or kidney transplantation |
Parallel with other therapeutics |
| Polymyositis, dermatomyositis |
First or 2nd line |
| Autoimmune uveitis |
First or 2nd line |
| Systemic lupus erytematosus |
In some patients effective |
| Autoimmune liver disease |
In some patients effective |
| Severe rheumatoid arthritis |
In some patients effective |
| Graves ophthalmology |
In some patients effective |
| Asthma: high dose steroid dependent |
Steroid sparing effect |
| Recurrent spontaneous abortion |
First line |
| Alzheimer disease |
Studies ongoing, promising |
| Chronic fatigue syndrome |
Uncertain |
| |
|
|
IVIG use in neurology
|
|
| Guillain-Barré syndrome |
First line |
| Chronic, demyelinating polyneuropath CIDP |
First line |
| Multifocal motor neuropathy MMN |
First line |
| Myasthenia gravis |
2nd line |
| Stiff person syndrome |
2nd line |
| Lampert-Eaton myasthenic syndrome LEMS |
2nd line |
| Demyelinating polyneuropathies |
In some patient effective |
| Inclusion body myositis |
In some patient effective |
| Multiple sclerosis: relapsing-remitting form, postpartum period in women during breast feeding |
In some patient effective |
| |
|
|
Use of IVIG in dermatology
|
|
| Toxic epidermal necrolysis, Steven-Johnson syndrome |
First or 2nd line |
| Pemphigus: |
First or 2nd line |
| Vulgaris |
|
| Foliacus |
|
| Cicatricial |
First or 2nd line, steroid sparing effect |
| Chronic urticaria |
First or 2nd line |
New developments and research in ITP
As can be seen, ITP and the majority of chronic inflammatory and autoimmune disorders are burdened with complex immunopathological and clinical heterogeneities. In addition the innumerable antibody specificities in IVIG concentrate and their complex interactions with the immune system (fig. 3), all contribute to the moderate progress in understanding the management of immunologic diseases. Drawing up evidence based clinical indications is often difficult. Treatment recommendations, guidelines [60–62] and harmonisation of terminology [63] are, therefore, welcome for the prescribing physician.
In recent years the importance of pathologic platelet production due to autoantibodies against megakaryocytes in ITP [64] and the new possibility of stimulation of platelet production by thrombopoietin receptor agonists (for review see [65]) may challenge the progress of management – at least in patients with severe refractory, chronic ITP. The new treatment rationale is based on profound, controlled clinical studies of selected patient cohort, done by the pharmacologic industry. Thrombopoietin receptor agonists stimulate megakaryocytes and the platelet increase in peripheral blood circulation appears 5–8 days after beginning the treatment. However, as soon as the administration of thrombopoietin receptor agonists is withheld, the majority of patients drop their platelet counts to baseline levels. In patients with chronic ITP and continuous risk of bleedings the new treatment modality probably has to be given for undetermined duration. The questions of long term adverse effects (mainly thrombo-embolic events and bone marrow fibrosis) of thrombopoietin receptor agonists are not yet answered. Long term observations and registries of patients under thrombopoietin receptor agonist are requested. The new treatment possibility with its time lag of platelet increase may change the management strategy of prevention of bleeding in chronic ITP, while in patients with ITP and acute bleeding IVIG administration characterised by the rapid platelet increase will remain the indication for stopping bleeding.
In general, rare disorders demand prospective evidence based studies. Such studies are complex asking for various endpoints, need high number of patients and are expensive. As a first step in this direction we established the International Cooperative ITP Study (ICIS) group in 1997: An international network of physicians and scientists collaborate in performing prospective databases and studies (see http://www.itpbasel.ch ) that may elucitate the natural history of ITP and define less heterogeneous patient subgroups for controlled treatment and management trials. The first results of ICIS are promising after registration of more than 6000 patients from 70 centres worldwide (published articles see http://www.itpbasel.ch ).
The ongoing long term Pediatric and Adult Registry of Chronic ITP (PARC ITP) focuses on the mentioned endpoints. In addition genetics, co-morbidities, management and adverse effects of treatment will be studied. The intitial data comparison between more than 2000 adults and children of the PARC Registry demonstrated, that there are significant similarities between the two groups, i.e. concerning initial platelet counts [66]. This is in contrast to published data in the past.
Conclusion
Over three decades disease treatments using IVIG have opened the discussion of efficacious immune modulation in patients with inflammatory and autoimmune disorders which are characterised by wide ranging heterogeneity. The complex mechanisms of immunomodulation by IVIG provoke continuous clinical and laboratory research with the aim to find more evidence related indication for the biologic product. ITP was not only the first, but still remains a valuable model in this direction.
Acknowledgment: The author thanks Urs Nydegger, Bern, for his reading and corrections of this manuscript.
References
1 Imbach P, Barandun S, Baumgartner C, Hirt A, Hofer F, Wagner HP. High‑dose intravenous immunoglobulin therapy of refractory, in particular idiopathic thrombocytopenia in childhood. Helv Paediat Acta. 1981;46:81–6.
2 Imbach P, Barandun S, d’Apuzzo V, Baumgartner C, Hirt A, Morell A, et al. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet. 1981;1:1228–31.
3 Imbach P, Wagner HP, Berchtold W, Gaedicke G, Hirt A, Joller P, et al. Intravenous immunoglobulin versus oral corticosteroids in acute immune thrombocytopenic purpura in childhood. Lancet. 1985;2:464–8.
4 Gugler E. Die kindlichen Thrombopenien. In: Rossi E, ed. Päd Fortbildungskurse. Basel, Switzerland: Karger, 1964;11–12:143–58.
5 Barandun S, Imbach P, Morell A, Wagner HP. Traitement du purpura thrombocytopénique par des immunoglobulines. Méd et Hyg. 1982;40:1774–8.
6 Fehr J, Hofman V, Kappeler U. Transient reversal of thrombocytopenia in idiopathic thrombocytopenic purpura by high-dose intravenous gamma globulin. N Engl J Med. 1982;306:1254–8.
7 Newland AC, Treleaven JG, Minchinton RM, Waters AH. High-dose intravenous IgG in adults with autoimmune thrombocytopenia. Lancet. 1983;1(8316):84–7.
8 Bussel JB, Hilgartner M. The use of and mechanism of action of high dose intravenous immunoglobulin in the treatment of immune haematologic disease. Br J Haematol. 1984;56:l–7.
9 Cohn EJ, Strong LE, Hughes WL, et al. Preparation and properties of serum and plasma proteins; a system for the separation into fractions of the protein and lipoprotein components of biological tissues and fluids. J Am Chem Soc. 1946;68:459–75.
10 Oncley JL, Melin M, et al. The separation of the antibodies, isoagglutinins, prothrombin, plasminogen and beta1-lipoprotein into subfractions of human plasma. J Am Chem Soc. 1949;71(2):541–50.
11 Barandun S, Kistler P, Jeunet F, Isliker H. Intravenous administration of human gamma-globulin. Vox Sang. 1962;7:157–74.
12 Hooper JA. Intravenous immunoglobulins: evolution of commercial IVIG preparations. Immunol Allergy Clin North Am. 2008;28:765–78.
13 Haessig A. 50 Jahre Blutspendedienst des Schweizerischen Roten Kreuzes. Schweiz Med Wochenschrift. 1991;121:156.
14 Kistler P, Friedli H. Ethanol precipitation. In: Curling JM, ed. Methods of plasma protein fractionation. London: Academic Press 1980;3–16.
15 Kistler P, Nitschmann H. Large scale production of human plasma fractions. Eight years experience with the alcohol fractionation procedure of Nitschmann, Kistler and Lergier. Vox Sang. 1962;7:414–24.
16 Seite JF, Shoenfeld Y, Youninou P, Hillion S. What is the contents of the magic draft IVIg? Autoimmun Rev. 2008;7:435–9.
17 Sorensen R. Expert opinion regarding clinical and other outcome considerations in the formulary review of immune globulin. J Manag Care Pharm. 2007;13:278–83.
18 Carbone J. Adverse reactions and pathogen safety of intravenous immunoglobulin. Curr Drug Saf. 2007;2:9–18.
19 Schleis TG. The process: new methods of purification and viral safety. Pharmacotherapy. 2005;25(11 pt 2):73S–77S.
20 Bjoro K, Froland SS, Yun Z, Samdal HH, Haaland T. Hepatitis C infection in patients with primary hypogammaglobulinemia after treatment with contaminated immune globulin. N Engl J Med. 1994;331:1607–11.
21 Bonilla FA. Intravenous immunoglobulin: adverse reactions and management. J Allergy Clin Immunol. 2008;122:1238–39.
22 Ryan ME, Webster ML, Statler JD. Adverse effects of intravenous immunoglobulin therapy. Clin Pediatr. (Phila) 1996;35:23–31.
23 Caress JB, Cartwright MS, Donofrio PD, Peacock JE Jr. The clinical features of 16 cases of stroke associated with administration of IVIg. Neurology. 2003;60:1822–4.
24 Dalakas MC. High-dose intravenous immunoglobulin and serum viscosity: risk of precipitating thromboembolic events. Neurology. 1994;44:223–6.
25 Imbach P, Lazarus A, Kühne T. Intravenous immunoglobulins induce potentially synergistic immunomodulations in autoimmune disorders. Vox Sanguinis. 2010;98:385–94.
26 Negi VS, Elluru S, Siberil S, Graff-Dubois S, Mouthon L, Kazatchkine MD, et al. Intravenous immunoglobulin: an update on the clinical use and mechanisms of action. J Clin Immunol. 2007;27:233–45.
27 Debré M, Bonnet MC, Fridman WH, Carosella E, Philippe N, Reinert P, et al. Infusion of Fc gamma fragments for treatment of children with acute immune thrombocytopenic purpura. Lancet. 1993;342(8877):945–9.
28 Park-Min KH, Serbina NV, Yang W, Ma X, Krystal G, Neel BG, et al. FcgammaRIII-dependent inhibition of interferon-gamma responses mediates suppressive effects of intravenous immune globulin. Immunity. 2007;26(1):67–78.
29 Samuelsson A, Towers T, Ravetch J. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–6.
30 Chang J, Shi PA, Chiang EY, Frenette PS. Intravenous immunoglobulins reverse acute vaso-occlusive crises in sickle cell mice through rapid inhibition of neutrophil adhesion. Blood. 2008;111:915–23.
31 Gianella-Borradori A, Hirt A, Lüthy A, Wagner HP, Imbach P. Haemophilia due to factor VIII inhibitors in a patient suffering from an autoimmune disease. Treatment with intravenous immunoglobulin. A case report. Blut. 1984;48:403–7.
32 Sultan Y, Kazatchkine MD, Maisonneuve P, Nydegger UE. Anti-idiotypic suppression of autoantibodies to factor VIII (antihaemophilic factor) by high-dose intravenous gammaglobulin. Lancet. 1984;2(8406):765–8.
33 Rossi F, Kazatchkine MD. Antiidiotypes against autoantibodies in pooled normal human polyspecific Ig. J Immunol. 1989;143:4104–9.
34 Berchtold P, Date GL, Tani P, McMillan R. Inhibition of autoantibody binding to platelet glycoprotein Iib ⁄ IIIa by anti-idiotype antibodies in intravenous gammaglobulin. Blood. 1989;74:2414–7.
35 Kondo N, Ozawa T, Mushiake K, Motoyoshi F, Kameyama T, Kasahara K, et al. Suppression of immunoglobulin production of lymphocytes by intravenous immunoglobulin. J Clin Immunol. 1991;11:152–8.
36 Le Pottier L, Sapir T, Bendaoud B, Youinou P, Shoenfeld Y, Pers JO. Intravenous immunoglobulin and cytokines: focus on tumor necrosis factor family members BAFF and APRIL. Ann N Y Acad Sci. 2007;1110:426–32.
37 Semple JW, Freedman J. Increased antiplatelet T helper lymphocyte reactivity in patients with autoimmune thrombocytopenia. Blood. 1991;78(10):2619–25.
38 Semple JW, Bruce S, Freedman J. Suppressed natural killer cell activity in patients with chronic autoimmune thrombocytopenic purpura. Am J Hematol. 1991;37(4):258–62.
39 Semple JW, Milev Y, Cosgrave D, Mody M, Hornstein A, Blanchette V, et al. Differences in serum cytokine levels in acute and chronic autoimmune thrombocytopenic purpura; relationship to platelet phenotype and antiplatelet T-cell reactivity. Blood. 1996;87:4245–54.
40 Kessel A, Ammuri H, Peri R, Pavlotzky ER, Blank M, Shoenfeld Y, et al. Intravenous immunoglobulin therapy affects T regulatory cells by increasing their suppressive function. J Immunol. 2007;179:5571–5.
41 Ephrem A, Chamat S, Miquel C, Fisson S, Mouthon L, Caligiuri G, et al. Expansion of CD4+CD25+ regulatory T cells by intravenous immunoglobulin: a critical factor in controlling experimental autoimmune encephalomyelitis. Blood. 2008;111:715–22.
42 Teeling JL, Jansen-Hendriks T, Kuijpers TW, de Haas M, van de Winkel JG, Hack CE, et al. Therapeutic efficacy of intravenous immunoglobulin preparations depends on the immunoglobulin G dimmers: studies in experimental immune thrombocytopenia. Blood. 2001;98:1095–9.
43 Siragam V, Brine D, Crow AR, Song S, Freedman J, Lazarus AH. Can antibodies with specificity for soluble antigens mimic the therapeutic effects of intravenous IgG in the treatment of autoimmune disease? J Clin Invest. 2005;115:155–60.
44 Siragam V, Crow AR, Brine D, Song S, Freedman J, Lazarus AH. Intravenous immunoglobulin ameliorates ITP via activating Fcgamma receptors on dendritic cells. Nat Med. 2006;12:688–92.
45 Bayry J, Lacroix-Desmazes S, Carbonneil C, Misra N, Donkova V, Pashov A, et al. Inhibition of maturation and function of dendritic cells by intravenous immunoglobulin. Blood. 2003;101:758–65.
46 Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol. 2008;26:513–33. Review.
47 Hansen RJ, Balthasar JP. Intravenous immunoglobulin mediates an increase in anti-platelet antibody clearance via the FcRn receptor. Thromb Haemost. 2002;88:898–9.
48 Li N, Zhao M, Hilario-Vargas J, Prisayanh P, Warren S, Diaz LA, et al. Complete FcRn dependence for intravenous Ig therapy in autoimmune skin blistering disease. J Clin Invest. 2005;115:3440–50.
49 Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, et al. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem. 2001;276(9):6591–604.
50 Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313(5787):670–3.
51 Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475(7354):110–3.
52 Lutz HU, Stammler P, Bianchi V, Trüeb RM, Hunziker T, Burger R, et al. Intravenously applied IgG stimulates complement attenuation in a complement-dependent autoimmune disease at the amplifying C3 convertase level. Blood. 2004;103:465–72.
53 Basta M, Van Goor F, Luccioli S, Billings EM, Vortmeyer AO, Baranyi L, et al, Metcalfe DD. F(ab)’2-mediated neutralization of C3a and C5a anaphylatoxins: a novel effector function of immunoglobulins. Nat Med. 2003;9:431–48.
54 Peerschke EI, Yin W, Ghebrehiwet B. Complement activation on platelets: implications for vascular inflammation and thrombosis. Mol Immunol. 2010;47(13):2170–5.
55 Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, et al. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science. 1998;282:490–3.
56 Lemieux R, Bazin R, Ne’ron S. Therapeutic intravenous immunoglobulins. Mol Immunol. 2005;42:839–48. Review.
57 Hartung HP. Advances in the understanding of the mechanism of action of IVIg. J Neurol. 2008;255(Suppl 3):3–6. Review.
58 Dalakas, et al. Clinical Use of IVIG in Neurology in: Immunoglobulin Therapy. A.H. Lazarus, J.W. Semple eds. 2010, AABB Press. Pages 80–95.
59 Jolles S, Hughes J. Use of IGIV in the treatment of atopic dermatitis, urticaria, scleromyxedema, pyoderma gangrenosum, psoriasis, and pretibial myxedema. Int Immunopharmacol. 2006;6:579–91. Review.
60 George JN, Woolf SH, Raskob GE, Wasser JS, Aledort LM, Ballem PJ, et al. Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American Society of Hematology. Blood. 1996;88(1):3–40. Review.
61 Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168–86. Epub 2009 Oct 21. Review.
62 Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA; American Society of Hematology. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–207.
63 Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–93.
64 McMillan R, Wang L, Tomer A, Nichol J, Pistillo J. Suppression of in vitro megakaryocyte production by antiplatelet autoantibodies from adult patients with chronic ITP. Blood. 2004;103(4):1364–9.
65 Imbach P, Crowther M. Thrombopoietin-receptor agonists for primary immune thrombocytopenia. N Engl J Med. 2011;365(8):734–41.
66 Kuhne T, Berchtold W, Michaels LA, Wu R, Donato H, Espina B, et al. Newly diagnosed immune thrombocytopenia in children and adults, a comparative prospective observational registry of the Intercontinental Cooperative Immune Thrombocytopenia Study Group. Haematologica. 2011;96(12):1831–7.



