Use of abatacept in rheumatoid arthritis
DOI: https://doi.org/10.4414/smw.2012.13581
Johannes
von Kempis, Jean
Dudler, Paul
Hasler, Diego
Kyburz, Alan
Tyndall, Pascal
Zufferey, Peter M.
Villiger
Summary
Abatacept (CTLA-Ig), a modulator of T-lymphocyte activation, has been approved by the Swiss health regulatory agency Swissmedic for the treatment of active rheumatoid arthritis (RA). This article summarises the key trial findings for this biologic agent in RA in different situations such as early erosive rheumatoid arthritis (RA), biologic-naïve RA, RA before and after the use of methotrexate or TNF-inhibitors and includes safety information from these trials. Based on these data, recommendations for clinical practice in Switzerland are made by a panel of experts.
Recommendations based on current evidence
Introduction
Abatacept was approved by the Swiss health regulatory agency Swissmedic in August 2007 and is reimbursed by health insurance plans for the treatment of active rheumatoid arthritis (RA) “not sufficiently responsive to disease modifying antirheumatic drugs (DMARD) such as methotrexate (MTX) or TNF inhibitors”. Abatacept may be used as a first line biologic agent in Switzerland.
Experts from leading Swiss rheumatological centres have convened for a joint analysis of the relevant, including the most recent, published phase 2 and 3 as well as open extension trials.
This article summarises the key trial findings for abatacept in RA in different situations such as early erosive RA, biologic-naïve RA, RA before and after the use of MTX or TNF inhibitors and includes safety information from these trials.
The article closes with the experts’ conclusions and recommendations, based on the clinical data presented and the experts’ experience with abatacept in clinical practice.
Mode of action of abatacept
Abatacept’s mode of action is distinct from all other available DMARDs and biologics [1]. The molecule is a human protein designed to selectively inhibit T-cell activation, a process that plays a central role in the immunopathogenesis of RA [2]. Subsequent to antigen recognition via the major histocompatibility complex on antigen-presenting cells by the T-cell receptor [3, 4], T-cells require a second, so-called co-stimulatory signal for full activation (fig. 1). Abatacept, as a humanised CTLA-4-immunoglublin-Fc (CTLA-4-Ig) fusion protein, selectively modulates T-cell co-stimulation. It prevents activation of T-cells by binding to the natural ligands CD 80 and CD86. In consequence, CD80 and CD86 cannot interact with CD28 on the T lymphocyte. As an important indirect effect within the inflammatory cascade, the production of cytokines and autoantibodies is inhibited (fig. 1) [2]. Studies confirmed the broad influence of abatacept on both serum and synovial biomarkers, with reductions in the levels of IFNγ, TNF-α, IL-6, matrix metalloproteinase (MMP)-1 and -2, RF and other cytokines [5, 6].
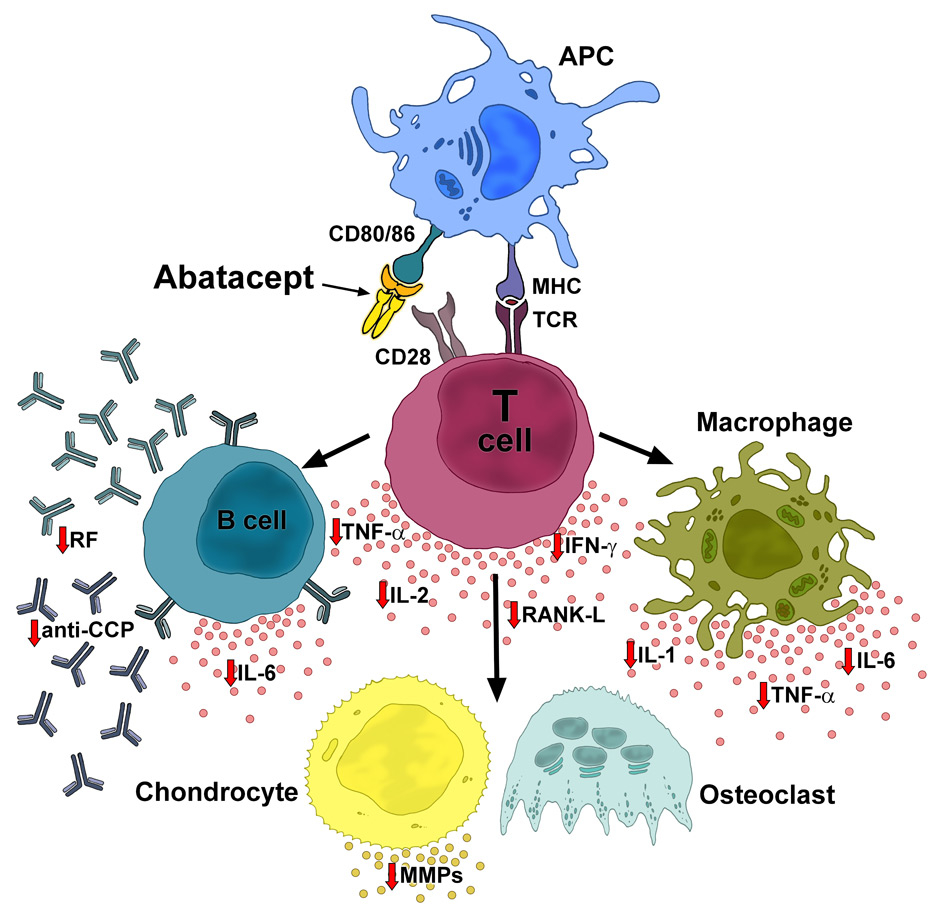
Figure 1
Mode of action of abatacept. By binding to CD80/86, abatacept blocks the interaction of CD80/86 and CD28 on the APC and T cell, respectively. The co-stimulatory pathway leading to T-cell activation is inhibited and processes in the inflammatory cascade are downregulated, resulting in normalized levels of cytokines and autoantibodies, and inhibition of osteoclast activity. APC: antigen presenting cell (e.g., macrophages, dendritic cells, B cells); MHC: major histocompatibility complex; TCR: T-cell receptor; RF: rheumatoid factor; TNF: tumour necrosis factor; IFN: interferon; IL: interleukin; anti-CCP: anti-cyclic citrullinated peptide; RANK: receptor activator of NF-κB; MMPs: matrix metalloproteinases.
Abatacept in early erosive RA with poor prognostic factors
The AGREE trial evaluated the efficacy and safety of abatacept in MTX-naive patients with early RA (mean disease duration 6 to 7 months) and poor prognostic factors [7]. A total of 509 patients were randomised 1:1 to receive abatacept plus MTX or placebo plus MTX for 1 year. Mean DAS28 (CRP) was 6.3 at study entry and radiographic evidence of joint destruction was present in all patients. All patients were RF and/or anti-CCP positive. At year 1, disease activity as well as damage was significantly lower in the patients with active treatment.
The overall drug retention rate was 94.3% in the second year [8]. This value is extraordinarily high. It could be explained in part if a high percentage of patients were recruited in countries with limited access to other biologic agents. 91.1% of patients on abatacept plus MTX who showed no radiographic progression at the end of year 1 remained non-progressors at the end of year 2. Similar to studies with TNF inhibitors, the positive effects of abatacept on the inhibition of radiographic progression was independent of disease activity or physical functions [9].
Abatacept in biologic-naïve patients
Abatacept in combination with MTX in biologic-naïve patients with active RA and an inadequate response to MTX has been assessed in one phase IIb study and two large phase III studies.
In the AIM trial, the clinical response after 6 months was significantly higher with abatacept as compared to placebo, and the ACR response rates continued to improve up to 12 months only in the abatacept arm [10, 11]. Over 90% of the patients receiving abatacept plus MTX who achieved moderate disease activity after 3 months had a sustained or even improved outcome regarding low disease activity (LDAS) or DAS28 (ESR)-defined remission at 6 and 12 months.
In the ATTEST study, patients with an inadequate response to MTX continued taking MTX and additionally received abatacept, infliximab, or placebo [12]. After 6 months of treatment, abatacept plus MTX significantly reduced the signs and symptoms of RA compared with placebo plus MTX. Similar results were seen in the infliximab plus MTX group. However, the proportions of patients achieving good EULAR response, LDAS, and DAS28 (ESR)-defined remission continued to increase with abatacept plus MTX, whereas the response to infliximab-based treatment stabilised. After 12 months, a greater reduction in DAS28 (ESR) was observed with abatacept plus MTX than with infliximab plus MTX (–2.88 vs. –2.25).
Radiographic progression
In the AIM trial, radiographic data were available for 92% of patients [10]. At the end of year 1, the slowing of joint destruction was significantly greater with abatacept plus MTX as compared to placebo plus MTX. The analysis of radiographic data from AIM over 5 years indicates that abatacept plus MTX may have an increasing beneficial effect on structural damage over time in the majority of initially responding patients [13]. Importantly, almost half of the patients treated with abatacept in the study remained free of progression over 5 years (change in radiographic total score of ≤0 vs. baseline). The chance of remaining free of progression within one more year increased with the length of the previous period of non-progression.
Abatacept in patients failing prior anti-TNF therapy
Several trials investigated abatacept in patients without prior therapy with other biologics.
As expected, therapy with abatacept plus MTX resulted in significantly greater improvements in ACR response and DAS28 remission rates than MTX alone [14]. As shown in the double-blind period of the ATTAIN trial in patients with long-standing disease and failure of at least one previous anti-TNF therapy, therapy with abatacept plus MTX resulted in significantly greater improvements in ACR response and DAS28 remission rates than MTX alone [15]. After completing the 6-month double-blind period, patients entering an open-label period continued to improve in disease status over the subsequent 4 years. The efficacy of abatacept after anti-TNF failure was also investigated in the open-label long-term extension of the ATTEST trial [16]. Of the 27 patients who were in high disease activity (HDAS) after 1 year of infliximab treatment, 81.5% showed moderate (MDAS) or low (LDAS) disease activity or were in remission 1 year after initiation of treatment with abatacept.
In the ARRIVE trial, the safety, tolerability and efficacy of switching directly from anti-TNF to abatacept was compared to a scheme with a washout period of anti-TNF [17]. In the washout group, anti-TNF treatment was stopped at least 2 months prior to abatacept treatment, while patients in the direct switch group received the first abatacept dose at the next scheduled anti-TNF therapy dose. The outcome in terms of safety, tolerability, and efficacy was comparable with or without a washout, thus supporting a direct switch to abatacept after anti-TNF treatment as an option in clinical practice.
Onset of action
In the AGREE trial, Durez and colleagues evaluated the time course of improvement in ACR core components with abatacept plus MTX or MTX alone over 12 months in patients with early RA and poor prognostic factors [18]. The median duration to achieve 50% improvement in individual ACR core components was 1 to 4 months in the abatacept plus MTX group (fig. 2). CRP levels, joint counts and physician’s assessment of disease activity were the first ACR core components to demonstrate meaningful improvements within 2 months.
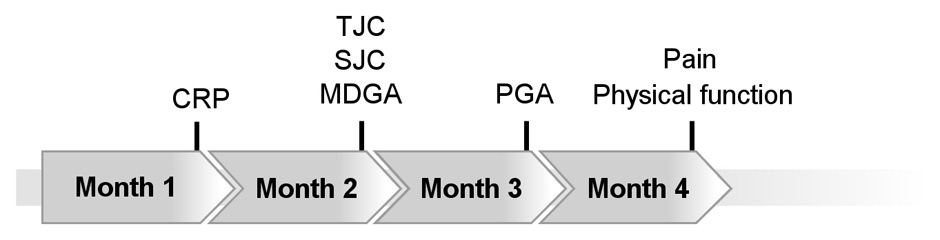
Figure 2
Median time to at least 50% improvement in individual ACR core components (LOCF). Figure based on data of the AGREE study as reported by Durez et al. [18]. CRP: C-reactive protein; TJC: tender joint count; SJC: swollen joint count; MDGA: physician’s global assessment; PGA: patient’s global assessment.
Predictability of clinical outcome based on evaluation at 3 months
Westhovens and colleagues investigated the predictive value of achieving a particular disease state with abatacept after 3 months with respect to the outcome after 1 year [19]. Most patients achieving remission at the end of month 3 maintained this state at the end of month 12 (89.7%). Two-thirds of the patients with a good response to treatment at month 3 (LDAS) achieved remission at month 12 (67.9%). Of the patients with a moderate response to treatment at month 3 (MDAS), 61.4% achieved LDAS or remission at month 12.
Maintenance therapy with abatacept
Westhovens and colleagues examined the sustained reduction of disease activity, inhibition of X-ray progression and improvements in physical function and health-related quality of life (HRQoL) in patients treated with abatacept [20]. The analysis was based on 378 patients entering the long-term extension phase of the AIM trial after completing the first year of double-blind treatment. The proportion of patients with low disease activity at the end of year 1 and sustaining it from year 1 to year 5 was 71.2%, while 60.3% of patients in remission at the end of year 1 remained in remission also from year 1 to year 5. Similar results were obtained for HAQ-DI normalisation rates and radiographic non-progression. Overall, approximately two-thirds of patients maintained clinical remission, radiographic non-progression, and normalisation of physical function and HRQoL over 5 years of treatment. The authors concluded that abatacept can provide sustained disease modification and restore normal physical function in patients with RA.
Safety of abatacept
Serious adverse events
Data from eight clinical trials comprising 4,149 patients with 12,132 patient-years of exposure up to 7 years demonstrate abatacept’s good tolerability [21]. The types and the frequency of safety events were consistent between short term and long term treatment periods, indicating that the safety profile of abatacept remains stable. The incidence of serious adverse events was comparable to that of placebo. This is in line with a recent Cochrane review which reports a significantly lower risk of serious adverse events with abatacept than with most other biologics [22].
Severe and opportunistic infections
Smitten and colleagues evaluated the risk of serious infections (opportunistic infections included) in 4,149 RA patients enrolled in abatacept clinical trials with a total of 11,658 patient-years of exposure [23]. The incidence rates (IRs) of serious infection and hospitalised infection were 2.87 and 2.64/100 patient-years respectively. The most common serious infections were pneumonia, urinary tract infection and cellulitis. There were few opportunistic infections with seven cases of tuberculosis. The frequency of serious infections remained relatively stable over time.
Malignancies
In the same cohort of patients the IRs (per 100 patient-years) of total malignancies, non-melanoma skin cancer (NMSC), solid tumours, lung cancer and lymphoma were 1.41, 0.74, 0.57, 0.15 and 0.07/100 patient-years respectively [23]. On the basis that the expected incidence rates for certain malignancies such as lung cancer and lymphoma are generally higher in RA patients, Smitten et al. conclude from their comparison with data derived from normal populations and cohorts of RA patients not treated with biologics that the reported malignancy rates were not higher in patients treated with abatacept. Hochberg and colleagues analysed the data of a total of 12,132 patient-years of exposure and reported similar results [21].
Acute infusion events
In the long-term safety analysis, Hochberg and colleagues assessed the IRs of acute infusion reactions with abatacept in 3,755 patients with a total exposure of roughly 9,300 patient-years [21]. The IRs of acute infusion events in the short term (6–12 months) were comparable between abatacept (11.6%) and placebo (9.8%). The proportion of patients experiencing infusion events decreased considerably in year 2 (2.01%) and continued to decrease steadily over time.
Combination with anti-TNFα
Weinblatt and colleagues investigated the efficacy and safety of abatacept in combination with etanercept in 121 patients with active RA during a 1-year randomised, placebo controlled, double-blind phase, followed by an open-label long-term extension lasting 2 years, entered by 80 patients [24]. The combination of abatacept and etanercept was associated with a significantly increased risk for serious adverse events at 1 year compared to placebo and etanercept, while the clinical benefit was limited. Based on this trial, the manufacturer, Swissmedic and EMA recommend not combining abatacept with an anti-TNF agent.
Vaccinations
A study in healthy volunteers suggested that abatacept may blunt immune responses to vaccination [25]. In a sub-study of the ARRIVE trial, Schiff and colleagues evaluated the immune response to influenza immunisation in 20 abatacept patients with active RA [26]. They were vaccinated 7 days prior to an abatacept dose with the current WHO trivalent influenza vaccine. A total of 55, 50 and 35% patients mounted a response to the H1N1, H3N2 and influenza B strains respectively. Ribeiro and colleagues investigated the humoral response to H1N1 influenza vaccination in 11 RA patients treated with abatacept plus MTX and in matching controls (33 RA patients treated with MTX and 33 healthy controls) [27]. Only 9% of patients treated with abatacept plus MTX achieved seroprotection as compared to 58% of patients with MTX alone (19/33) and 70% of healthy subjects (23/33). Schiff and colleagues investigated the response to pneumococcal vaccine in 21 RA patients treated with abatacept in a sub-study of the ARRIVE trial [28]. 81% of patients mounted an immune response to at least one serotype.
In summary, abatacept appears to significantly suppress vaccination responses, particularly to influenza antigens. Data from patient registries will be necessary to define the protective effect of influenza vaccination during treatment with abatacept.
Surgical interventions
Practical concerns revolve mainly round intra- or postoperative infections and wound healing. Theoretically, abatacept may contribute to both. However, there have been no reports on postoperative infections or on delayed wound healing so far. As controlled studies do not specifically address such questions, patient registries will need to fill these gaps.
Pregnancy
To date there are no data on the effect of abatacept on human pregnancy. It should be borne in mind that abatacept crosses the placenta. In animal studies, no teratogenic effects have been registered in doses up to 20fold the doses used in humans. The manufacturer advises against pregnancy during therapy and recommends discontinuation of abatacept at least 10 weeks before a planned pregnancy.
Anti-nuclear and anti-DNA antibodies
Schiff and colleagues investigated the incidence of anti-nuclear (ANA) and anti-DNA antibody seropositivity and immunogenicity in RA patients treated with abatacept [29]. The proportion of patients with active treatment who developed ANA and anti-DNA antibodies was 3–5 times lower as compared to those treated with placebo. Interestingly, no antibodies against abatacept were observed during this period.
Application mode and frequency
Abatacept is given intravenously at a dosage of approximately 10 mg/kg (<60 kg: 500 mg, >60 kg: 750 mg, >100 kg: 1000 mg). It is solubilised in 10 ml of aqua ad iniectabilia and then dispersed in 90 mL isotonic NaCl. Infusions last 30 minutes. The second and third infusions are scheduled at biweekly, all following administrations at monthly intervals.
|
Table 1: Overview of clinical abatacept trials referred to in this publication. |
|
Clinical Phase
|
Study
|
Study duration and design
|
Patient population
|
Patients treated
|
| IIb |
IM101-100
|
12 months, double-blind |
MTX inadequate responders |
339 |
| III |
AIM
|
12 months, double-blind |
MTX inadequate responders |
638 |
|
ATTEST
|
12 months, double-blind |
MTX inadequate responders |
431 |
|
ATTAIN
|
6 months, double-blind |
Anti-TNF inadequate responders |
391 |
|
ASSURE
|
12 months, double-blind |
DMARD inadequate responders |
1441 |
|
ARRIVE
|
6 months, open-label |
Anti-TNF inadequate responders |
1285 |
| IIIb |
AGREE
|
12 months, double-blind |
MTX-naive patients with poor prognostic factors |
509 |
Conclusions and recommendations
Efficacy and safety
Abatacept is effective in MTX-naïve patients and in patients with inadequate response to non-biologic DMARDs or to TNF inhibitors. The efficacy of abatacept is comparable to other biologic agents. While TNF inhibitors are still widely used and recommended as first choice biologics in the treatment of RA [30], the available data may support the use of abatacept as a first line biologic agent. From a clinical perspective, the favourable safety profile of abatacept, in particular regarding serious infections, is of interest. However, it has to borne in mind that rare side effects, e.g. effects on malignancies, call for long-term data from patient registries. Currently, we recommend the use of abatacept in combination with MTX or – in the case of MTX side effects or intolerance – in combination with another conventional DMARD, as there is not sufficient data to support the use of abatacept in monotherapy.
Onset of action
Disease activity parameters such as CRP or swollen/tender joints may start to improve within two months after initiation of abatacept treatment in patients with early RA. However, clinical effectiveness should not be evaluated before 3 months of treatment.
Abatacept as first-line biologic
Treatment should be stopped if the patient remains active (HDAS, i.e. DAS >5.1) at the end of 3 months of treatment, unless there is evidence of important improvement, e.g., a drop in activity of at least 20% in DAS28. In the event of an intercurrent problem such as surgery or an infection, leads to interruption of therapy, the time period of 3 months should be extended accordingly. On the other hand, if the patients have achieved low disease activity (LDAS), the chance of achieving remission in the coming months is good (67.9%) and treatment should be continued. The same applies to patients with moderate disease activity after 3 months, given the likelihood of achieving low disease activity or remission of 61.4%. However, response should be monitored every 3 months, and therapy should be stopped in the absence of further improvement.
Abatacept as second or third-line biologic
When used after previous failure of other biologics, the treatment should be continued after 3 months even if the patient is still in high disease activity. In this case a treatment assessment and decision should be made after 6 months.
Glucocorticoids
Glucocorticoids should be introduced or increased in dosage to bridge the time to a clinically meaningful effect of abatacept. We also recommend intra-articular injections into joints with persistent arthritis during treatment with abatacept.
Surgical interventions
The interval between the last infusion of abatacept and the scheduled surgery must be decided on an individual basis. A host of factors may influence the chosen interval, including activity of joint disease, concomitant immunosuppression (e.g. with glucocorticoid therapy), diabetes mellitus, history of infections, type of surgical procedure and so forth. In uncomplicated cases with the RA in remission, we recommend planning surgery at the point in time of the following infusion and delaying the continuation of abatacept therapy until wound healing is complete.
Pregnancy
Due to the lack of data, no statement on the compatibility of abatacept and pregnancy can be made. It should also be borne in mind that abatacept crosses the placenta and will accumulate in the foetus if administered during pregnancy. The manufacturer advises against pregnancy during therapy and recommends discontinuation of abatacept at least 10 weeks before a planned pregnancy.
Acknowledgements: The authors would like to thank Frauke Förger, MD, for revising the chapter on pregnancy and Thomas Handschin, MD, for his editorial assistance.
References
1 Choy EH. Selective modulation of T-cell co-stimulation: a novel mode of action for the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2009;27(3):510–8.
2 Orencia® (Abatacept): Swiss prescribing information for physicians.
3 Pettit AR, Thomas R. Dendritic cells: the driving force behind autoimmunity in rheumatoid arthritis? Immunol Cell Biol. 1999;77(5):420–7.
4 Janeway C. Immunobiology: The immune system in health and disease. 6th ed, New York: Garland Science Publishing. 2005:319–65; 557–612.
5 Buch MH, Boyle DL, Rosengren S, Saleem B, Reece RJ, Rhodes LA, et al. Mode of action of abatacept in rheumatoid arthritis patients having failed tumour necrosis factor blockade: a histological, gene expression and dynamic magnetic resonance imaging pilot study. Ann Rheum Dis. 2009;68(7):1220–7.
6 Weisman MH, Durez P, Hallegua D, Aranda R, Becker JC, Nuamah I, et al. Reduction of inflammatory biomarker response by abatacept in treatment of rheumatoid arthritis. J Rheumatol. 2006;33(11):2162–6.
7 Westhovens R, Robles M, Ximenes AC, Nayiager S, Wollenhaupt J, Durez P, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis. 2009;68(12):1870–7.
8 Westhovens R, Durez P, Genant H, Robles M, Becker JC, Covucci A, et al. Disease remission, normalized physical function and radiographic non-progression are achieved by the majority of patients with early rheumatoid arthritis treated with abatacept + methotrexate: results from the 2-year AGREE trial. Poster SAT0178, EULAR Annual Congress, June 16–19, 2010.
9 Keystone E, Westhovens R, Moniz Reed D, Covucci A, Wells A. Radiographic progression correlates well with patient-reported RAPID3 disease activity levels in methotrexate (MTX).naive patients with early rheumatoid arthritis (RA): insights from the AGREE study. Arthritis & Rheumatism. 2010;62(10 Suppl):S470.
10 Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud-Mendoza C, et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2006;144:865–76.
11 Dougados M, Kremer JM, Le Bars M, Reed D, Park S, Vatsanos G, et al. Abatacept improves disease activity status over time in patients with rheumatoid arthritis and an inadequate response to methotrexate [abstract]. Ann Rheum Dis. 2009;68:573.
12 Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S, et al. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis. 2008;67:1096–103.
13 Genant HK, Peterfy C, Westhovens R, Becker JC, Vratsanos G, Zhou X, et al. Abatacept increases the proportion of patients who remain free from structural damage progression through 5 years in methotrexate inadequate responders with rheumatoid arthritis. Poster FRI0253, EULAR Annual Congress, June 10–13, 2009.
14 Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353(11):1114–23.
15 Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353(11):1114–23.
16 Schiff M, Keiserman MW, Moniz Reed D, Le Bars M, Becker J-C, Zhao C, et al. An increasing proportion of patients achieve a low disease activity state or remission when switched from infliximab to abatacept regardless of initial infliximab treatment response: results from the ATTEST trial. Poster 1659 (392), ACR/ARHP Annual Scientific Meeting, October 16–21, 2009.
17 Schiff M, Pritchard C, Huffstutter JE, Rodriguez-Valverde V, Durez P, Zhou X, et al. The 6-month safety and efficacy of abatacept in patients with rheumatoid arthritis who underwent a washout after anti-tumour necrosis factor therapy or were directly switched to abatacept: the ARRIVE trial. Ann Rheum Dis. 2009;68(11):1708–14.
18 Durez P, Bathon J, Becker JC, Covucci A, Moniz Reed D, Westhovens R. Time course of improvement in ACR core components in patients with early rheumatoid arthritis treated with abatacept plus methotrexate. Poster FRI0201, EULAR Annual Congress, June 16–19, 2010.
19 Westhovens R, Moniz Reed D, Becker J-C, Vratsanos G, Yazici Y. Early improvements in disease activity with abatacept continue to increase or are maintained over time in methotrexate-naïve patients with early rheumatoid arthritis and poor prognostic factors. Poster SAT0109. EULAR Congress, 10–13 June, 2009.
20 Westhovens R, Dougados M, Hall S, Moniz Reed D, Becker J-C, Teng J, et al. Disease remission, radiographic non-progression and normalization of function achieved at year 1 are sustained long-term in a majority of patients: 5-year outcomes with abatacept in biologic-naïve patients. Poster 1657 (390). ACR/ARHP Annual Scientific Meeting, October 16–21, 2009.
21 Hochberg MH, Westhovens R, Aranda R, Kelly S, Khan N, Qi K, et al. Long-term safety of abatacept: integrated analysis of clinical program data of up to 7 years of treatment. Poster 390, ACR/ARHP Annual Scientific Meeting, November 7–11, 2010.
22 Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, MacDonald JK, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview (Review). The Cochrane Library. 2011, Issue 2.
23 Smitten A, Westhovens R, Hochberg M, Torbeyns A, Becker J-C, Aranda R. Serious infections and malignancies during long-term exposure to abatacept. Poster SAT0163, EULAR Annual Congress, June 16–19, 2010.
24 Weinblatt M, Schiff M, Goldman A, Kremer J, Luggen M, Li T, et al. Selective costimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: a randomised clinical trial. Ann Rheum Dis. 2007;66:228–34.
25 Tay L, Leon F, Vratsanos G, Raymond R, Corbo M. Vaccination response to tetanus toxoid and 23-valent pneumococcal vaccines following administration of a single dose of abatacept: a randomized, open-label, parallel group study in healthy subjects. Arthritis Research & Therapy. 2007;9:R38.
26 Schiff M, Saewert M, Bahrt K, Genovese MC. Response to influenza vaccine in rheumatoid arthritis patients with an inadequate response to anti-TNF therapy treated with abatacept in the ARRIVE trial. Poster FRI 943/175, ACR Congress, November 6–11, 2007.
27 Ribeiro A, Guedes L, Moraes J, Saad C, Calich A, Aikawa N, et al. Abatacept in association with traditional DMARDS severely impairs humoral response to pandemic A H1N1 influenza vaccination in rheumatoid arthritis patients. Ann Rheum Dis. 2011;70(Suppl3):458. Abstract FRI0342.
28 Schiff M, Kaell A, Tay L, Vratsanos G, Bahrt K. Response to pneumococcal vaccine in rheumatoid arthritis patients with an inadequate response to anti-TNF therapy treated with abatacept in the ARRIVE trial. Poster SAT0029, EULAR, June 13–16, 2007.
29 Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S, et al. Incidence of anti-nuclear and anti-DNA antibody seropositivity, and immunogenicity, in patients with rheumatoid arthritis treated with abatacept or infliximab. Poster SAT0030, EULAR, June 13–16, 2007.
30 Jean Dudler, Burkhard Möller, Beat A. Michel, Peter M. Villiger. Biologics in rheumatoid arthritis (RA) – recommendations for Swiss practice. Swiss Med Wkly. 2011;141:w13189.

