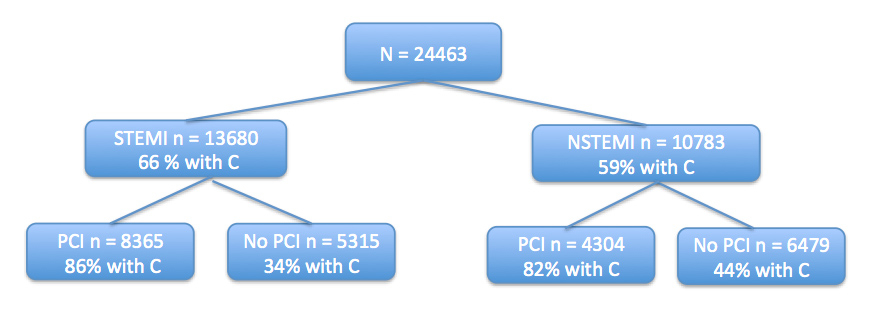
Figure 1
Flow chart of the patient regarding STEMI, NSTEMI and PCI or no PCI and percentage of patients treated with clopidogrel ( C ).
DOI: https://doi.org/10.4414/smw.2012.13573
Data from the Swiss registry AMIS-Plus
Abbreviations
ACS Acute Coronary Syndrome
PCI Percutaneous Coronary Intervention
ASA Acetyl Salicylic Acid
MACE Major Adverse Cardiac Event (death, re-infarction, stroke)
MM medically managed patients (patients not receiving PCI)
STEMI ST segment Elevation Myocardial Infarction
NSTEMI Non-ST segment Elevation Myocardial Infarction
OR Odds ratio
Current treatment guidelines issued by the European Society of Cardiology (ECS) and the American Heart Association (AHA) recommend that, unless contraindicated, a combination therapy that includes acetyl salicylic acid (ASA) and clopidogrel should be administered to acute coronary syndrome (ACS) patients with non-ST segment elevation (NSTEMI) [1] as well as to ACS patients with STEMI [2]. This treatment is important in preventing short-term mortality after the first event and acts prophylactically for later major adverse cardiac events (MACE), even when no immediate percutaneous coronary intervention (PCI) is performed [3].
A 2008 analysis of data gathered between January 2001 and December 2005 from a large US registry has shown not only that clopidogrel administration to NSTEMI patients immediately after hospital admission significantly reduces in-hospital mortality, but that, even as late as 2005, a considerable portion of patients in the USA were not receiving treatment that fully complied with acknowledged guidelines [4]. Positive clinical benefit for clopidogrel use has been demonstrated for patients undergoing PCI [5]; furthermore, significantly lower 1-year cardiovascular mortality rates were reported in Canada when prescription rates increased after reimbursement authorisation [6]. At the same time, results from a smaller French registry suggest that the clinical outcome after one year does not significantly differ for NSTEMI and STEMI patients [7].
The aim of this study was to obtain further evidence of the clinical benefit of guideline adherence in terms of mortality and MACE in Switzerland, and to investigate compliance with treatment guidelines.
AMIS (Acute MyocardialInfarction in Switzerland) Plus is a national Swiss registry for patients with acute coronary syndrome (STEMI, NSTEMI, and unstable angina). AMIS is hosted by the Institute of Social and Preventive Medicine at the University of Zurich (now termed AMIS Plus with enhanced data collection). Emphasis is placed on the evaluation of risk factors (family history of coronary artery disease (CAD), diabetes, hypertension, dyslipidemia, smoking), diagnostics, urgent therapy strategies, treatment and long-term outcome of acute coronary syndrome. All data acquisition and analysis tasks are performed in strict accordance with Good Clinical Practice (GCP) guidelines. Details have been described elsewhere [8] and are available from http://www.amis-plus.ch. Data are entered in a web-database at the time of hospitalisation. MACE were defined as death, myocardial infarction or re-infarction and stroke.
This investigation uses the data from all ACS patients enrolled in the AMIS Plus. The data analysed focused on the impact of clopidogrel, given within 24 hours of admission, on mortality and MACE. Observed treatment regimens were also compared with established treatment guidelines. MACE were defined as composed end points of re-infarction, cerebrovascular events and/or death. The null hypothesis tested was that there is no difference in mortality and MACE because of early clopidogrel administration.
The AMIS data centre uses SPSS software for data analysis (SPSS Inc., Chicago, Illinois; Version 17.0). Proportions were compared using Fisher's Exact Test. Odds ratio (OR) with 95% confidence interval were calculated (16). All statistical tests were two-sided. A p value <0.05 was considered significant. A multivariate logistic regression model based on backwards logistic regression methodology was used to determine in-hospital mortality predictors from the following set of variables: age, sex, Killip classes, prior CAD, diabetes current smoking, clopidogrel, primary PCI and resuscitation prior admission.
The registry included data from 30,243 ACS patients from 76 Swiss hospitals. For 24,463 (81%) of these patients, data on early clopidogrel (<24 h of admission) administration were available (fig. 1). Some 15,525 (51%) of the total cohort were administrated clopidogrel within 24h of admission (table 1). Patients receiving early clopidogrel treatment (300 mg loading dose followed by 75 mg/day) were significantly younger (mean age 64 years vs. 69 years,p <0.001), there were more smokers (40%, vs. 33%, p <0.001), and more males (76 vs. 67%, p <0.001). A total of 4.7% of the patients of the early clopidogrel group were in Killip class III and IV versus 10% of the other group (p <0.001; table 1). Of note, aspirin was given to all patients and almost 2/3 them had a body mass index above 25. A total of 48% of Swiss patients did not receive primary PCI (including dilation of coronary arteries) and are henceforth termed MM (medically managed) patients.

Figure 1
Flow chart of the patient regarding STEMI, NSTEMI and PCI or no PCI and percentage of patients treated with clopidogrel ( C ).

Figure 2
Effect of early clopidogrel administration (within 24h of admission) on 24h mortality, in-hospital mortality, and MACE. The effect of clopidogrel is statistically significant with p < 0.0001 in Fisher's exact test in all categories..
Early clopidogrel administration is beneficial for 24h mortality, in-hospital mortality and MACE as shown in table 2 and figure 1.
Multivariate analysis shows that some clinical parameters were associated with an increased mortality (table 4). Early clopidogrel administration and PCI were the only treatment that lowered mortality as shown by this analysis.
In-hospital mortalities were similar but slightly higher for patients with primary PCI (8.0%) alone when compared to early clopidogrel alone (6.8%) (p =0.24). MACE were equally frequent between primary PCI patients without early clopidogrel and patients without primary PCI but early clopidogrel administration (10% versus 9.3%, p = 0.55).
Mortality was similar for patients with primary PCI and no clopidogrel (3.4%) and patients without primary PCI and early clopidogrel administration (3.2%, p = 0.82). MACE were more frequent in patients without early clopidogrel and 1°PCI versus no primary PCI and early clopidogrel treatment (5.2% versus 4.2%, p = 0.26). In both STEMI and non-STEMI the lower mortality rate and incidence of MACE emerged from the group of patients treated with a combination of primary PCI and early clopidogrel administration (table 3).
| Table 1: Clopidogrel as immediate therapy in patients admitted for acute coronary syndrome between 2000 and 2008. | |||
| Clopidogrel no(n = 8,978) | Clopidogrel yes (n = 15,525) | p | |
| Gender male | 6,006/8,978 (66.9) | 11,774/15,525 (75.8) | <0.001 |
| Age in years range mean (SD) | 22-101 69.0 (13.6) | 21-99 63.6 (12.7) | <0.001 |
| Diabetes | 1,972/8,594 (22.9) | 2,731/14,914 (18.3) | <0.001 |
| Current smoking | 2690/8199 (32.8) | 5,878/14,557 (40.4) | <0.001 |
| Hypertension | 5,254/8,443 (62.2) | 8,619/14,810 (58.2) | <0.001 |
| Dyslipidemia | 4,447/7,694 (57.8) | 8,259/13,983 (59.1) | 0.072 |
| Overweight (BMI >25) | 4,633/7,474 (62.0) | 8,664/13,202 (65.6) | <0.001 |
| Killip classes n | 8,940 | 15,450 | <0.001 |
| I–II | 8,040 (89.9) | 14,725 (95.3) | |
| III–IV | 900 (10.1) | 725 (4.7) | |
| Regular medication before admission | |||
| Aspirin, ASA | 3,603/8,775 (41.1) | 5,758/15,117 (38.1) | <0.001 |
| Clopidogrel* | 119/2,838 (4.2) | 1,262/10,060 (12.5) | <0.001 |
| Oral anticoagulant | 808/8,740 (9.2) | 665/15,000 (4.4) | <0.001 |
| Beta-blocker | 2,867/8,737 (32.8) | 4,579/15,032 (30.5) | <0.001 |
| ACE inhibitor or Angiotensin II receptor antagonist | 2,862/8,744 (32.7) | 4,654/15,046 (30.9) | 0.004 |
| Ca-channel blocker | 1,248/8,670 (14.4) | 1,840/14,952 (12.3) | <0.001 |
| Nitrates | 1,324/8,724 (15.2) | 1,263/14,978 (8.4) | <0.001 |
| Diuretics | 2,279/8,749 (26.0) | 2,465/15,016 (16.4) | <0.001 |
| Statins | 2,077/8,732 (23.8) | 4,237/15,063 (28.1) | <0.001 |
| Table 2a: Isolated effect of early clopidogrel treatment on clinical outcome. | ||||
| N | OR | 95% CI | p | |
| 24 h mortality | 24,487 | 0.20 | 0.16–0.24 | <0.0001 |
| In-hospital mortality | 24,503 | 0.31 | 0.27–0.34 | <0.0001 |
| MACE | 24,036 | 0.35 | 0.32–0.39 | <0.0001 |
| Table 2b: Effect of early clopidogrel treatment, adjusted for age, gender and STEMI. | ||||
| N | OR | 95% CI | p | |
| 24 h mortality | 24,447 | 0.25 | 0.20–0.31 | <0.0001 |
| In-hospital mortality | 24,463 | 0.41 | 0.36–0.46 | <0.0001 |
| MACE | 23,996 | 0.45 | 0.40–0.49 | <0.0001 |
| Table 3: In-hospital mortality and MACE. All proportions are statistically significantly different with respect to early clopidogrel administration (p <0.0001, Fisher's exact test). | ||||||
| In-hospital mortality | STEMI Clopidogrel | NSTEMI Clopidogrel | All Clopidogrel | |||
| Yes | No | Yes | No | Yes | No | |
| All | 370/9,099; 4.1% | 568/4,581; 12.4% | 154/6,409; 2.4% | 352/4,374; 8.0% | 528/15,525; 3.4% | 926/8,978; 10.3% |
| 1°PCI | 246/7,275; 3.4% | 87/1,090; 8.0% | 62/3,560; 1.7% | 25/732; 3.4% | 310/10,833; 2.9% | 112/1,828; 6.1% |
| No 1°PCI | 124/1,824; 6.8% | 481/3,491; 13.8% | 92/2,837; 3.2% | 326/3,625; 9.0% | 218/4,669; 4.7% | 813/7,126; 11.4% |
| MACE | STEMI Clopidogrel | NSTEMI Clopidogrel | All Clopidogrel | |||
| Yes | No | Yes | No | Yes | No | |
| All | 5.7% | 14.6 % | 3.4% | 9.9% | 4.7% | 12.3% |
| 1°PCI | 4.8% | 10.0 % | 2.8% | 5.2% | 4.1% | 8.1% |
| No 1°PCI | 9.3% | 16.1 % | 4.2% | 10.8% | 6.2% | 13.5% |
| Table 4: Independent predictors for in-hospital mortality. | |||
| OR | 95% CI | p | |
| Gender | 1.01 | 0.86–1.18 | 0.93 |
| Killip I Killip II Killip III Killip IV | Ref. 2.39 4.52 17.77 | 2.00–2.84 3.60–5.68 13.42–23.54 | <0.001 <0.001 <0.001 |
| Prior CAD | 1.02 | 0.87–1.18 | 0.83 |
| Diabetes | 1.30 | 1.10–1.54 | 0.002 |
| Current smoking | 1.16 | 0.96–1.39 | 0.115 |
| Clopidogrel | 0.57 | 0.48–0.69 | <0.001 |
| Age | 1.07 | 1.06–1.08 | <0.001 |
| Primary PCI | 0.70 | 0.57–0.87 | = 0.001 |
| Resuscitation | 4.98 | 3.86–6.44 | <0.001 |
The AMIS Plus registry with its large database on treatments and outcomes provides a comprehensive picture of AMI management and its development over the years in Switzerland. However, when statistical comparisons are made between therapy dependent outcomes, one must always keep in mind that treatments are not randomised: they are assigned by the treating physicians in a real-world environment according to an individual assessment based on the patient's particular situation and the facilities offered by the admitting hospital. Baseline comparability of patients receiving different treatments is therefore not ensured, and extensive multivariate analyses for predictors would have to be conducted in order to produce stand-alone valid statistical results. The extent of these differences with respect to early clopidogrel administration in terms of demographic properties, pre-existing pathologies, and previous treatments is shown in table 1. The prevalence of the individual risk factors, even smoking, was closely similar to what has been reported in the OPERA study [13], except for hypertension, which was lower in the French investigation (47%). It remains open whether the same blood pressure limits were applied. Our numbers are almost identical for STEMI (29% vs. 29%) and lower for NSTEMI patients (27% vs. 48%) as those reported in the OPERA study [13]. Despite the methodological shortcomings, strong and consistent positive results concerning mortality and, similarly, MACE, cannot possibly be explained by those differences found between the treatment groups cited above. For all ACS patients, the best results are thus achieved with ASA and early clopidogrel, and, when indicated, in combination with primary PCI. Clopidogrel consistently reduces the incidence of mortality as well as MACE for all ACS patients. This was shown when clopidogrel was given in addition to aspirin, like in the CURE [7, 9] CREDO [10] and COMMIT [11] trials. Our results confirm these observations and emphasise the need of early clopidogrel administration and primary PCI. Similar data were recently reported from the GRACE [12] registry with a significant benefit of dual anti-platelet therapy for patients with NSTEMI. These authors, also, conclude to an underuse of this strategy especially in Canadian patients [13]. The observation made in the OPERA [14] study that the outcome after one year is not significantly different for STEMI and NSTEMI cannot be confirmed by the AMIS Plus data.
The finding that NSTEMI patients are less likely to receive early clopidogrel treatment can, to a degree, be explained by the economic pressure exerted by health authorities, and the opinion that these patients are at a lower risk for recurring cardiovascular events. Caution should be applied here in view of the 12-month risk reported in the OPERA study [14] From 2002 to 2005, early clopidogrel administration in Switzerland was closely similar to the levels reported from the CRUSADE study [4] (i.e., 30–50%). It can be noted with satisfaction that adherence to the recommendations stipulated by the acknowledged treatment guidelines has significantly improved in recent years, and continues to improve with each passing year. However under use of clopidogrel has recently been reported in the GRACE registry [13] showing that efforts should still be made to increase these numbers. Treatment guidelines are, in general, followed better in hospitals with catheter labs (which are usually larger and more centrally located), though guideline adherence has increased with time in recent years in all hospitals.
Acknowledgements:Present Steering Committee: P. Erne, President, Lucerne; O. Bertel, Zurich; F. Eberli, Zurich; M. Essig, Zweisimmen; F. Gutzwiller, Zurich; P. Hunziker, Basel; P-F. Keller, Geneva; M. Maggiorini, Zurich; G. Pedrazzini, Lugano; D. Radovanovic, Zurich, H. Rickli, St. Gallen; J-C. Stauffer, Fribourg; P. Urban, Geneva; S. Windecker, Bern. Data Center: D. Radovanovic, Head, N. Duvoisin, C. Bähler, J. Piket, E. Doukas.
AMIS Plus Participants 2000–2008: The following hospitals participated in the AMIS registry (in alphabetical order): Affoltern am Albis, Bezirkspital (F- Hess); Altdorf, Kantonsspital (R. Simon); Altstätten, Kantonales Spital (P. J. Hangartner); Aarau, Kantonsspital (P. Lessing); Baden, Kantonsspital (U. Hufschmid); Basel, Kantonsspital (P. Hunziker); Basel, St. Claraspital (C. Grädel); Bern, Beau-Site Klinik (A. Schönfelder); Bern, Inselspital (S. Windecker); Biel, Spitalzentrum (H Schläpfer); Brig-Glis, Oberwalliser Kreisspital (D. Evéquoz); Bülach, Spital (A. Vögele); Burgdorf, Regionalspital Emmental (D. Ryser); Chur, Kreuzspital (R. Jecker); Davos, Spital (G. Niedermaier); Dornach, Spital (A. Droll / T. Hongler); Einsiedeln, Regionalspital (S. Stäuble); Flawil, Kantonales Spital (J. Haarer); Frauenfeld, Kantonsspital (H. P. Schmid); Fribourg, Hôpital cantonal (B. Quartenoud); Frutigen, Spital (K. Bietenhard); Genève, Hôpitaux universitaires (HUG) (J.-M. Gaspoz / P. F. Keller); Glarus, Kantonsspital (W. Wojtyna); Grenchen, Spital (B. Oertli / R. Schönenberger); Heiden, Kantonales Spital (R. Waldburger); Herisau, Kantonales Spital (M. Schmidli); Interlaken, Spital (E. M. Weiss); La Chaux-de-Fonds, Hôpital (H. Zender); Lachen, Regionalsspital (C. Steffen); Langnau im Emmental, Regionalspital (A. Hugi); Laufenburg, Gesundheitszentrum Fricktal (E. Koltai); Lugano, Cardiocentro Ticino (G. Pedrazzini); Luzern, Luzerner Kantonsspital (P. Erne); Männedorf, Kreisspital (T. Luterbacher); Mendrisio, Ospedale regionale (A. Pagnamenta); Meyrin, Hôpital de la Tour (P. Urban); Moutier, Hôpital du Jura bernois (C. Stettler); Münsingen, Regionales Spital Zentrum (F. Repond); Münsterlingen, Kantonsspital (F. Widmer); Muri, Kreisspital für das Freiamt (H. Lusser); Nyon, Group. Hosp. Ouest lémanique (R. Polikar); Olten, Kantonsspital (S. Bassetti); Rheinfelden, Gesundheitszentrum Fricktal (H. U. Iselin); Rorschach, Kantonales Spital (M. Giger); Samedan, Spital Oberengadin (P. Egger); Sarnen, Kantonsspital Obwalden (T. Kaeslin); Schaffhausen, Kantonsspital (R. Frey); Schlieren, Spital Limmattal (T. Herren); Schwyz, Spital (P. Eichhorn); Scuol, Ospidal d'Engiadina Bassa (C. Neumeier, G. Flury); Solothurn, Bürgerspital Solothurn (A. Grêt / R. Schöneneberger); St. Gallen, Kantonsspital (H. Rickli); Sursee, Luzerner Kantonsspital (S. Yoon); Tiefenau, Tiefenauspital (P. Loretan); Thun, Spital (U. Stoller); Thusis, Krankenhaus (U. P. Veragut); Uster, Spital (E. Bächli); Uznach, Kantonales Spital (A. Weber); Wädenswil, Schwerpunktspital Zimmerberg-Horgen (B. Federspiel / M. Weisskopf); Walenstadt, Kantonales Spital (D. Schmidt / J. Hellermann); Wetzikon, GZO Spital (M. Graber); Winterthur, Kantonsspital (A. Haller); Wolhlusen, Luzerner Kantonsspital (M. Peter); Zofingen, Spital (S. Gasser); Zollikerberg, Spital (P. Siegrist / R Fatio); Zug, Kantonsspital (M. Vogt / D. Ramsay); Zürich, Klinik im Park (O. Bertel); Zürich, Universitätsspital Zürich (M. Maggiorini); Zürich, Stadtspital Triemli (F. Eberli); Zürich, Stadtspital Waid (S. Christen).
The authors want to thank Dr Bucher for his technical assistance in drafting the manuscript.
1 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2011:32:2999–3054.
2 Van de Werf F, Bax J, Betriu A, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation. Eur Heart J. 2008; 29:2909–45.
3 Husted S, Evidence-based prescribing and adherence to antiplatelet therapy – how much difference do they make to patients with atherothrombosis? Int J Cardiol. 2009; 134:150–9.
4 Alexander D, Ou FS, Roe MT, et al. Use of and in hospital outcomes after early clopidogrel therapy in patients not undergoing an early invasive strategy for treatment of non-ST-segment elevation myocardial infarction: Results from Can Rapid risk stratification of Unstable angina patients Suppress Adverse outcomes with Early implementation of the American College of Cardiology/American Heart Association guidelines (CRUSADE). Am Heart J. 2008; 156:606–12.
5 Silber S, Albertsson P, Avilés FF, et al. Guidelines for percutaneous coronary interventions. Eur Heart J. 2005; 26:804–47.
6 Jackevicius CA, Tu JV, Demers V, et al. Cardiovascular outcomes after a change in prescription policy for clopidogrel. N Engl J Med. 2008; 359:1802–10.
7 The CURE Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–500.
8 Outcome of patients with acute coronary syndrome in hospitals of different sizes. A report from the AMIS Plus Registry. Radovanovic D, Urban P, Simon R, Schmidli M, Maggiorini M, Rickli H, Stauffer JC, Seifert B, Gutzwiller F, Erne P; AMIS Plus Investigators. Swiss Med Wkly. 2010;140(21-22):314–22.
9 Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study Lancet. 2001;358:527–33.
10 Sean C. Beinart, Paul Kolm, Emir Veledar, Zefeng Zhang, Elizabeth M. Mahoney, Olivier Bouin, et al. Long-term cost effectiveness of early and sustained dual oral antiplatelet therapy with clopidogrel given for up to one year after percutaneous coronary intervention: Results from the Clopidogrel for the Reduction of Events During Observation (CREDO) Trial. J Am Coll Cardiol. 2005;46:761–9.
11 Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation N Engl J Med. 2001;345:494–502.
12 Steinhubl SR, Berger PB, Mann 3rd JT, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411–20.
13 Banihashemi B, Goodman SG, Yan RT, Welsh RC, Mehta SR, Montalescot G, et al.; Global Registry of Acute Coronary Events (GRACE/GRACE(2)) Investigators. Underutilization of clopidogrel and glycoprotein IIb/IIIa inhibitors in non-ST-elevation acute coronary syndrome patients: the Canadian global registry of acute coronary events (GRACE) experience. Am Heart J. 2009;158:917–24.
14 Montalescot G, Dallongeville J, van Belle E, et al. STEMI and NSTEMI: are they so different? 1 year outcomes in acute myocardial infarction as defined by the ESC/ACC definition (the OPERA registry). Eur Heart J. 2007;28:1409–17.
15 Dangas G, Mehran R, Guagliumi G, Caixeta A, Witzenbichler B, Aoki J, et al.; HORIZONS-AMI Trial Investigators. Role of clopidogrel loading dose in patients with ST-segment elevation myocardial infarction undergoing primary angioplasty: results from the HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trial. J Am Coll Cardiol. 2009;54:1438–46.
16 Bland JM and Altman DG. Statistics Notes: The odds ratio. BMJ. 2000;320:1468.
Funding / potential competing interests: The authors declare no conflict of interest. The AMIS Plus project is, among others, sponsored by Bristol-Myers Squibb AG and Sanofi-Aventis SA, holders of marketing authorisations for Plavix® (Clopidogrel) in Switzerland.
Funding Sources 2008: Main sponsors: Astra-Zeneca, Bayer-Schering, Biotronik, Daiichi-Sankyo/Lilly, Invatec, A. Menarini, Medtronic, St. Jude Medical, all in Switzerland. Donators: Abbott, Biosensors, Bristol-Myers Squibb, GlaxoSmithKline, Johnson & Johnson, MerckSharp & Dohme-Chibret, Essex, Novartis, Pfizer, sanofi-aventis, Servier, SPSS and Takeda, all in Switzerland. The interpretation of the data and the decision to submit the manuscript were made by members of the Steering Committee of the study, independently of the funding sources.