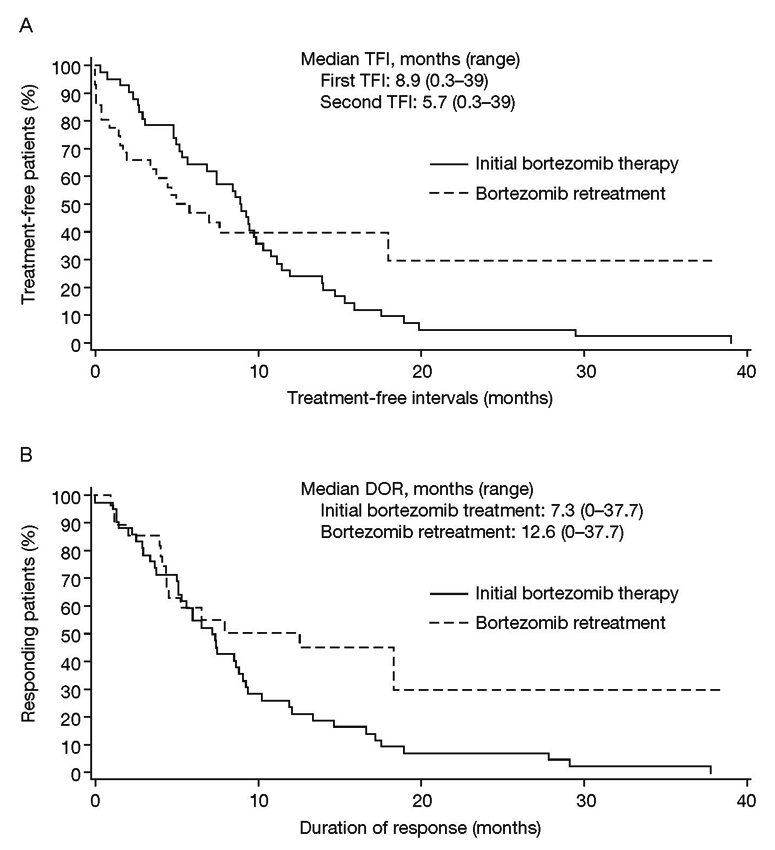
Figure 1
Bortezomib treatment-free interval (A), duration of response (B).
DOI: https://doi.org/10.4414/smw.2012.13562
A multicentre retrospective survey in Switzerland1
1 Prior presentation of this study: Taverna C, Voegeli J, Trojan A, Olie RA, Von Rohr A. Bortezomib Retreatment In Patients With Relapsed Multiple Myeloma In Switzerland. International Myeloma Workshop 2011. Haematologica. 2011;96(suppl 1):S86, abstract P-193.
Although a number of treatment options are available for multiple myeloma (MM), it remains an incurable, progressively relapsing disease; patients typically undergo multiple lines of therapy and have progressively shortening remission times [1]. While treatment regimens are often changed with each new line of therapy, retreatment with previously employed agents may be of benefit. Bortezomib (VELCADE®) has been approved by the Swiss Agency for Therapeutic Products (Swissmedic, http://www.swissmedic.ch ) for the treatment of MM in the frontline setting in combination with melphalan-prednisone and in patients with relapsed/refractory MM who have received at least one prior therapy [2].
A number of studies have provided evidence that retreatment of relapsed/refractory MM with a second course of bortezomib or bortezomib-based therapycould be effective, even in heavily pre-treated patients [3–10]. (Throughout the manuscript, the mention of "bortezomib therapy" refers to bortezomib mono-therapy, the combination of bortezomib with dexamethasone or other bortezomib-based combinations, see table 2.) In these studies, response rates ranged from 21 to 80% [10]. Thus retreatment with bortezomib appears to be a feasible treatment approach in patients with relapsed MM [10]. A retrospective survey of patients with MM in 36 centres in Germany and Switzerland showed an overall response rate (ORR) of 63% when retreating patients with bortezomib mono-therapy or a combination of bortezomib with dexamethasone [5]. Toxicity in these bortezomib-retreated patients was similar to that observed with initial therapy. The Swiss data reported in the current study were taken from the original survey [5], partly updated upon extended follow-up, and also include additional patients who were treated since the initial analysis. In the current study the efficacy results were also analysed for patients treated with other combination schedules, besides bortezomib-dexamethasone, since such combinations are increasingly being used in daily clinical practice. All patients had responded to bortezomib therapy, presented with progressive or relapsed disease, and were then retreated with bortezomib after a treatment-free interval (TFI).
This was a retrospective, multicentre, open, non-interventional, single-arm survey conducted at 26 centres in Switzerland. Inclusion criteria included patients with relapsed MM aged ≥18 years, previous treatment with bortezomib therapy resulting in complete response (CR), near CR (nCR), or partial response (PR), and a completed retreatment regimen with bortezomib after relapse or disease progression. Information related to any MM-specific therapy that patients received between the two courses of bortezomib treatment, was also obtained.
Data were extracted from patient case report forms and clinical records between September 26th, 2005, and December 29th, 2009. The full safety population was used for tolerability analysis and included all patients in participating centres in Switzerland who had disease progression following initial bortezomib therapy and were undergoing bortezomib retreatment due to recurrent disease. The per-protocol (PP) population included all patients who responded to prior bortezomib therapy without major violations of the selection criteria. The following parameters were documented from the clinical records of eligible patients: demographic and disease-related data; data from each bortezomib exposure, including dose and duration of treatment, best response, and clinical benefit (CR, nCR, PR, stable disease [SD]) after first and second bortezomib therapy. Response criteria were used for assessment, and administration of concomitant medication and confirmation of disease progression following initial bortezomib therapy were documented. Adverse drug reactions (ADR) attributed to bortezomib were documented for up to 30 days following the last dose of bortezomib. Dose and schedule of bortezomib administration and criteria for assessment of response were at the discretion of the treating physician. The first TFI was defined as the time between the end of previous bortezomib therapy and the start of bortezomib retreatment or the start of MM-specific interim therapy. The second TFI was defined as the time between the end of bortezomib retreatment and the start of next therapy after bortezomib retreatment.
Prior to statistical analysis, data were checked for plausibility by visual inspection and by SAS software. Implausible data were corrected by manual checks, technical corrections and queries. For the final analysis, some queries were not resolved by the time of data cut-off and were considered as missing values. For categorical data, absolute and relative frequencies were calculated. Non-adjusted frequencies included missing values and adjusted frequencies excluded missing values. Incidence rates were calculated with exact 95% confidence intervals (CI). For continuous data, the mean, standard deviation, median, minimum, maximum, and quartiles were calculated. Kaplan-Meier (KM) estimates for median, mean, standard error (SE) and quartiles were calculated for the duration of the second response, the second TFI, for overall survival (OS), and for time to first and second progression. Survival time (KM) methods were used for analyses of time intervals that included censored data. For other time intervals (time to second response, time to first response, duration of first response, first TFI) the mean, standard deviation, minimum, maximum, and quartiles were calculated by standard methods.
Of the 29 patients from Switzerland included in the initial survey report [6], updated data are presented here for 9 of these patients, and 14 additional patients are included in this analysis. Deviations from the study plan were reported for 1 patient who did not have a PR or better following prior bortezomib, thus, 42 patients were included in the PP population. Patient demographic and disease characteristics for the PP population are shown in table 1. The median age of patients at initiation of bortezomib retreatment was 63 years. Patients had received a median of 2 therapies (range 1–11) before initial bortezomib treatment. The most common prior therapies were vincristine-adriamycin-dexamethasone, thalidomide-dexamethasone, and melphalan-prednisone. Prior stem cell transplant was performed in 31% of patients. All 43 patients were included in the safety population.

Figure 1
Bortezomib treatment-free interval (A), duration of response (B).
Patients in the PP population received a median of 4 cycles of bortezomib (range 2–19) as the initial treatment (table 2). Most patients (83.3%) were treated with 1–6 cycles. During initial bortezomib treatment, 50% of patients received concomitant dexamethasone (table 2). All patients had achieved a PR or better with initial bortezomib therapy, and one-third achieved CR or nCR (table 3). With initial bortezomib treatment, the median time to response was 2.4 months (range 0.7–6.4), the median first TFI was 8.9 months (range 0.3–39.0; fig. 1A), the median duration of first response (DOR) after initial bortezomib therapy was 7.3 months (range 0–37.7; fig. 1B), and the median time to progression (TTP) was 10.7 months (range 2.7–54.4 months) for the first progression.
Between initial bortezomib treatment and retreatment, 12 patients (28.6%) received MM-specific interim therapy. Interim therapies, either as single agents or in combination, included thalidomide or lenalidomide in 4 patients, dexamethasone in 5 patients, and autologous stem cell transplant in 2 patients.
| Table 1: Patient demographic and disease characteristics. | |
| Parameter | Patients (N = 42)* |
| Median age at initial diagnosis, years (range) | 60 (38–87) |
| Median age at retreatment, years (range) | 63 (40–89) |
| Male, n (%) | 22 (52.4) |
| Median time from diagnosis to initial bortezomib therapy, months (range) | 34.1 (2.3–159.2) |
| Myeloma type, n (%) | |
| IgG IgA IgD Light chain Non-secretory | 22 (52.4) 11 (26.2) 1 (2.4) 6 (14.3) 2 (4.8) |
| Median number of prior therapies (range) | 2 (1–11) |
| Prior therapies, n (%) | |
| Vincristine-adriamycin-dexamethasone | 23 (54.8) |
| Thalidomide-dexamethasone | 21 (50.0) |
| Melphalan-prednisone | 14 (33.3) |
| Autologous/allogeneic stem cell transplant | 13 (31.0) |
| Dexamethasone | 12 (28.6) |
| Thalidomide | 9 (21.4) |
| α-interferon | 3 (7.1) |
| Doxorubicin-vincristine-dexamethasone | 2 (4.8) |
| Other | 16 (38.1) |
| *Per-protocol population. SD, standard deviation. | |
| Table 2: Exposure to bortezomib initial therapy and retreatment, as well as other anti-MM therapy taken concomitantly with bortezomib treatment. | ||
| Patients (N = 42)* | ||
| Parameter | Initial bortezomib treatment | Bortezomib retreatment |
| Number of cycles, median (range) | 4 (2–19) | 3 (1–19) |
| Cycles, n (%) | ||
| 1–3 | 19 (45.2) | 24 (57.1) |
| 4–6 | 16 (38.1) | 14 (33.3) |
| 7–9 | 5 (11.9) | 3 (7.1) |
| ≥10 | 2 (4.8) | 1 (2.4) |
| Dose, mg/m² (BSA) | ||
| 1.3 | 34 (81.0) | 33 (78.6) |
| 1.0 | 6 (14.3) | 7 (16.7) |
| Other | 2 (4.8) | 2 (4.8) |
| Concomitant dexamethasone | 21 (50.0) | 27 (64.3) |
| Other concomitant therapy | N/A | 20 (47.6) |
| Antineoplastic and immunomodulating agents | N/A | 6 (14.3%) |
| Musculoskeletal system | N/A | 10 (23.8%) |
| * Per-protocol population BSA, body surface area; SD, standard deviation. | ||
Patients received a median of 2 therapies (range 1–11) prior to bortezomib retreatment (table 1) and a median of 3 cycles of bortezomib (range 1–19) as retreatment; 90.4% received 1–6 cycles (table 2). At retreatment, 27 patients (64.3%) received concomitant dexamethasone, and 47.6% of patients received other concomitant medications during bortezomib retreatment, including 14.3% who received concomitant anti-neoplastic or immunomodulating agents (table 2). The ORR (CR + nCR + PR) was 64.3% (95% CI: 48.0–78.4) (table 3). The clinical benefit rate (CR + nCR + PR + SD) was 83.3%. Response rates to bortezomib retreatment stratified by response to initial bortezomib are shown in table 4. Of 14 patients who had CR or nCR with initial bortezomib therapy, 12 (85.7%) responded to bortezomib retreatment. Of 7 patients who achieved CR with initial bortezomib therapy, 3 experienced a repeat CR with bortezomib retreatment. One of seven patients with an initial nCR had a repeat nCR, while 6 of these patients had PR with bortezomib retreatment. Out of 28 patients who had PR on initial treatment, 2 responded with nCR and 13 responded with PR on retreatment.
The response rate was examined according to first TFI (≤6 months vs >6 months), and use of concomitant dexamethasone with bortezomib retreatment (yes vs. no) (table 5). The response rate to bortezomib retreatment in the subgroup with first TFI >6 months was higher than that in the subgroup with first TFI ≤6 months (74.1% vs. 46.7%, p = 0.10). The response rate in patients who received concomitant dexamethasone therapy at retreatment appeared lower than in patients who did not (57.1% vs. 78.6%, p = 0.31). The median time to response with bortezomib retreatment was 2.8 months (range 1.3–6.7). The median second TFI (after bortezomib retreatment) was 5.7 months (range 0.3–39; fig. 1A). A second TFI longer than 6, 9 and 12 months was experienced by 35.7%, 23.8%, and 19.0% of patients, respectively. The median DOR after bortezomib retreatment was 12.6 months (range 0–37.7; fig. 1B). At the time of data cut-off, 35% of patients (95% CI: 20.6, 51.7) remained in response. Median TTP after bortezomib retreatment was 10.5 months (range 0.4–≥39.5 months).
| Table 3: Response rates with bortezomib retreatment (per-protocol population, N = 42). | |||
| Response to previous bortezomib therapy, N (%) | Response to bortezomib retreatment | ||
| N (%)* | 95% CI | ||
| ORR | 42 (100) | 27 (64.3) | 48.0–78.4 |
| CR | 7 (16.7) | 5 (71.4) | 29.0–96.3 |
| nCR | 7 (16.7) | 7 (100.0) | 59.0–100 |
| PR | 28 (66.7) | 15 (53.6) | 33.9–72.5 |
| ORR, overall response rate; CR, complete response; nCR, near-complete response; PR, partial response. *% calculated based on n in the response to previous bortezomib therapy category | |||
| Table 4: Response rates to bortezomib retreatment stratified by response to initial bortezomib treatment (per-protocol population, N = 42). | |||||
| Best response to bortezomib retreatment | |||||
| Best response to previous bortezomib therapy | CR | nCR | PR | SD | PD |
| CR (N = 7) | 3 | 1 | 1 | 1 | 1 |
| nCR (N = 7) | 1 | 6 | |||
| PR (N = 28) | 2 | 13 | 7 | 6 | |
| CR, complete response; nCR, near CR; PR, partial response. | |||||
The median OS after first diagnosis of MM was 9.3 years, and the median OS after previous bortezomib therapy was 3.5 years. After retreatment with bortezomib, the median OS was 1.7 years. At the time of data cut-off, 14 patients had died, 4 had progressive disease (PD) as best response, 4 had SD, 5 had PR, and 1 had CR.
Safety analyses were based on the safety population (N = 43). Eleven patients (25.6%) experienced a total of 19 ADRs (table 6). The most common ADR were nervous system disorders (including peripheral neuropathy [PN]) and blood and lymphatic system disorders, which were reported by 5 and 3 patients, respectively. A total of 5 out of 19 ADR (66.7%) resolved completely. Five events were assessed as definitely or probably related to bortezomib treatment, and 13 as possibly related to bortezomib treatment, and for one event the causal relationship was not assessed by the investigator. Bortezomib was discontinued in 6 patients. ADRs resulting in discontinuation were lung infection, unknown drug toxicity and PN, abdominal pain and nausea, and thrombocytopenia (all 1 each), and PN (n = 2). All but PN had resolved at the time of data analysis. A total of 6 patients reported a total of 9 suspected serious ADRs, and of these 6 patients, 3 discontinued bortezomib. Serious ADRs in the other 3 patients were thrombocytopenia (n = 1); PN, neutropenia and asthenia (n = 1), and cardiovascular disorder (n = 1). The outcome of the cardiovascular disorder was fatal.
| Table 5: Response rates to bortezomib retreatment stratified by first TFI (after initial bortezomib) and concomitant use of dexamethasone during retreatment (per-protocol population, N = 42). | ||
| Subgroup | Response to bortezomib retreatment | |
| N (%) | 95% CI | |
| TFI to initial bortezomib treatment | ||
| ≤6 months (N = 15) | 7 (46.7) | 21.3–73.4 |
| >6 months (N = 27) | 20 (74.1) | 53.7–88.9 |
| Concomitant or maintenance dexamethasone treatment | ||
| Yes (N = 28) | 16 (57.1) | 37.2–75.5 |
| No (N = 14) | 11 (78.6) | 49.2–95.3 |
| TFI, treatment-free interval. | ||
| Table 6: Adverse drug reactionsa occurring in ≥5% of patients (safety population, N = 43). | ||
| Primary system organ class (PSOC) | Events, n | Patients, n (%) |
| Any adverse drug reaction | 19 | 11 (25.6) |
| Nervous system disordersb | 5 | 5 (11.6) |
| Blood and lymphatic system disorders | 3 | 3 (7.0) |
| General disorders and administration site conditions | 3 | 3 (7.0) |
| a Adverse drug reactions for which a causal relationship to bortezomib treatment could not be ruled out. b Including peripheral neuropathy, peripheral sensory neuropathy, neuropathy peripheral, neuropathy, polyneuropathy. | ||
This multicentre, retrospective survey represents the first non-interventional review of bortezomib retreatment exclusively in the Swiss clinical practice setting in patients who had responded to previous bortezomib treatment for MM. Bortezomib is currently approved in Switzerland for the first-line treatment of MM patients in combination with melphalan-prednisone (MP) and for the treatment of relapsed/refractory MM patients who have received at least one previous line of therapy [11].
The ORR presented here is in agreement with the results of various other studies in this setting, in which ORR ranged from 21 to 80% with the use of bortezomib or bortezomib-based combinations [3–10]. The clinical benefit rate including SD was 83.3% in this study. Based on the data from this Swiss survey, retreatment with bortezomib in patients with relapsed MM is an effective option for patients who have previously responded to bortezomib therapy.
According to the recent treatment recommendations issued in Switzerland for treatment of patients in the relapsed/refractory setting [2], the choice of treatment at relapse is affected by the efficacy and toxicity of the prior treatment. For remissions >6–12 months, a repetition of the initial therapy may be appropriate and provide acceptable toxicity. Alternatively, for short duration remission (<6 months), a switch in treatment may be indicated. This recommendation is supported by the findings in the current analysis: the ORR for retreatment of patients with a TFI >6 months was 74.1% compared to 46.7% for patients with a TFI ≤6 months. Although the difference was not statistically significant, probably due to the small sample size, our results are supported by the findings of Hrusovsky et al. [5].
Decreasing duration of clinical benefit has previously been reported with successive lines of therapy [1] and is generally believed to be due to an increase in disease resistance after each course of treatment [1, 9, 12, 13]. In our analysis, in patients who responded to both initial bortezomib treatment and bortezomib retreatment, the clinical benefit of bortezomib retreatment was comparable to that of initial bortezomib treatment in terms of time to response, DOR and TTP.
Bortezomib retreatment was well tolerated with only 19 ADRs reported in 11 patients, which is lower than in reported clinical trials of bortezomib in relapsed, refractory MM [3]. The most common toxicities were PN and thrombocytopenia. The rates of both these ADRs were low in the present study. Due to the retrospective analysis in this clinical practice setting, toxicities may be under-reported.
Management of bortezomib-related toxicity is important to allow prolonged duration of therapy [14, 15]. Recent studies have evaluated alternative schedules and formulations of bortezomib in previously untreated MM. In two studies of once-weekly (days 1, 8, 15, and 22 of a 5-week cycle) versus twice-weekly (days 1, 4, 8, and 11 of a 3-week cycle) bortezomib in newly-diagnosed MM [16, 17] once-weekly regimens were better tolerated than the standard twice-weekly regimen. Within the VMP arm of the GIMEMA MM0305 trial, grade 3/4 sensory PN was 7% with once-weekly dosing compared with 28% for twice-weekly dosing with similar overall response rates (79% and 86%, respectively) [16]. Mateos et al. [17] also reported that less intensive weekly dosing was associated with lower rates of grade 3/4 PN (8%), compared with VISTA (13%) and, with maintenance therapy, resulted in an increased CR rate of 42%, compared with 30% reported in VISTA [14]. Likewise, subcutaneous administration of bortezomib in relapsed/refractory patients has shown similar systemic bortezomib exposure to intravenous dosing, with comparable response rates and an improved safety profile, especially a reduced PN rate [18]. The s.c. administration of bortezomib may offer the potential for further optimizing bortezomib therapy and retreatment in patients with relapsed MM.
Limitations of this study are its small size and retrospective design, but as such, it provides information on a set of relatively uniform treatment practices in a defined population that may be compared with real-world results from other countries.
In conclusion, bortezomib retreatment was well-tolerated and effective in relapsed MM patients in the Swiss clinical setting who have previously responded to bortezomib, and particularly for those who experienced an initial TFI of more than 6 months.
Acknowledgements:The authors acknowledge all physicians in Switzerland who participated in this retrospective survey. The authors acknowledge Stephen Mosley and Catherine Crookes of FireKite for their medical writing support in the development of this manuscript, which was funded by Millennium Pharmaceuticals, Inc. and Janssen Global Services.
1 Kumar SK, Therneau TM, Gertz MA, et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc. 2004;79:867–74.
2 Taverna C, Bargetzi M, Betticher D, et al. Integrating novel agents into multiple myeloma treatment – current status in Switzerland and treatment recommendations. Swiss Med Wkly. 2010;140:w13054.
3 Conner TM, Doan QD, Walters IB, LeBlanc AL, Beveridge RA. An observational, retrospective analysis of retreatment with bortezomib for multiple myeloma. Clin Lymphoma Myeloma. 2008;8:140–5.
4 Wolf J, Richardson PG, Schuster M, LeBlanc A, Walters IB, Battleman DS. Utility of bortezomib retreatment in relapsed or refractory multiple myeloma patients: a multicenter case series. Clin Adv Hematol Oncol. 2008;6:755–60.
5 Hrusovsky I, Emmerich B, von RA, et al. Bortezomib retreatment in relapsed multiple myeloma – Results from a retrospective multicentre survey in Germany and Switzerland. Oncology. 2011;79:247–54.
6 Petrucci MT, Blau I, Corradini P, et al. Efficacy and safety of retreatment with bortezomib in patients with multiple myeloma: Interim results from RETRIEVE, a prospective international phase 2 study. 95 (Suppl 2). Abstract 0377 ed. 2010.
7 Ciolli S, Leoni F, Casini C, Bosi A. Feasibility and efficacy of bortezomib re-treatment in multiple myeloma. Haematologica. 2007;92:Abstract 0260.
8 Rubio-Martinez A, Recasens V, Soria B, Montanes MA, Rubio-Escuin R, Giraldo P. Response to retreatment on relapse multiple myeloma patients previously treated with bortezomib. Haematologica 2008;93:Abstract 0649.
9 Sood R, Carloss H, Kerr R, et al. Retreatment with bortezomib alone or in combination for patients with multiple myeloma following an initial response to bortezomib. Am J Hematol. 2009;84:657–60.
10 Mohty B, El-Cheikh J, Yakoub-Agha I, Avet-Loiseau H, Moreau P, Mohty M. Treatment strategies in relapsed and refractory multiple myeloma: a focus on drug sequencing and “retreatment” approaches in the era of novel agents. Leukemia. 2012;26:73–85.
11 Velcade. Summary of product characteristics EMEA Janssen-Cilag International NV. Summary of product characteristics 2010.
12 Drewinko B, Alexanian R, Boyer H, Barlogie B, Rubinow SI. The growth fraction of human myeloma cells. Blood. 1981;57:333–8.
13 Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–27.
14 Mateos MV, Richardson PG, Schlag R, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol. 2010;28:2259–66.
15 Richardson PG, Sonneveld P, Schuster MW, et al. Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guideline. Br J Haematol. 2009;144:895–903.
16 Bringhen S, Larocca A, Rossi D, et al. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood. 2010;116:4745–53.
17 Mateos MV, Oriol A, Martinez-Lopez J, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncol. 2010;11:934–41.
18 Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431–40.
Funding / potential competing interests:Research support by Janssen-Cilag AG; medical writing support was funded by Millennium Pharmaceuticals, Inc., and Janssen Global Services. Ch. Taverna is an advisory board member and R. A. Olie is an employee of Janssen-Cilag AG.