The correlation between carotid-femoral pulse wave velocity and composition of the aortic media in CAD patients with or without hypertension
DOI: https://doi.org/10.4414/smw.2012.13546
Beian
You, Lin
Shen, Jifu
Li, Yuguo
Chen, Xinghua
Gu, Haiqing
Gao
Summary
OBJECTIVES: To investigate the influence of hypertension on large artery elasticity and the microstructure of the ascending aortic media in patients with coronary artery disease (CAD), and the association between arterial compliance and composition of the ascending aorta.
METHODS: 60 patients with CAD who underwent coronary artery bypass graft surgery were divided into two groups: 30 patients in a hypertension group and 30 patients in a non-hypertension group. Carotid-femoral pulse wave velocity (cfPWV) was measured by an automatic device (Complior, Artech, France). The severity of coronary atherosclerosis was assessed after selective coronary angiography using the Gensini score system. A quantitative study was conducted on ascending aorta specimens by histological and computer image analysis.
RESULTS: cfPWV of the hypertension group was higher than that of the non-hypertension group. The relative content of collagen in the ascending aortic media of the hypertension group was higher than that of the non-hypertension group, while the relative content of elastin in the ascending aortic media of the hypertension group was lower than that of the non-hypertension group. cfPWV showed a positive correlation with relative contents of collagen in the ascending aorta and a negative correlation with relative contents of elastin in the ascending aorta in the two groups.
CONCLUSIONS: Hypertension may raise the contents of collagen and decrease the contents of elastin in the ascending aortic media of patients with CAD, which in turn may decrease the patients’ large artery compliance. cfPWV may reflect the quantitative changes of collagen and elastin in the ascending aortic media in CAD patients independently of hypertension.
Introduction
Analysis of arterial stiffness and of pressure wave reflection has received increasing attention over the past few decades. Among the methods used to describe arterial stiffness, pulse wave velocity (PWV) has been the most investigated in a clinical setting. It has been shown that carotid-femoral pulse wave velocity (cfPWV)is a well-accepted surrogate measure of aortic stiffness [1–4] and a significant predictor of cardiovascular risk in diseased [2, 4–6] and older healthy populations [7].
cfPWV is usually measured by a foot-to-foot velocity method. Briefly, waveforms are obtained transcutaneously over the right common carotid artery and the right femoral artery, and the time delay (t) is measured between the feet of the two waveforms. The distance (D) covered by the waves is assimilated to the distance measured between the two recording sites. cfPWV is measured as D/t, where D is measured in metres, and t in seconds. Although studies have demonstrated that many cardiovascular risk factors, such as aging, hypertension, type 2 diabetes, hypercholesterolaemia, sedentary lifestyle and cigarette smoking can raise PWV, the precise mechanisms of arterial stiffening are not completely understood.
It has been thought that structural changes within the medium of arteries play an important role in elevated PWV. The human aorta contains vascular smooth muscle, is rich in elastin and, with advancing age, increasing relative amounts of collagen [8]. A delicate balance among collagen, elastin, and smooth muscle in the aortic wall is clearly essential for a compliant aorta. Thus, we postulated there must be a close relationship between cfPWV and composition of the human aorta. However, the correlation between cfPWV and smooth muscle, elastin, or collagen of the aortic media has not been published at the time of the present study. We therefore assessed aortic stiffness by arterial tonometry, investigated the histological structure of the ascending aorta media and studied the relationship between structural changes in the aortic media and cfPWV in patients with coronary artery disease (CAD) undergoing coronary artery bypass graft (CABG) surgery. This study was designed to investigate the pathological basis of the increased stiffness of large arteries reflected in cfPWV.
Patients and methods
Patient population
Hypertension is an independent risk factor for cardiovascular events and is associated with increased arterial stiffness [9]. Sixty consecutive patients with CAD undergoing CABG surgery in Shandong University’s Qilu Hospital were included from December 2009 to May 2010. They were divided into two groups: hypertension group (n = 30) and non-hypertension group (n = 30). Hypertension was defined as diastolic blood pressure (DBP) ≥90 mm Hg, or systolic blood pressure (SBP) ≥140 mm Hg, or use of antihypertensive medication [10]. If the patients were taking antihypertensives for a different indication, such as angina pectoris or chronic heart failure, they were still eligible for the non-hypertension group.
All subjects gave informed consent. The study was approved by the Ethics Board of Shandong University’s Qilu Hospital (ethics review committee study number:1048). Patients with arteriosclerosis obliterans, valvular heart diseases, arrhythmia, or heart failure, or documented ejection fraction <45%, were excluded due to inaccuracy in pulse wave recording and analysis. Patients with diabetes mellitus, Ehlers-Danlos syndrome, Marfan syndrome, and related disorders were excluded.
Medical history and examinations
Clinical characteristics including age, occupation, medical drug and smoking history and family medical history were recorded (table 1).
SBP and DBP were analysed as the first and fifth Korotkoff phases [11] on the right arm with subjects seated. Mean values were obtained after 3 repeated tests with a 5-minute interval. Body mass index (BMI), total cholesterol (TC), triglyceride (TG) and fasting plasma glucose (FPG) were measured.
Carotid-femoral pulse wave velocity measurement
cfPWV was measured by an automatic device (Complior, Artech, France). Briefly, two pressure waveforms were transcutaneously recorded at the base of the neck for the right common carotid artery and over the right femoral artery. PWV was determined by the foot-to-foot velocity. Pulse transit time was determined as the average of 10 consecutive beats. The straight-line distances between the sternal-notch and both waveform measurement sites were determined, and path length was taken as the difference between the two distances. cfPWV was calculated as the ratio of distance to transit time.
Assessment of coronary atherosclerosis by quantitative coronary angiography
Selective coronary angiography was performed by radial or femoral approaches using catheters 6F or greater. Quantitative coronary angiographic (QCA) analysis was performed using the computer-based edge-detection coronary angiography analysis system. Coronary angiograms were obtained in multiple views matched after intracoronary injection of nitrates. The severity of coronary atherosclerosis was assessed by Gensini score [12], which was computed by assigning a severity score to each coronary stenosis according to the degree of luminal narrowing and its anatomical importance.
Tissue collection and histological investigation
At CABG operation, a 4.5-mm opening was created with a puncher in the anterior wall of the ascending aorta, and the freshly harvested specimen of the ascending aortic wall was fixed in buffered 10% formalin for 48 h, dehydrated in graded ethanol solutions and embedded in paraffin. The paraffin-embedded specimens were sectioned at 5 μm and separately stained with Masson and Weigert’s solution for further light microscopic examination.
Statistical methods
All data analyses were performed using SPSS® version 11.5 (SPSS® Inc., Chicago, IL, USA) for Windows®. An independent-sample T test was used to compare continuous data and a χ2 test to compare categorical variables between two groups. Bivariate analyses were performed to study associations between cfPWV and the relative content of elastin, collagen, and smooth muscle of the aortic media. P <0.05 was considered statistically significant.
|
Table 1: Characteristics of patients in two groups. |
| |
Non-hypertension group (n = 30)
|
Hypertension group (n = 30)
|
P value
|
| Gender (male/female) |
20/10 |
17/13 |
0.426 |
| Age (years) |
64.77 ± 6.47 |
63.63 ± 7.81 |
0.543 |
| BMI (kg/m2) |
25.22 ± 2.36 |
25.37 ± 1.30 |
0.772 |
| SBP (mm Hg) |
120.00 ± 9.65 |
135.00 ± 8.20 |
<0.01 |
| DBP (mm Hg) |
69.17 ± 7.78 |
70.17 ± 7.71 |
0.619 |
| PP (mm Hg) |
50.83 ± 7.55 |
64.83 ± 9.60 |
<0.01 |
| HR (bpm) |
68.83 ± 7.31 |
67.00 ± 8.63 |
0.378 |
| FPG (mmol/L) |
5.23 ± 0.44 |
5.06 ± 0.50 |
0.166 |
| TC (mmol/L) |
4.66 ± 0.89 |
4.85 ± 0.88 |
0.413 |
| TG (mmol/L) |
1.86 ± 0.67 |
1.63 ± 0.41 |
0.104 |
| History of smoking (yes/no) |
14/16 |
12/18 |
0.602 |
| ACEIs or ARBs (yes/no) |
9/21 |
15/15 |
0.114 |
| CCBs (yes/no) |
7/23 |
13/17 |
0.100 |
| β-blockers (yes/no) |
20/10 |
16/14 |
0.292 |
| Statins (yes/no) |
11/19 |
16/14 |
0.194 |
| ASA (yes/no) |
24/6 |
21/9 |
0.371 |
| Nitroglycerin (yes/no) |
5/25 |
3/27 |
0.706 |
| Gensini score |
82.03 ± 14.67 |
87.60 ± 18.53 |
0.202 |
| cfPWV (m/s) |
13.01 ± 1.48 |
15.38 ± 1.78 |
<0.01 |
| Smooth muscle (%) |
22.01 ± 3.88 |
22.12 ± 3.18 |
0.910 |
| Collagen (%) |
42.16 ± 3.03 |
45.95 ± 3.84 |
<0.01 |
| Elastin (%) |
19.25 ± 2.73 |
17.48 ± 3.46 |
0.031 |
| SBP = systolic blood pressure; DBP = diastolic blood pressure; PP = pulse pressure; HR = heart rate; bpm = bump per minute; BMI = body mass index; FPG = fasting plasma glucose; TC = total cholesterol; TG = triglyceride; ACEIs = angiotensin converting enzyme inhibitors; ARBs = angiotensin receptor blockers; β blockers = beta blockers; CCBs = calcium channel blockers; ASA, aspirin. |
Results
Clinical characteristics
The clinical data for all subjects of the two groups are shown in table 1. There was no significant difference between the two groups in clinical characteristics except for SBP and PP.
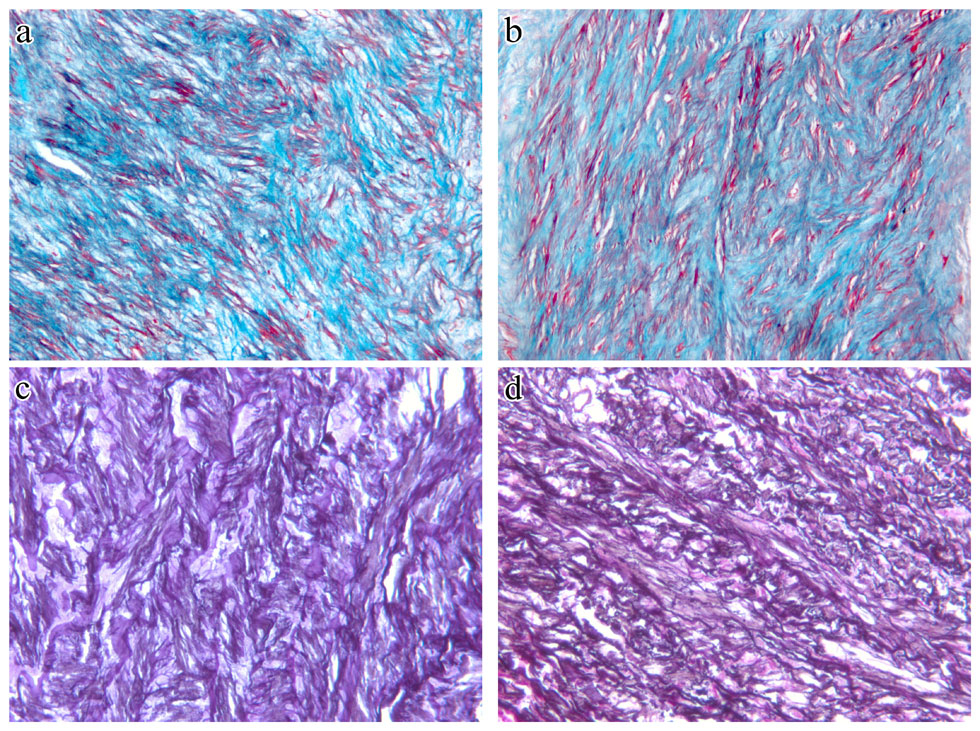
Figure 1
Representative sections of aorta stained with Masson’s demonstrate increased collagen and disorganisation of smooth muscle and collagen in the media of the aortic wall of hypertension patients (a), as compared with non-hypertension patients (b). Collagens are stained green and smooth muscle is stained red. Magnification x200. Representative sections of aorta stained with Weigert’s demonstrate decreased elastin and disorganisation of the elastic network in the media of the aortic wall of hypertension patients (c), as compared with non-hypertension patients (d). Elastins are stained black. Magnification x200.
Angiographic results
The minimum Gensini score of the non-hypertension group was 52 and the maximum 107. The minimum Gensini score of the hypertension group was 49 and the maximum 119. There were no significant differences in Gensini score between the two groups. These data are shown in table 1.
Comparison of cfPWV between the hypertension group and the non-hypertension group
The difference in cfPWV between the two groups was evaluated by independent-sample T test. These data are shown in table 1. cfPWV of the hypertension group was significantly higher than that of the non-hypertension group (15.38 ± 1.78 vs. 13.01 ± 1.48 m/s, P <0.01).
Morphological alterations in smooth muscle, collagen and elastin of the aortic media
On Masson’s-stained specimen slides, disorganisation of smooth muscle and focal accumulations of collagen were visible along the medial aorta of the hypertension group compared with those of the non-hypertension group (fig. 1a and fig. 1b). Weigert’s-stained cross sections of the ascending aortic media in the hypertension group frequently exhibited focal breakdown or discontinuous segments of elastic fibres compared with those of the non-hypertension group (fig. 1c and fig. 1d).
Quantitative analysis indicated that there was a significant decrease in the relative content of elastin and a significant increase in the relative content of collagen in the aortic media of the hypertension group compared with those of the non-hypertension group. There was no significant difference between the two groups in smooth muscle content of the ascending aortic media. These data are shown in table 1.
Correlation between cfPWV and the relative contents of smooth muscle, collagen and elastin of the aortic media
cfPWV showed a positive correlation with the relative contents of collagen (r = 0.576, P <0.01) and a negative correlation with the relative contents of elastin (r = –0.669, P <0.01), but no significant correlation with the relative contents of smooth muscle (r = –0.089, P= 0.638) in the media of the ascending aorta in the non-hypertension group (fig. 2a). Similarly, cfPWV showed a positive correlation with the relative contents of collagen (r = 0.539,P <0.01) and a negative correlation with the relative contents of elastin (r = –0.475, P <0.01), but no significant correlation with the relative contents of smooth muscle (r = 0.086, P= 0.652) in the media of the ascending aorta in the hypertension group (fig. 2b).
Discussion
Elevated cfPWV and its related factors
Carotid femoral pulse wave velocity, a measure of aortic stiffness, was assessed in this study and an independent-sample T test showed that CAD patients in the hypertension group showed significantly higher cfPWV than those in the non-hypertension group, which indicated that aortic wall stiffness in the hypertension group was more marked than that in the non-hypertension group. The elevated cfPWV in the hypertension group can be partly ascribed to high blood pressure. In hypertension, cfPWV is an independent predictor of both cardiovascular and all-cause mortality [3]. On the other hand, greater arterial stiffness in large arteries can also cause hypertension, the mechanism of which may be that aortic stiffness is a cause of premature return of reflected waves in late systole [13, 14]. Thus, increased aortic stiffness is associated with increased after-load. Consequently, after-load elevation raises SBP. In addition to hypertension, a range of established cardiovascular risk factors, including aging, type 2 diabetes, hypercholesterolaemia, sedentary lifestyle, cigarette smoking and elevated serum uric acid level, may raise PWV [15–20]. Hence cfPWV in the non-hypertension group was still higher than the normal level of cfPWV [21]. Moreover, some gene mutation diseases such as mitochondrial myopathy, encephalopathy, lactic acidosis, stroke-like episodes syndrome and Marfan syndrome may also increase aortic stiffness [22].
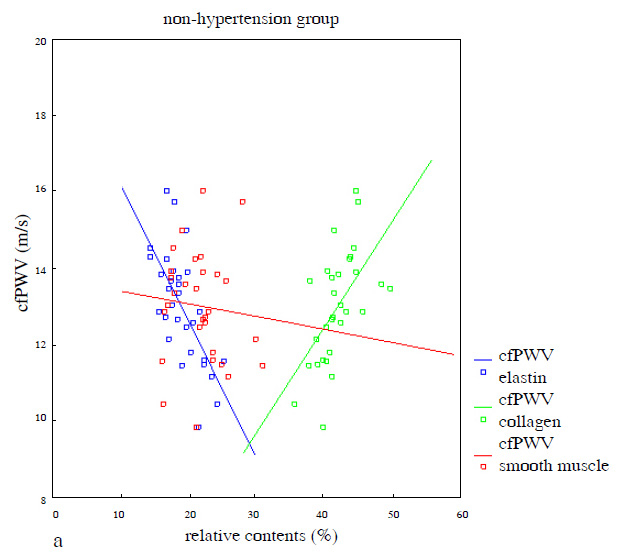
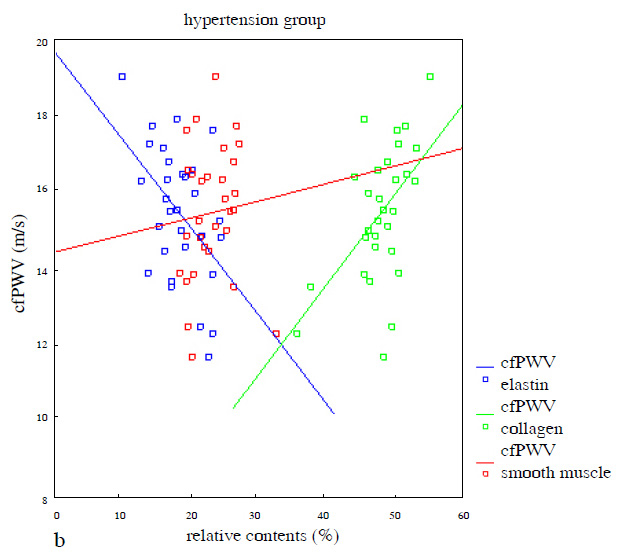
Figure 2
a) Correlation between cfPWV and relative contents of elastin (r = –0.669, 95%CI: –0.517~–0.206, P<0.01), collagen (r = 0.576, 95%CI: 0.127~0.436, P <0.01) and smooth muscle (r = ‒0.089, 95%CI: –0.181~0.113, P= 0.638) of the aortic media in the non-hypertension group; b) Correlation between cfPWV and relative contents of elastin (r = –0.475, 95%CI: –0.419~–0.069, P <0.01), collagen (r = 0.539, 95%CI: –0.098~–0.400, P<0.01) and smooth muscle (r = 0.086, 95%CI: –0.167~0.263, P= 0.652) of the aortic media in the hypertension group (simple linear regression was used in fig. 2).
Structural changes in the aortic media due to hypertension
Generalised narrowing in smaller arteries (arteriosclerosis) has long been recognised as the major pathophysiological change in essential hypertension. In contrast, structural changes to the large arteries in the development of hypertension have not been well documented. Using virtual histological intravascular ultrasound imaging, Kwon et al. found that the maximal calcium percent of the coronary artery plaque correlated significantly with brachial-ankle pulse wave velocity [23]. To determine the structural bases for increased aortic wall stiffness due to hypertension in this study, ascending aortic wall specimens were obtained from CAD patients undergoing CABG surgery and morphological alterations of the ascending aortic media were investigated. Vascular smooth muscle cells, the most abundant cell type in the aortic media, are the main source of extracellular matrix (ECM) proteins, including collagen, elastin, gelatin and proteoglycans in the arterial tunica media. Proper amounts of each of these individual ECM proteins are essential for arterial integrity to withstand the outward forces exerted by blood pressure. Collagen and elastin are the most abundant ECM proteins of the aortic wall, and they are responsible for the characteristic mechanical properties – tensile strength and elasticity [24, 25]. Study has shown that serum alterations in collagen turnover that favour collagen type I synthesis are related to increased aortic stiffness in treated hypertensive individuals [26].
Our data showed that the percentages of collagen in the ascending aortic media of the hypertension group were significantly higher than in the non-hypertension group, while the percentages of elastin in the ascending aortic media of the hypertension group were significantly lower than in the non-hypertension group. In addition, qualitative changes such as disorganisation of smooth muscle and collagen and focal fragmentation of elastic fibres were also visible along the aortic media of the hypertension group, as compared with the non-hypertension group. Hence the elevated cfPWV in the hypertension group can be ascribed to increased collagen contents and decreased elastin contents, as well as alterations in microstructures of the two components of the aortic media. These findings suggest that changes in relative contents of collagen and elastin, as well as structural alterations to the two components in the aortic media, are important factors determining the functional properties of large arteries in CAD patients.
Correlation between cfPWV and composition of the aortic media
Bivariate analysis showed that cfPWV correlated positively with the relative contents of collagen and negatively with the relative contents of elastin in the media of the ascending aorta in the two groups. There was no significant correlation between cfPWV and the percentage of the medial surface occupied by smooth muscle. The findings indicated that cfPWV may quantitatively reflect changes in collagen and elastin in the ascending aortic media in CAD patients independently of hypertension.
Conclusion
In conclusion, we found there were significant linear relations between cfPWV and the percentage of medial surface occupied by elastic fibres, as well as between cfPWV and the percentage of medial surface occupied by collagen in CAD patients with or without hypertension. These results indicated that the reduced aortic elasticity in hypertension is partly ascribable to decreased elastin, increased collagen and disorganisation of smooth muscle, collagen and elastic fibres of the aortic media. Our findings are an important step in elucidating the pathological basis of large artery stiffness caused by cardiovascular risk factors such as hypertension. Future studies should focus on elucidating the signalling pathways whereby these pathological changes occur. These investigations may result in the development of new medications to effectively treat cardiovascular disease.
Limitations
There are several limitations to this study. Specimens of the ascending aortic wall in this study may not have been collected from exactly the same anatomical position because they were obtained from CAD patients undergoing CABG surgery, which may have introduced bias into our results. In addition to histological structure, calcification of the aorta has an effect on elasticity. Apart from the drugs included in this study, some other medicines may also influence arterial properties. Additionally, the small sample size may have limited the power of this study. Moreover, the mechanism of increased cfPWV is not explained only by documentation of structural changes, since the signalling pathways whereby these changes occur have not been explained by this study. It should be emphasised that all patients in this study had CAD, which rendered biopsy easier. It is unclear whether the results in the present study apply to other patients.
Acknowledgements: This study is a joint effort of many investigators and staff members whose contribution is gratefully acknowledged. We also thank the Department of Cardiology, the Department of Geriatrics, and the Department of Cardiosurgery of Qilu Hospital, Shandong University, and most importantly, the patients who participated in this study.
References
1 Carmel MM, Michael S, Margaret M, John Y, Gordon DL, Ann R, et al. An analysis of prospective risk factors for aortic stiffness in men: 20-year follow-up from the Caerphilly prospective study. Hypertension. 2010;56:36–43.
2 Blacher J, Asmar S, Djane S, London G, Safar M. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111–7.
3 Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–41.
4 Guerin A, Blacher J, Pannier B, Marchais S, Safar M, London G. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation. 2001;103:987–92.
5 O’Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45:652–8.
6 Baoying L, Haiqing G, Xiaoli L, Yuanping L, Min W. Correlation between brachial-ankle pulse wave velocity and arterial compliance and cardiovascular risk factors in elderly patients with arteriosclerosis. Hypertens Res. 2006;5:309–14.
7 Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–63.
8 Faber M, Moller-Hou G. The human aorta. V. Collagen and elastin in the normal and hypertensive aorta. Acta Pathol Microbiol Scand. 1952;31:377–82.
9 Liu ZR, Ting CT, Zhu SX, Yin FC. Aortic compliance in human hypertension. Hypertension. 1989;14:129–36.
10 The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (The JNC 7 Report). JAMA. 2003;289:2560–72.
11 Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716.
12 Gensini,GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606–7.
13 Stefanadis C, Dernellis J, Vlachopoulos C, Tsioufis C, Tsiamis E, Toutouzas K, et al. Aortic function in arterial hypertension determined by pressure-diameter relation: Effects of diltiazem. Circulation. 1997;96:1853–8.
14 Hashimoto J, Ito S. Pulse pressure amplification, arterial stiffness, and peripheral wave reflection determine pulsatile flow waveform of the femoral artery. Hypertension. (United States), 2010;56(5):926–33.
15 Benetos A, Adamopoulos C, Bureau JM, Temmar M, Labat C, Bean K, et al. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation. 2002;105:1202–7.
16 Vaitkevicius PV, Fleg JL, Engel JH, O’Connor FC, Wright JG, Lakatta LE, et al. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–62.
17 Schram MT, Henry RM, van Dijk RA, Kostense PJ, Dekker JM, Nijpels G, et al. Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes: the Hoorn Study. Hypertension. 2004;43:176–81.
18 Lehmann ED, Watts GF, Gosling RG. Aortic distensibility and hypercholesterolaemia. Diabet Med. 1992;9:114–9.
19 Kubozono T, Miyata M, Ueyama K, Hamasaki S, Kusano K, Kubozono O, et al. Acute and chronic effects of smoking on arterial stiffness. Circ J. 2011;75:698–702.
20 Vlachopoulos C, Xaplanteris P, Vyssoulis G, Bratsas A, Baou K, Tzamou V, et al. Association of serum uric acid level with aortic stiffness and arterial wave reflections in newly diagnosed, never-treated hypertension. Am J Hypertens (United States), 2011;24(1):33–9.
21 Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors:”?" Reference Values for Arterial Stiffness’ Collaboration. Eur Heart J. 2010;31(19):2338–50. Epub 2010 Jun 7.
22 Nemes A, Geleijnse ML, Sluiter W, Vydt TC, Soliman OI, van Dalen BM, et al. Aortic distensibility alterations in adults with m.3243A>G MELAS gene mutation. Swiss Med Wkly. 2009;139(7-8):117–20.
23 Kwon JE, Mintz GS, Kim SW, Oh MS, Min YJ, Kim HK, et al. Relationship between coronary artery plaque composition by virtual histology intravascular ultrasound analysis and brachial-ankle pulse wave velocity in patients with coronary artery disease. Coron Artery Dis. 2011;22(8):565–9.
24 Jensen LT, Host NB. Collagen: scaffold for repair or execution. Cardiovasc Res. 1997;33:535–9.
25 Clark JM, Glagov S. Transmural organization of the arterial media: the lamellar unit revisited. Arteriosclerosis. 1985;5:19–34.
26 Stakos DA, Tziakas DN, Chalikias GK, Mitrousi K, Tsigalou C, Boudoulas H. Associations between collagen synthesis and degradation and aortic function in arterial hypertension. Am J Hypertens. (United States), 2010;23(5):488–94.


