Figure 1
Study design.
DOI: https://doi.org/10.4414/smw.2012.13561
Assessing the quality of healthcare has become increasingly important over time [1–5]. The demand for improvements in terms of transparency of healthcare quality from different stakeholders such as insurance companies, patient organisations and health policy-makers is increasing [1, 3–10].
Since the publication of “To err is human: building a safer health care system” in 1999, public interest in patient safety has been growing across the world. Patient safety has become a growing economic and political objective [4, 6, 10, 11]. Solid epidemiological data have shown that iatrogenic adverse events are a major problem [5, 9, 10, 12]. Adverse events are defined as unintended complications caused by medical mismanagement rather than by the patient’s underlying disease [11, 13, 14]. According to a study of the Swiss Patient Safety Foundation involving 3,983 patients, adverse events occurred in approximately 21.4% of all hospitalised patients [15]. In studies originating from France and Canada, adverse events were found in 2.9–16.6% of hospitalised patients and resulted in death in 20–57% of cases [11, 13]. In the United States, approximately 98,000–180,000 deaths per year are estimated to be caused by adverse events [3, 5, 16, 17], of which around 90% are thought to be due to failed control systems and procedures [11, 13, 16]. Approximately 37–51% of adverse events have been judged to be preventable [13]. According to Hayward et al., medical errors constitute the fifth most common cause of death in the United States [43].
In Switzerland, quality management originates in the health insurance act, which became effective in 1996 [18, 19]. Quality management includes organisational measures to improve products, processes and performances of all types. Healthcare providers and stakeholders are required to perform quality assessments, but no further specifications were made with regard to the time of introduction or the form this performance should take [18]. Up to now, the act has not been fully implemented due to a lack of coordination between the different actors [9, 18, 20].
To ameliorate patient safety, a systematic analysis of medical procedures as well as an organisation designed to coordinate quality surveillance is needed [2, 13, 16, 21, 22].
Switzerland currently lacks a detailed overview of how quality management is implemented and its effects on medical procedures and patients’ concerns [9, 18, 20]. Quality initiatives are performed by medical societies, the government, hospitals, insurance companies and patient organisations [9].
The aim of the present study was to examine the systematics of quality management in Switzerland by assessing the actors and collected parameters of current quality initiatives.
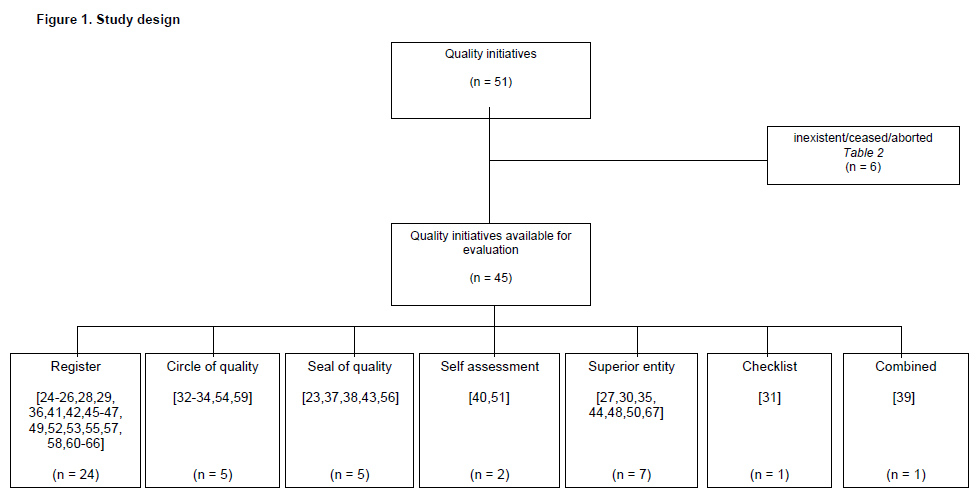
In summer 2011, we contacted in writing all the medical societies in Switzerland, the Federal Office of Public Health ( http://www.bag.admin.ch ), the Swiss Medical Association (FMH, http://www.fmh.ch ) and the head of Swiss medical insurance providers, in order to obtain detailed information on current quality initiatives. In addition, we conducted a Web-based search using academic (Pubmed) and public search engines (Google) with the terms “quality initiative”, “patient safety programme”, “patient satisfaction assessment”, “quality project” and “Switzerland”. All quality initiatives featuring standardised parameter assessment were included. The exclusion criteria were inconsistent assessment of parameters (e.g., a random sample) and a short assessment period (less than one year).

Figure 1
Study design.
After completion of the search we sent a quantitative questionnaire to the person in charge of each identified quality initiative (fig. 1). Based on 15 free text questions on general information and 14 questions on the implementation of parameters (yes/no), information was obtained on the quality indicators, periodicity and type of statistical evaluation, permission to access the data and overall cost of each initiative (see supplementary material). The questionnaire was pretested among a small group of doctors for readability and acceptability. If inconclusive or incomplete information was obtained, additional data were acquired by means of a telephone interview when possible. If an answer was refused, the data were entered on the basis of information from the quality initiative’s public website (registered accordingly in table 1). The person in charge was contacted a maximum of three times.
Information on scientific output was obtained from each quality initiative’s public website and the search engines Google and Pubmed with the terms “name of quality initiative” and “database”. Scientific output was defined as the publication of data in a peer-reviewed journal or a dissertation accepted by a university.
| Table 1: Quality initiatives in Switzerland (ordered alphabetically). | |||||||||
| ACREDIS | Seal of quality | 2006 | 2,500 | NR | Yes | 5760 | No | Yes | [23] |
| ADS (Anästhesiedatenbank der Schweiz) | Register | 1996 | 249,399 | 36 | Yes | NR | No | Yes | [24] |
| AMIS Plus | Register | 1997 | 30,088 | 36 | Yes | 40 | Yes | Yes | [25] |
| Anaphylaxie Register NORA | Register | 2006 | NR | NR | Yes | 15 | Yes | Yes | [26] |
| ANQ (Nationaler Verein für Qualitätsentwicklung) | Superior | 2000 | NR | NR | Yes | NR | No | Yes | [27] |
| AQC (Arbeitsgemeinschaft für Qualitätssicherung in der Chirurgie) | Register | 1995 | 6,000,000 | 70 | Yes | 5 | Yes | Yes | [28] |
| Arbeitsgemeinschaft Schweizer Frauenkliniken (ASF-Statistik) | Register | 1981 | 2,000,000 | 55 | Yes | 4 | Yes | Yes | [29] |
| BFS Statistik (Federal Statistical Office) | Superior | 1998 | NR | 313 | Yes | NR | No | Yes | [30] |
| Checkliste SGOT-SSOT (Checkliste Schweizer Gesellschaft für Orthopädie und Traumatologie ) | Checklist | 2010 | NR | NR | NR | NR | No | Yes | [31] |
| Cirdoc | Circle of quality | 2006 | NR | 125 | No | 10 | No | Yes | [32] |
| Cirrnet (Critical Incident Reporting and Reacting Network) | Circle of quality | 2006 | NR | NR | NR | NR | No | no | [33] |
| CIRS (Critical Incident Reporting System ) | Circle of quality | 2006 | 209 | 14 | Yes | 30 | Yes | Yes | [34] |
| DIN ISO 9001:2008 | Superior | 1983 | NR | 200 | Yes | NR | Yes | Yes | [35] |
| D-SENT (Database-surgical and endoscopic novel technologies) | Register | NR | NR | NR | NR | NR | No | no | [36] |
| EQUAM | Seal of quality | 1999 | NR | NR | NR | NR | No | No | [37] |
| Good Medical Practice | Seal of quality | 1983 | NR | 17 | Yes | NR | No | Yes | [38] |
| H+ Qualité | Combined | 2005 | NR | NR | NR | NR | No | No | [39] |
| Hippokratest | Self assessment | 2007 | NR | NR | No | NR | No | Yes | [40] |
| KIMSA (Kooperatives Integrationsmanagement der Suva mit Ärztenetzen) | Register | 2008 | NR | 380 | Yes | 10 | No | Yes | [41] |
| Mammaregister | Register | 2010 | NR | NR | Yes | 1 | No | Yes | [42] |
| MDSi (Der minimale Datensatz der SGI ) | Seal of quality | 2007 | NR | NR | NR | NR | No | No | [43] |
| MECON | Superior | 1998 | 48,914 | 28 | Yes | NR | No | Yes | [44] |
| Nephrologie Register | Register | 2006 | 6,200 | 78 | NR | NR | No | Yes | [45] |
| OP-Statistik Plastic Surgery | Register | 2009 | NR | 35 | Yes | 1 | No | Yes | [46] |
| Outcome Messung in der Psychiatrie | Register | 2002 | 9,000 | 26 | Yes | 30 | NR | Yes | [47] |
| Q-Monitoring | Superior | 2009 | NR | NR | no | 15 | No | Yes | [48] |
| Q-Reporting(Qualitätsreporting) | Register | 2008 | 1,500,000 | 120 | Yes | Yes | Yes | [49] | |
| qtools | Superior | 2008 | 105 | 4 | NR | No | Yes | [50] | |
| Quali-med-net | Self assessment | 2005 | NR | NR | no | NR | Yes | Yes | [51] |
| Qualitätsindikatoren der Schweizer Akutspitäler | Register | 2009 | NR | NR | Yes | No | Yes | [52] | |
| Quomex | Register | 2006 | 600 | 1 | Yes | 10 | Yes | Yes | [53] |
| Réseau Delta | Circle of quality | 1992 | NR | NR | NR | NR | No | No | [54] |
| SALTC (Swiss Association of Laparoscopic and Thoracoscopic Surgery) | Register | 1990 | NR | NR | NR | NR | Yes | No | [55] |
| sanaCERT | Seal of quality | 2001 | NR | NR | NR | NR | No | No | [56] |
| SCQM (Swiss Clinical Quality Management in Rheumatic Diseases) | Register | 1996 | NR | NR | NR | NR | Yes | No | [57] |
| SIRIS | Register | 2002 | 10,000 | 10 | Yes | 0.5 | No | Yes | [58] |
| SIS (Störungen im System) | Circle of quality | NR | NR | NR | NR | NR | No | No | [59] |
| Spine Tango | Register | 2002 | 7,061 | 1 | Yes | 1.5 | Yes | Yes | [60] |
| Swiss Noso | Register | 2009 | 28,097 | NR | Yes | NR | Yes | Yes | [61] |
| Swiss Spine | Register | 2005 | 10,000 | 10 | Yes | 2 | Yes | Yes | [62] |
| Swissvasc Registry | Register | 2003 | 25,000 | 22 | Yes | 5 | Yes | Yes | [63] |
| TARC (Traumaregistry of acute care) | Register | 2008 | 800 | 1 | Yes | NR | Yes | No | [64] |
| TARN (Trauma Audit & Research Network) | Register | 2009 | 400 | 1 | NR | NR | Yes | No | [65] |
| Ticino Cancer Registry | Register | 2010 | NR | NR | Yes | NR | No | Yes | [66] |
| Verein Outcome | Superior | 2000 | 600,000 | 80 | Yes | 60 | Yes | Yes | [67] |
| Response = answer obtained from the person in charge of the quality initiative. NR = not reported. | |||||||||
In all, 51 quality initiatives were found, but six no longer existed, had been terminated in the planning phase, had ceased or were non-existent in Switzerland (table 2). A total of 45 quality initiatives remained available for the present report (table 1).
The oldest quality initiative was the Arbeitsgemeinschaft Schweizer Frauenkliniken (ASF), which was conducted by the Swiss Society of Gynaecology and Obstetrics and was founded in 1981 [29]. Most of the quality initiatives were instituted in the first decade of the 21st century (n = 31) [23, 26, 27, 31–34, 39–43, 45–53, 56, 58, 60–67], and 24 were initiated between 2005 and 2011 [23, 26, 31–34, 39–43, 45, 46, 48–53, 61, 63–66]. A quality control section featured in 27 initiatives for data monitoring purposes [23–30, 34, 35, 38, 41, 42, 44, 46, 47, 49, 52, 53, 58, 60–64, 66, 67]. The time required to register a patient’s data varied between the quality initiatives from half a minute [58] for an implant register to 5,760 minutes for a seal of quality [23].
| Table 2: Quality initiatives inexistent/ceased/aborted. | ||
| N° | Name | Status |
| 1 | ARPAZ (Arbeitsgemeinschaft für Patientenzufriedenheit) | Ceased |
| 2 | Autopsie | Inexistent in Switzerland |
| 3 | Betriebsinterner Umgang mit Zwischenfällen | Ceased |
| 4 | Collaborative Breakthrough Series | Inexistent in Switzerland |
| 5 | Reanimationsdatenbank der Schweiz | Ceased |
| 6 | Schweizer Brustkrebsregister | Aborted in planning phase |
Regarding the implementation of the 45 quality initiatives available for our study, 19 were powered by medical societies [24, 25, 28, 29, 31, 32, 36, 42, 43, 45–47, 55, 57, 58, 60–63], five were hospital-based [26, 44, 53, 64, 65], 11 were launched by non-medical societies [23, 27, 33–35, 38, 39, 48, 49, 51, 67], two by the government [30, 52], two by insurance companies or related institutions [37, 41] and six by non-specified institutions [40, 50, 54, 56, 59, 66]. The costs of 14 of the 45 quality initiatives were covered by the medical society or hospital in charge [24, 28, 29, 34, 42, 44–46, 49, 53, 60–62, 64]. Another two quality initiatives were funded entirely by the government [30, 52] and one by an insurance company [41]. Governmental co-funding existed in one case [27], whereas seven quality initiatives were partly financed by sponsors [32, 33, 37, 50, 57, 63, 67]. Two quality initiatives were funded entirely by private sponsors [25, 66], such as pharmaceutical companies and private hospitals. Self-financing existed in four cases [23, 38, 40, 51]. Two initiatives were financed by a non-medical society [35, 48]. For 12 out of 45 quality initiatives, the funding source remained elusive [26, 31, 36, 39, 43, 47, 54–56, 58, 59, 65].
In total, 24 medical registers [24–26, 28, 29, 36, 41, 42, 45–47, 49, 52, 53, 55, 57, 58, 60–66], five seals of quality [23,37,38,43,56], five circles of quality [32–34, 54, 59], two self-assessment tools [40, 51] and seven superior entities [27, 30, 35, 44, 48, 50, 67] were found. One checklist [31] and one combined project [39] were also found. Of the medical registers that were found, 13 were run by surgical or anaesthesiological societies [24, 28, 29, 36, 42, 46, 53, 55, 58, 60, 62, 64, 65], while eight were concerned with medical or psychiatric illnesses [25, 26, 45, 47, 57, 61, 63, 66]. Three initiatives were not associated with a specific speciality [41, 49, 52]. Over half of the medical registers (16/24) collected data on patients’ general characteristics (e.g., sex, age, diagnosis, comorbidity, therapies carried out and their related morbidity and mortality) [26, 28, 29, 41, 42, 45, 47, 49, 52, 53, 58, 60–63, 66], while no data were obtained in two cases [25, 46]. Whether or not data on patients’ general characteristics were obtained remains unknown in six cases [24, 36, 55, 57, 64, 65]. Of the 24 medical registers, the duration of the treatment was assessed by 13 [25, 28, 29, 45, 47, 49, 52, 53, 60–63, 66], the patients’ satisfaction at the end of the treatment by four [47, 53, 60, 62], quality of life after an intervention/treatment by six [25, 28, 47, 53, 60, 62] and whether or not the patient was able to go back to work by four [25, 47, 60, 62]. The cost of treatment was evaluated by four initiatives [28, 41, 47, 53].
Seals of quality are awarded to hospitals, specific units or outpatient facilities which passed an extensive test with a variety of parameters ranging from up-to-date medical standards to high-level patient satisfaction and well-maintained medical facilities [23, 37, 38, 43, 56]. Self-assessment tools provide opportunities to compare specific knowledge and treatment strategies with others [40, 51]. Superior entities form the basis of quality initiatives, providing parameters with which to assess and conduct a quality initiative or to monitor how quality management is established. Their practical implementation relies on the user. They are assigned by either federal or cantonal authorities to coordinate the implementation of quality initiatives [27, 30, 35, 44, 48, 50, 67]. A checklist is intended to monitor operational sequences [31]. One example of a combination of different types of quality initiative is H+ qualité, which consists of different sub-programmes, including patients’ satisfaction and a Web-based hospital comparison programme [39].
Statistical evaluation was performed and a data report released by 24 quality initiatives [23–30, 34, 35, 38, 39, 47–49, 51–53, 57, 58, 60–62, 64], of which 17 released annual, semiannual or quarterly reports [24, 26, 27, 29, 30, 34, 39, 47–49, 52, 57, 58, 60–62, 64]. In one case the release periodicity was two years [51]. For six initiatives, no detailed information on periodicity was available [23, 25, 28, 35, 38, 53]. Nine of the quality initiatives did not publish their data [32, 40–42, 44, 46, 50, 63, 67]. In 12 cases, no information was available on whether or not a data report was released [31, 33, 36, 37, 43, 45, 54–56, 59, 65, 66]. Scientific processing existed in 18 of the 45 quality initiatives [25, 26, 28, 29, 34, 35, 49, 51, 53, 55, 57, 60–65, 67], while 14 of the 18 academically active projects were medical registers [25, 26, 28, 29, 49, 53, 55, 57, 60–65], of which eight were launched by groups specialising in surgery [28, 29, 53, 55, 60, 62, 64, 65].
In Switzerland, healthcare quality management is conducted by a wide variety of operators with different backgrounds and goals. Of the parameters assessed, only a few studies focused on patient satisfaction, post-treatment care and cost evaluation. There has been very little scientific evaluation of the data they obtained.
In agreement with the current literature, we found a wide variety of quality initiatives, conducted by various institutions and lacking adequate safety policies [68–70]. Measuring quality is important in improving medical care and preventing adverse events [1, 2, 4, 13, 70–73]. During the last decade, efforts to improve the quality of care have been increasing across the globe [2, 73, 74]. The concept of continuous quality improvement arose and has become more and more important [75, 76], as our findings confirmed. Care is growing increasingly complex as patients are treated by several providers. Structural changes which allow better communication and coordination are assuming growing importance [13]. Similarly to our study, the methodological approaches and objectives of quality initiatives are highly variable [68, 72, 77]. Quality initiatives may share a name but use totally different methods, measurements or resource investments [77, 78]. The effectiveness of quality improvement interventions is therefore variable, depending on the context in which they are used [78]. This makes it difficult to interpret the results in terms of a comparison of the effectiveness of quality initiatives [77].
The literature on the standardisation of quality improvement is divided in terms of its possible benefits. Walshe et al., for example, state that standardisation would still lead to highly variable outcomes [78]. Nevertheless, a framework of baseline components should be implemented in all quality initiatives if they are to be made more comparable [77, 78].
As national quality indicators are lacking in Switzerland, there is, according to the Organisation for Economic Co-operation and Development [79], a want of transparency and effectiveness with regard to quality. This may be the result of the many individual care providers who are conducting their own quality management programmes. There is still a deficiency in terms of coordination between the individual projects, despite the growing importance of coordination in the light of increasing healthcare costs [79].
We showed that the potential of registers as a quality control tool has been widely recognised in Switzerland. Medical registers are known to improve care and outcomes for patients with specific diseases while implementing evidence-based and well-researched guidelines [69, 71, 75]. They make it possible to analyse patterns of care and monitor patient safety [69, 71]. Also, they can be used to evaluate the effectiveness of healthcare interventions [69, 76]. Fonarow compared American registers of heart failure and detected a trend towards reduced in-hospital mortality, post-discharge deaths and rehospitalisation rates, as well as a significant reduction in the length of hospital stay due to the use of registers [69]. Often the same Web-based platform is used by different hospitals or practices [69, 71, 73]. This makes it possible to provide continuous feedback to each operator, as well as progressional reflection and improvement on the internal quality of care [76]. Furthermore, it provides the option of benchmarking personal results against the other participating institutions [14, 69, 76].
In order to optimise quality of care it is important to capture post-discharge outcomes, as the continuous adoption of treatment guidelines improves long-term clinical outcomes [13, 71]. We found that post-discharge care and outcomes are barely assessed in Switzerland. Further efforts in the context of primary care in the hospital setting are needed.
Patients’ satisfaction with their treatment is rarely assessed. Patient feedback evaluation has two main objectives: first, to monitor performance, and second, to improve the quality of care [80]. However, the effects are inconsistent. Whereas a study in the United Kingdom found an improvement in the quality of care through patient feedback, a French study showed that a patient-centred quality improvement initiative did not lead to systematic amelioration of care [80–82]. The most common problem lies in the non-specificity of patient declarations [80]. Evaluation of patient satisfaction might contribute to an improvement in the quality of care, but it needs to be combined with other measurements [80].
In spite of increasing efforts to improve patient safety, only a few systems exist for reporting of errors and adverse events [70], which is in agreement with our results. As data are generally scarce, an evaluation of current patient safety or trends over time is impossible [70]. Medical errors are often under-reported due to fear of reprisal and concerns regarding lawsuits [12, 73, 76]. An open culture of discussion in an open-minded environment might therefore be a first step towards increasing the incident report rate [70, 73]. According to Farley et al., the reporting system should capture both adverse events and critical incidents [70]. A wide range of staff throughout hospital or practice should participate in the reporting and discussion process with guaranteed anonymity [12, 70].
The public release of performance data is becoming increasingly common [4, 8]. We found that more than half of the initiatives disclosed their data. In the literature, contradictory opinions regarding public release of performance data are discussed [1, 4, 8, 74]. According to Lester et al., publication of performance data can lead to measure fixation, tunnel vision or misinterpretation if the indicators measure a specific process while missing the larger objective [83]. Even if the data represent the reality, differences in the external environment make misinterpretation likely [84]. Nevertheless, according to Fung et al., public release of performance data improves quality by providing greater transparency and accountability of healthcare providers through two approaches [74]: first, by improving the providers’ motivation to increase the quality of care, and second, by allowing comparison between the different initiatives on offer [74, 85]. However, public disclosure of performance data is rather insignificant as a factor influencing consumer choice [8, 85]. Personal experience or recommendations by neighbours or friends have been found to be more important [1]. An argument in favour of the public disclosure of performance data is its use as a tool to control costs and regulate the healthcare system [8, 85].
According to Rubenstein et al., promoting quality management programmes to improve the scientific approach to healthcare delivery is important [68]. In addition, a scientific advance is required with regard to cost containment [68]. We found that scientific evaluation and the publication of the data assessed are scarce in Switzerland. Similarly, quality projects throughout the world are currently underrepresented in the literature [68]. Although the results of such research would be of vital interest, evidence from clinical practice is lacking [16, 86].
There are some limitations to our study. As data were collected using a public search engine, it cannot be determined with certainty whether or not all of the existing quality initiatives were included and how representative the sample is. With regard to the voluntary basis of the replies, incompleteness of information cannot be excluded.
We collected no data on whether the initiative was performed in the outpatient care setting or the hospital setting. Therefore, no further specifications regarding improvements to specific settings can be made. As our questionnaire was based mainly on hospital-derived quality initiatives, it is possible that important features of programmes in the outpatient setting have not been assessed. It was not determined whether data were disclosed publicly or internally. Therefore, no conclusions can be drawn regarding the amount of public data disclosure in Switzerland. Even though our questionnaire asked about financial contributors, it is possible that not all sponsors were declared.
The wide variety and the large number of 45 recorded quality initiatives provides a promising basis for effective healthcare quality management in Switzerland. However, only a part of the quality initiatives assessed are coordinated by superior entities, while the specifics are rarely known and difficult to explore. Swiss healthcare costs are still increasing, meaning that quality management and evaluation of the effectiveness and cost of all types of treatment has become increasingly important. Only a few of the existing quality initiatives focus on cost evaluation. Moreover, while barely any initiatives conduct post-hospitalisation follow-ups in order to measure long-term outcomes, this would be of great importance in evaluating long-term efficiency of care. Finally, emphasis should be placed on the hitherto inadequate scientific output of quality initiatives, as research is a means to improve patient satisfaction and quality of care. A continuous comparison of the results obtained with the current literature would facilitate continuing medical progress.
In short, an independent national supervisory authority should be appointed to effectively influence all quality initiatives and their transparency and coordination.
Acknowledgments: All authors contributed equally to this work. The authors thank the following persons for providing information on their initiatives: L. Bachmann, MD; A. Bircher, MD; A. Borodini, MD; M. Brüesch, MD; P. Busch, MD; R. Capaul Ammann, MD; W. Czerwenka, MD; J. Diebold, MD; D. Erni, MD; A. Exadaktylos, MD; O. Frank, MD; A. Forster, MD; A. Gehret, MD; St. Hägeli; C. Heim, MD; S. Hess; Th. Hess, MD; Ch. Kolling, MD; Ch. Leutert, MD; A. Lorenz, MD; U. Müller, MD; J. Nadig, MD; V. Nikolic; D. O`Riordan, MD; M. Peltenburg, MD; V. Pittet, MD; B. Preiswerk, MD; D. Radovanovic, MD; L. Rageth; Ch. Röder, MD; M. Rothenbühler; Ch. Ruef, MD; A. Scheiwiller, MD; Th. Schneider; Ch. Schuetz; P. Schwab; M. Schwitter; R. Sinniger, MD; R. Tschumi; HP. Vogt, MD; D. Wiedenhöfer; P. Wigger, MD; A. Wirthner; M. Wieser, MD; D. Zahnd, MD.
1 Groene O, Skau JK, Frølich A. An international review of projects on hospital performance assessment. Int J Qual Health Care. 2008;20:162–71. Epub 2008 Mar 13.
2 Brennan TA, Leape LL, Laird NM, Hebert L, Localio AR, Lawthers AG, et al.; Harvard Medical Practice Study I. Incidence of adverse events and negligence in hospitalized patients: results of the Harvard Medical Practice Study I. 1991. Qual Saf Health Care. 2004;13:145–51; discussion 151–2.
3 Hayward RA, Hofer TP. Estimating hospital deaths due to medical errors: preventability is in the eye of the reviewer. JAMA. 2001;286:415–20.
4 Davies HT. Public release of performance data and quality improvement: internal responses to external data by US health care providers. Qual Health Care. 2001;10:104–10.
5 Bates DW, Gawande AA. Error in medicine: what have we learned? Ann Intern Med. 2000;132:763–7.
6 Heller R, Wiedenhöfer D. Zunahme der Relevanz der Ergebnisqualität in der Schweiz [http://www.vereinoutcome.ch/pdf/Relevanz_Ergebnisqualitaet_Maerz%202009.pdf]. Last access July 13th, 2011.
7 Rosenbaum S. Law and the public's health. Medicare reform. Public Health Rep. 2003;118:162–4.
8 Marshall MN, Shekelle PG, Leatherman S, Brook RH. The public release of performance data: what do we expect to gain? A review of the evidence. JAMA. 2000;283:1866–74.
9 Hess K. Steuerung der Qualität in der medizinischen Versorgung. Care Management. 2008:36–8.
10 Beyer M, Chenot R, Erler A, Gerlach FM. Using quality indicators to measure the quality of general practice care in Germany. Z Evid Fortbild Qual Gesundhwes. 2011;105:13–20. Epub 2010 Oct 8. [Article in German]
11 Ksouri H, Balanant PY, Tadié JM, Heraud G, Abboud I, Lerolle N, et al. Impact of morbidity and mortality conferences on analysis of mortality and critical events in intensive care practice. Am J Crit Care. 2010;19:135–45.
12 Frey B, Schwappach D. Critical incident monitoring in paediatric and adult critical care: from reporting to improved patient outcomes? Curr Opin Crit Care. 2010 Oct 7. [Epub ahead of print]
13 Baker GR, Norton PG, Flintoft V, Blais R, Brown A, Cox J, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ. 2004;170:1678–86.
14 Michel P, Quenon JL, de Sarasqueta AM, Scemama O. Comparison of three methods for estimating rates of adverse events and rates of preventable adverse events in acute care hospitals. BMJ. 2004;328:199.
15 Schwappach DL, Frank O, Hochreutener MA. “New perspectives on well-known issues”: patients’ experiences and perceptions of safety in Swiss hospitals. Z Evid Fortbild Qual Gesundhwes. 2011;105:542–8. Epub 2010 Aug 3.
16 Clinton HR, Obama B. Making patient safety the centerpiece of medical liability reform. N Engl J Med. 2006;354:2205–8.
17 Baker R, Olesen F. Continuous quality improvement: a solution for primary care and care across the interface? Qual Health Care. 1999;8:3–4.
18 Heller R, Wiedenhöfer D. Qualitätsmessungen im Akutspital. Trendmonitor 2009:7–10.
19 Matthews F, Rageth L, Schöb O. Qualitätssicherung – mehr als Schlagworte. SAEZ 2004:1217–21.
20 Aebi M. Rolle und Aufbau von medizinischen Registern. SAEZ. 2010:977.
21 PAHT [ http://www.pathqualityproject.eu/what_is_path.html ]. Last access July 13th, 2011.
22 Annas GJ. The patient's right to safety – improving the quality of care through litigation against hospitals. N Engl J Med. 2006;354:2063–6.
23 ACREDIS. Unabhängiges Beratungszentrum für Plastische und Ästhetische Chirurgie Deutschland/Schweiz [http://www.acredis.com/]. Last access July 19th, 2011.
24 ADS. Anästhesiedatenbank Schweiz [http://www.iumsp.ch/ADS]. Last access July 19th, 2011.
25 AMIS Plus – National Registry of Acute Myocardial Infarction in Switzerland [http://www.amis-plus.ch/]. Last access July 19th, 2011.
26 Anaphylaxie-Register NORA [http://www.anaphylaxie.net/]. Last access July 19th, 2011.
27 ANQ. Nationaler Verein für Qualitätsentwicklung in Spitälern und Kliniken [http://www.anq.ch/]. Last access July 19th, 2011.
28 AQC. Arbeitsgemeinschaft für Qualitätssicherung in der Chirurgie [http://www.aqc.ch]. Last access July 19th, 2011.
29 ASF. Arbeitsgemeinschaft Schweizer Frauenkliniken [http://www.sevisa.ch]. Last access July 19th, 2011.
30 BfS. Federal Statistical Office [http://www.bfs.admin.ch/bfs/portal/de/index/infothek/erhebungen__quellen/blank/blank/mkh/01.html]. Last access July 19th, 2011.
31 Checkliste SGOT-SSOT [http://www.sgotssot.ch]. Last access July 19th, 2011.
32 Cirdoc. Umgang mit kritischen Ereignissen in der Arztpraxis [http://www.hawadoc.ch/de/hawaplus/hawaplus_dienstleistungen_cirdoc.asp]. Last access July 19th, 2011.
33 Cirrnet. Critical Incident Reporting & Reacting NETwork [http://www.cirrnet.ch/]. Last access July 19th, 2011.
34 CIRS. Critical Incident Reporting System [http://www.cirs.ch/]. Last access July 19th, 2011.
35 DIN ISO 9001:2008 [http://www.sqs.ch/branche_gesundheit]. Last access July 19th, 2011.
36 D-SENT. Database-surgical and endoscopic novel technologies [http://www.d-sent.ch/]. Last access July 19th, 2011.
37 EQUAM. Externe Qualitätssicherung in der Medizin [http://www.equam.ch/]. Last access July 19th, 2011.
38 Good Medical Practice. Zertifizierung von Qualitätsmanagementsystem zugeschnitten auf Arztpraxen [http://www.sqs.ch/index/leistungsangebot/lgmp.htm]. Last access July 19th, 2011.
39 H+ Qualité [http://www.hplusqualite.ch/]. Last access July 19th, 2011.
40 Hippokratest [http://www.hippokratest.com/]. Last access July 19th, 2011.
41 KIMSA. Kooperatives Integrationsmanagement der Suva mit Ärztenetzen [http://www.kimsa.ch/]. Last access July 19th, 2011.
42 Mammaregister [http://www.plastic-surgery.ch/index.php?lg=DE&sn=03&c=01&sc=01]. Last access July 19th, 2011.
43 MDSi. Der minimale Datensatz der SGI [http://www.sgi-ssmi.ch/sgi-konzept-mdsi.html?&L=3]. Last access July 19th, 2011.
44 MECON. Measure & consult GmbH [http://www.mecon.ch/]. Last access July 19th, 2011.
45 Nephrologie Register [http://www.sgn-ssn.ch/cms/website.php?id=/de/sgn-ssn/arbeitsgruppen.htm]. Last access July 19th, 2011.
46 OP-Statistik Plastic Surgery [http://www.plastic-surgery.ch/index.php?lg=DE&sn=03&c=01&sc=01]. Last access July 19th, 2011.
47 Outcome Messung in der Psychiatrie [http://www.psychiatrie-winterthur.ch/]. Last access July 19th, 2011.
48 Q-Monitoring ambulante Medizin CH [http://www.fmh.ch/themen/qualitaet/q_monitoring.html]. Last access July 19th, 2011.
49 Q-Reporting. Qualitätsreporting [http://www.initiative-qualitaetsmedizin.de/]. Last access July 19th, 2011.
50 qtools. Outcome Messung im Gesundheitswesen [http://www.it-work.ch/qtools/-de_vt56qex.html]. Last access July 19th, 2011.
51 Quali-med-Net [http://www.medswiss.net/]. Last access July 19th, 2011.
52 Qualitätsindikatoren im Schweizer Gesundheitswesen [http://www.bag.admin.ch/shop/00102/00542/index.html?lang=de]. Last access July 19th, 2011.
53 Quomex. Ergebnisforschung über chirurgische Methoden der oberen Extremität [http://www.schulthess-klinik.ch/app/article/index.cfm?fuseaction=OpenArticle&aoid=2762&lang=DE]. Last access July 19th, 2011.
54 Réseau Delta. Réseau de soins Delta [http://www.reseau-delta.ch]. Last access July 19th, 2011.
55 SALTC. Swiss Association for Laparoscopic and Thoracoscopic Surgery [http://www.saltc.ch/]. Last access July 19th, 2011.
56 sanaCERT. Schweizerische Stiftung für die Zertifizierung der Qualitätssicherung im Gesundheitswesen [http://www.sanacert.ch/sana.cgi]. Last access July 19th, 2011.
57 SCQM. Swiss Clinical Quality Management in Rheumatic Diseases [http://www.scqm.ch/]. Last access July 19th, 2011.
58 SIRIS. Schweizerisches Implantatregister [http://www.siris-implant.ch/]. Last access July 19th, 2011.
59 SIS. Störungen im System – Fehlerkultur in der Grosspraxis [http://www.praxis-bubenberg.ch/DE/]. Last access July 19th, 2011.
60 Spine Tango [http://www.eurospine.org/p311000381.html]. Last access July 19th, 2011.
61 SwissNoso [http://www.swissnoso.ch/de/swissnoso]. Last access July 19th, 2011.
62 Swiss Spine [http://www.spinesociety.ch/quality_assessment.php]. Last access July 19th, 2011.
63 SwissVasc [https://www.rehabnet.ch/SWISSVASC/]. Last access July 19th, 2011.
64 TARC. Traumaregistry of acute care [http://www.eurotrauma.net/site2/index.php?option=com_content&view=article&id=46&Itemid=55]. Last access July 19th, 2011.
65 TARN. Trauma Audit & Research Network [http://www.tarn.ac.uk/]. Last access July 19th, 2011.
66 Ticino Cancer Registry [http://www4.ti.ch/index.php?id=31170]. Last access July 19th, 2011.
67 Verein Outcome [http://www.vereinoutcome.ch/de/home/index.asp]. Last access July 19th, 2011.
68 Rubenstein LV, Hempel S, Farmer MM, Asch SM, Yano EM, Dougherty D, et al. Finding order in heterogeneity: types of quality-improvement intervention publications. Qual Saf Health Care. 2008;17:403–8.
69 Fonarow GC. Improving quality of care and outcomes for heart failure. Circ J. 2011;75:1783–90. Epub 2011 Jul 5.
70 Farley DO, Haviland A, Champagne S, Jain AK, Battles JB, Munier WB, et al. Adverse-event-reporting practices by US hospitals: results of a national survey. Qual Saf Health Care. 2008;17:416–23.
71 Fonarow GC, Abraham WT, Albert NM, Gattis Stough W, Gheorghiade M, Greenberg BH, et al.; OPTIMIZE-HF Investigators and Hospitals. Influence of a performance-improvement initiative on quality of care for patients hospitalized with heart failure: results of the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF). Arch Intern Med. 2007;167:1493–502.
72 Danz MS, Rubenstein LV, Hempel S, Foy R, Suttorp M, Farmer MM, et al. Identifying quality improvement intervention evaluations: is consensus achievable? Qual Saf Health Care. 2010;19:279–83. Epub 2010 Jul 14.
73 Abstoss KM, Shaw BE, Owens TA, Juno JL, Commiskey EL, Niedner MF. Increasing medication error reporting rates while reducing harm through simultaneous cultural and system-level interventions in an intensive care unit. BMJ Qual Saf. 2011 Jun 20. [Epub ahead of print]
74 Fung CH, Lim YW, Mattke S, Damberg C, Shekelle PG. Systematic review: the evidence that publishing patient care performance data improves quality of care. Ann Intern Med. 2008;148:111–23.
75 O’Neill SM, Hempel S, Lim YW, Danz MS, Foy R, Suttorp MJ, et al. Identifying continuous quality improvement publications: what makes an improvement intervention “CQI”? BMJ Qual Saf. 2011 Jul 4. [Epub ahead of print]
76 Brush JE Jr, Balakrishnan SA, Brough J, Hartman C, Hines G, Liverman DP, et al. Implementation of a continuous quality improvement program for percutaneous coronary intervention and cardiac surgery at a large community hospital. Am Heart J. 2006;152:379–85.
77 Lemmens KM, Nieboer AP, van Schayck CP, Asin JD, Huijsman R. A model to evaluate quality and effectiveness of disease management. Qual Saf Health Care. 2008;17:447–53.
78 Walshe K, Freeman T. Effectiveness of quality improvement: learning from evaluations. Qual Saf Health Care. 2002;11:85–7.
79 Qualitätsindikatoren der Schweizer Akutspitäler 2007, [http://www.bag.admin.ch/shop/00102/00542/index.html?lang=de...%20-%20Ähnliche%20Seiten]. Last access July 19th, 2011.
80 Reeves R, Seccombe I. Do patient surveys work? The influence of a national survey programme on local quality-improvement initiatives. Qual Saf Health Care. 2008;17:437–41.
81 Fitzpatrick R. Assessment of quality of life as an outcome: finding measurements that reflect individuals’ priorities. Qual Health Care. 1999;8:1–2.
82 Boyer L, Francois P, Doutre E, Weil G, Labarere J. Perception and use of the results of patient satisfaction surveys by care providers in a French teaching hospital. Int J Qual Health Care. 2006;18:359–64. Epub 2006 Aug 24.
83 Lester HE, Hannon KL, Campbell SM. Identifying unintended consequences of quality indicators: a qualitative study. BMJ Qual Saf. 2011 June 21. [Epub ahead of print]
84 Smith P. On the unintended consequences of publishing performance data in the public sector. Int J Pub Admin. 1995:277–30.
85 Schwappach DL, Schubert HJ. To disclose or not to disclose? Chances and risks of the publication of medical quality comparisons. Dtsch Med Wochenschr. 2007;132:2637–42. [Article in German]
86 Freemantle N, Grilli R, Grimshaw J, Oxman A. Implementing findings of medical research: the Cochrane Collaboration on Effective Professional Practice. Qual Health Care. 1995;4:45–7.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.