A population-based study on the patterns of use of different chemotherapy regimens in patients with early breast cancer
DOI: https://doi.org/10.4414/smw.2012.13571
Markus
Joerger, Silvia
Ess, Silvia
Dehler, Anita
Savidan, Christine
Bouchardy, Harald
Frick, Isabelle
Konzelmann, Beat
Thürlimann
Summary
BACKGROUND: There is considerable heterogeneity in the use of chemotherapy in early breast cancer (BC), despite international recommendations issued from the NCCN, NIH and the St.Gallen bi-annual conference.
METHODS: We included 1,535 patients from seven Swiss cancer registries between 2003 and 2005 receiving chemotherapy for stage I to III BC. Chemotherapy was categorised into (a) FAC/FEC, anthracyclines followed by CMF or anthracycline-taxane combinations (FAC-T) (781 patients) and (b) other chemotherapy regimens such as CMF/AC (EC) (754 patients). Predictors for choosing FAC-T over non-FAC-T chemotherapy were separately determined in all patients and in ER-negative patients (n = 496) by multivariate logistic regression analysis.
RESULTS: The use of FAC-T increased significantly over time, from 44% in 2003 to 55% in 2005. BC stage III (versus stage I-II) and nodal positivity were the predominant predictors for using FAC-T chemotherapy in the adjusted model (odds ratio (OR) 4.1, 95%-confidence intervals (CI) 2.6–6.3 and OR 3.0, 95%-CI 2.0–4.4, respectively). In high-risk ER-negative BC patients, poor histological differentiation was more important to choose FAC-T chemotherapy (OR 3.8, 95%-CI 1.9–7.5) than tumour stage or nodal status. The use of FAC-T chemotherapy varied substantially among the seven geographic regions, from 20% in rural Grisons-Glarus to 73% in Zurich.
CONCLUSIONS: Tumour biology is a predominant factor for choosing FAC-T over older chemotherapy regimens in patients with ER-negative early BC, but improvements should be made to reduce the substantial regional heterogeneity. Further epidemiological studies should assess how the use of FAC-T chemotherapy is affecting clinical outcome in patients with early BC and different risk profiles.
Introduction
Combination chemotherapy is indicated in non minimal-risk early breast cancer (BC), as there is a relevant reduction of the risk of recurrence that can be achieved with an acceptable level of treatment-related adverse events [1]. Over time, three generations of chemotherapy regimens have been evaluated, which are the cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) regimen [2], the anthracycline-based regimens [3–9], and the taxane-containing regimens [10–15]. According to the 2005 Oxford meta-analysis, adjuvant treatment with six months of adjuvant anthracycline-based chemotherapy reduces the annual BC mortality by about 38% for women younger than 50 years of age, and by about 20% for those aged 50–69 years [16]. One of the persisting controversies in the adjuvant treatment of early BC is the type of chemotherapy chosen for patients with oestrogen receptor (ER) negative early BC. Following the first publication in 2003 [11], accumulating data support the use of 5-fluorouracil/doxorubicin(epirubicin)/cyclophosphamide (FAC/FEC) and anthracycline-taxane containing chemotherapy in patients with ER-negative BC, resulting in improved clinical outcome compared to CMF or AC/EC chemotherapy regimens [11–15, 17–20]. This is also supported by more recent data from the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), indicating that by the addition of taxanes to antracycline-containing regimens, recurrence-free survival is improved in patients with ER-negative BC [21]. However, it is unclear how these data should be applied to the individual patient. Several treatment guidelines, including the St. Gallen consensus recommendations [22], the National Comprehensive Cancer Network (NCCN) Breast Cancer guidelines [23–24] and the National Institutes of Health (NIH) consensus recommendations [25], have been developed to help physicians to choose between the different chemotherapy regimens, based upon patient and tumour characteristics. The aim of the present study is to assess the patterns of use of different chemotherapy regimens, and more specifically assess the introduction of the more recent FAC/FEC and anthracycline-taxane containing chemotherapy in patients with ER-negative early BC.
Methods
Study population and data collection
For this study, we used data from a representative sample of 4820 women who had being diagnosed with invasive BC between January 1st, 2003 and December 31st, 2005. Patients were identified from seven population-based cancer registries covering roughly 3.5 Mio inhabitants, or 47% of the Swiss population (fig. 1). At the time of the study initiation, some regions in Switzerland were not covered by a cancer registry, but there was no indication that this introduced any bias to the data. Routine indicators of data completeness and quality of the participating registries were good, and patients identified on the basis of death certificates only were excluded from the study. The proportion of histological verification was over 90% [26]. A more detailed methodology of this study has been reported previously [27–28]. Based on the requirements of the European Society of Mastology (EUSOMA) Audit system on Quality of Breast Cancer Treatment criteria [29], a database was designed for data entry at the site of the cancer registries. Items included information on patient and tumour characteristics, diagnostic circumstances and treatments planed and delivered. Trained registry staff were centrally instructed and following this data items were abstracted from pathology reports, medical charts and questionnaires sent to treating physicians, and added to the database by trained staff. Registries could choose between collecting information on all registered and eligible cases diagnosed with BC between 2003 and 2005, or on a random sample of at least 500 cases. Five registries (Geneva, Valais, Ticino, St. Gallen-Appenzell, Grisons Glarus) collected information on all registered cases, and the registries from Basel and Zurich collected information on a random sample of 505 cases, using the methodology as described previously [30]. Tests of representativeness did not suggest any bias regarding patient age, BC stage and biology. Breast cancer staging used the 6th edition of the American Joint Committee on Cancer staging criteria [31]. From the final dataset, we included 1,535 patients ≤75 years of age with incident BC stage I-III, receiving either adjuvant (1,317 cases, 86%) or neo-adjuvant (218 cases, 14%) chemotherapy in a curative intention (fig. 1). There were 121 patients who were intended to receive chemotherapy, but in whom chemotherapy was never started, mostly due to patient refusal. These patients were defined as not having received chemotherapy. The study was accepted by the Cantonal Ethics Committee in St. Gallen, where the study centre is located.

Figure 1
Consort diagram.
* calculated from the breast cancer yearly average incidence rates in the periods 1999–2003 and 2004–2008 (http://www.nicer.org/Editor/files/ cancer_incidence.pdf)
BC = breast cancer, NSCT = nonstandard chemotherapy regimens.
Data analysis
Chemotherapy regimens were grouped into two categories as follows: (a) FAC/FEC for ≥6 cycles, anthracyclines followed by CMF or anthracycline-taxane combinations (FAC-T) (781 patients) and (b) other chemotherapy regimens such as CMF for 6 cycles, AC(EC) for 4 cycles or other regimens, including non-standard chemotherapy (non-FAC-T) (754 patients). Non-standard chemotherapy (NSCT) was defined as drugs or drug combinations that were not registered or not investigated for adjuvant use, or proved to be inferior when tested against standard regimens. The use of FAC-T and non-FAC-T chemotherapy was assessed over the years of BC diagnosis (2003 to 2004), using Wilcoxon-type tests for trend. Patient and tumour characteristics, socio-economic and health care provider-related factors were compared between patients receiving FAC-T chemotherapy versus those receiving non-FAC-T chemotherapy using two-sided Fisher’s exact test. Predictors for choosing FAC-T over non-FAC-T chemotherapy were separately determined in all patients receiving chemotherapy and in ER-negative patients (n = 496) using multivariate logistic regression analysis, adjusting for known prognostic factors such as tumour stage and biology. For the analysis of potential predictors for chemotherapy, women receiving FAC-T were defined as cases and women not receiving FAC-T as controls. Predictors for the use of NSCT were similarly assessed. The following parameters were assessed as potential predictors within multivariate regression analysis: HER2-status [a result of immuno-histochemical analysis of 3+ (IHC 3+), or a positive result on fluorescence in situ hybridisation (FISH) for HER2 gene amplification, histopathological grading (poor versus good/intermediate), tumour stage (stage I/II versus III), nodal status (nodal negative versus nodal positive), lymphovascular invasion (positive lymphovascular invasion versus no invasion), patient age (≤50 versus >50 years), affluence (highest quartile of median income versus others), type of health care insurance (private versus other), education (high school degree versus other) as derived from the type of occupation, breast surgeon’s annual yearly caseload (highest tertile versus others, with the highest tertile corresponding to ≥27 breast surgeries per year), case presentation at the local multidisciplinary tumour conference (MDTC), treatment within an institute participating in clinical research, and patient residence (urban/suburban versus rural residence, as defined by the National Bureau of Statistics [32]). The definition for HER2- and ER-status was identical in patients from all regions. Data for the type of health care insurance (HCI) were available for 84% of the patients, education for 74%, yearly surgical caseload for 72% and MDTC for 83% of the patients. The geographic heterogeneity of using FAC-T or Non-FAC-T chemotherapy was assessed across the regions of patient residence (according to the 7 recruiting cancer registries), using Fisher’s exact test. Finally, adherence to the full number of chemotherapy cycles (FAC-T or Non-FAC-T) was assessed using chi-square statistics. All tests of significance were two-sided, and p <0.05 was considered significant. With multivariate logistic regression analysis, introducing multiple prognostic factors such as tumour stage and biology, a p-value of 0.01 was considered significant. All statistical analyses were performed using STATA 11.0 software (STATA Corp, College Station, Texas, U.S.).
Results
Study population and chemotherapy regimens
FAC/FEC chemotherapy for ≥6 cycles was the most frequently used treatment schedule in 548 patients (36%), followed by 4 cycles of AC/EC in 481 patients (31%). FAC-T chemotherapy was given in 781 patients (51%, non-FAC-T in 754 patients (49%). The use of FAC-T chemotherapy increased significantly over time, from 44% in 2003 to 55% in 2005 (p = 0.002 for trend). NSCT was given in 105 patients (7%), including regimens such as leukeran/methotrexate/5-FU (LMF) in 21 cases, epirubicin/carboplatin/5-fluorouracil in 5 cases, navelbine in 14 cases, paraplatin and paclitaxel in 8 cases, capecitabine ± navelbine in 7 cases, single-agent epirubicin or liposomal doxorubicin in 8 cases, carboplatin/gemcitabine in 3 cases. The use of NSCT did not significantly change over time (5% in 2003 and 8% in 2005). Patient and tumour characteristics were significantly different between patients receiving FAC-T chemotherapy and those receiving non-FAC-T, with patients receiving FAC-T having a poorer risk profile (table 1). Patients who were treated in an institution participating in clinical research and living in an urban/suburban region were significantly more likely to receive FAC-T chemotherapy. FAC-T chemotherapy was more often used in the neo-adjuvant setting compared to non-FAC-T chemotherapy (19 vs. 9%, p <0.001). Overall, 496 out of the 1535 patients had ER-negative BC (32%), and 1026 patients had node-positive BC (67%). Within the major patient subgroups, FAC-T chemotherapy was most often given to patients ≤50 years of age with node-positive, ER-negative BC (81%) and least often to patients with well or moderately differentiated, ER-positive BC (22%) (table 2).
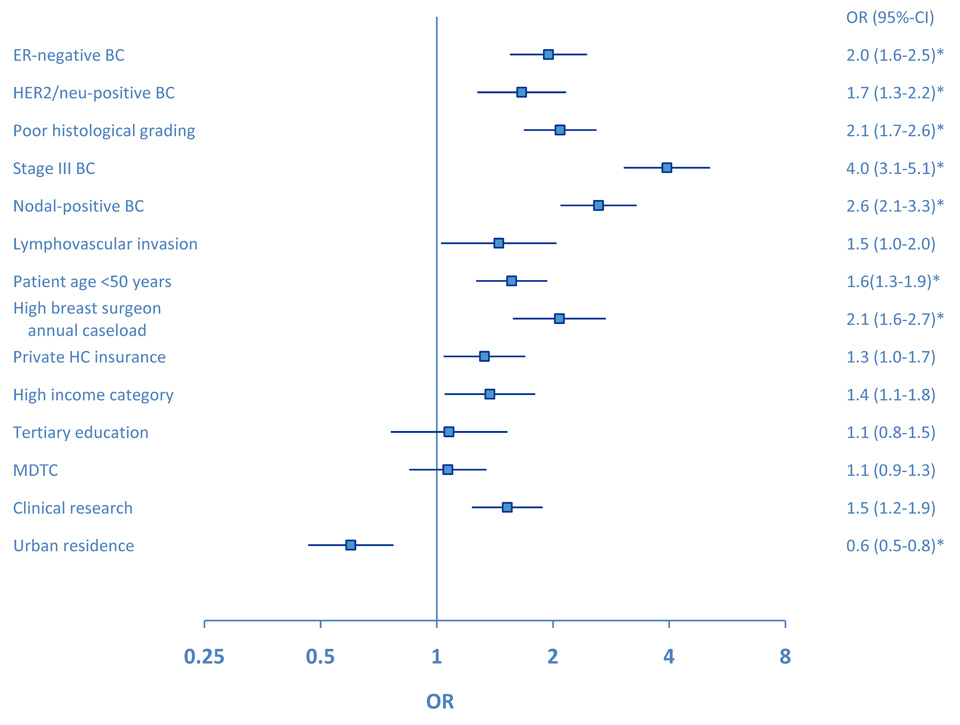
Figure 2
Forrest plot for the use of FAC-T chemotherapy compared to non-FAC-T chemotherapy in the overall study group (ER-positive and ER-negative early breast cancer). Clinical significance (p <0.01) from adjusted multivariate analysis is marked by an asterisk. OR = odds ratio, ER = oestrogen receptor, BC = breast cancer, MDTC = multidisciplinary tumour conference.
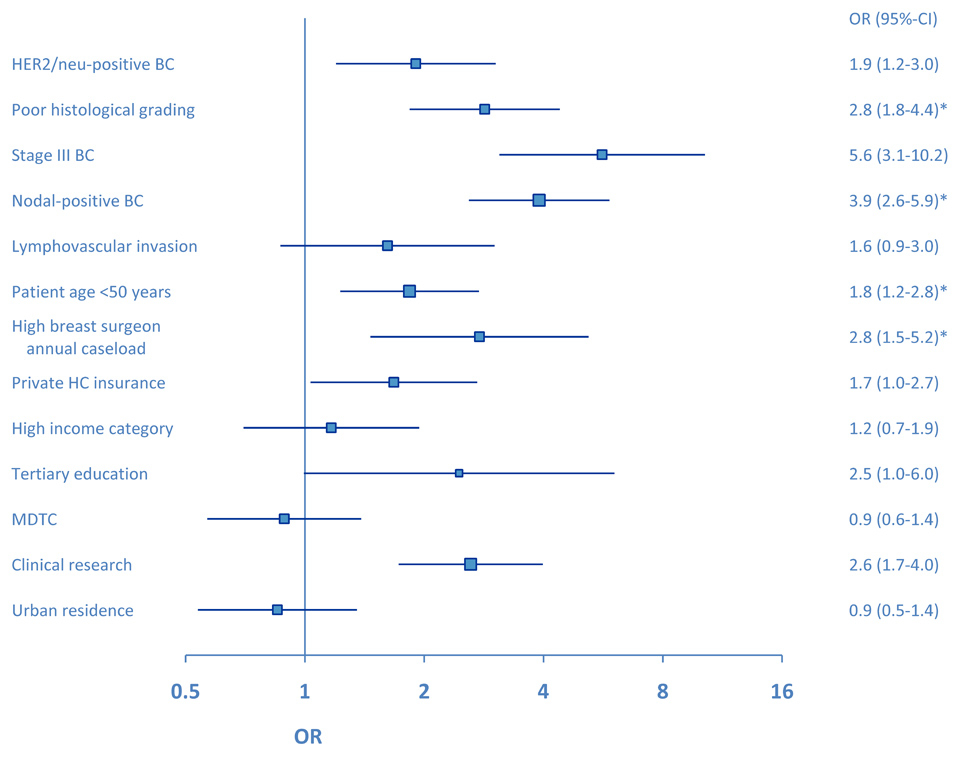
Figure 3
Forrest plot for the use of FAC-T chemotherapy compared to non-FAC-T chemotherapy in patients with ER-negative early breast cancer. Clinical significance (p <0.01) from adjusted multivariate analysis is marked by an asterisk. OR = odds ratio, BC = breast cancer, MDTC = multidisciplinary tumour conference.
Predictors of FAC-T and non-FAC-T chemotherapy
In all patients, tumour stage, biology and patient age were significant predictors for choosing FAC-T chemotherapy over non-FAC-T chemotherapy (fig. 2). In the adjusted multivariate model, BC stage III (versus stage I–II) and nodal positivity were the predominant predictors for using FAC-T chemotherapy (odds ratio (OR) 4.1, 95%-confidence intervals (CI) 2.6–6.3 and OR 3.0, 95%-CI 2.0–4.4, respectively). Patients with a rural residence were less likely to receive FAC-T compared to non-FAC-T chemotherapy (adjusted OR 0.4, 95%-CI 0.3–0.6). In high-risk ER-negative BC patients, poor histological differentiation was more important to choose FAC-T chemotherapy over non-FAC-T chemotherapy (adjusted OR 3.8, 95%-CI 1.9–7.5) than was tumour stage or nodal status (fig. 3). In ER-negative BC patients, tumour stage (adjusted OR 2.6, 95%-CI 0.9–6.8) and HER2-status (adjusted OR 1.8, 95%-CI 0.9–3.8) were no more significant as predictors for choosing FAC-T chemotherapy over non-FAC-T chemotherapy. Patients being treated by a breast surgeon with a high annual caseload were more likely to receive FAC-T chemotherapy compared to non-FAC-T chemotherapy (adjusted OR 4.2, 95%-CI 1.5–11.6). The use of FAC-T chemotherapy varied substantially among the seven geographic regions and ranged from 20% in rural Grisons-Glarus to 73% in urban Zurich. In the adjusted multivariate model, patients with residence in the regions of Geneva (OR 2.5, 95%-CI 1.9–3.3), Zurich (OR 3.9, 95%-CI 2.5–6.0) and St.Gallen-Appenzell (OR 1.6, 95%-CI 1.2–2.2) were significantly more likely to receive FAC-T compared to non-FAC-T chemotherapy. On the contrary, patients with residence in the regions of Ticino (OR 0.2, 95%-CI 0.2–0.3) and Grisons-Glarus (OR 0.2, 95%-CI 0.1–0.3) were less likely to receive FAC-T compared to non-FAC-T chemotherapy. In patients with ER-negative BC, the use of FAC-T chemotherapy ranged from 32% in Grisons-Glarus to 77% in urban French-speaking Geneva. In the adjusted model in ER-negative BC, patients with residence in the regions of Geneva (OR 3.5, 95%-CI 1.9–6.4), St.Gallen-Appenzell (OR 3.1, 95%-CI 1.7–5.4) were significantly more likely to receive FAC-T compared to non-FAC-T chemotherapy. On the contrary, patients with residence in the regions of Ticino (OR 0.2, 95%-CI 0.1–0.4) and Grisons-Glarus (OR 0.2, 95%-CI 0.1–0.5) were less likely to receive FAC-T compared to non-FAC-T chemotherapy.
|
Table 1: Patient and tumour characteristics according to chemotherapy intensity in all patients receiving chemotherapy. |
| |
FAC-T (n = 781)
|
Non-FAC-T (n = 754)
|
p-value
|
|
|
|
|
Patients
|
(%)
|
Patients
|
(%)
|
(χ2-Test)
|
| ER-status |
|
|
|
|
<0.001 |
| |
Positive |
477 |
(61) |
556 |
(74) |
| |
Negative |
300 |
(39) |
196 |
(26) |
| HER2-status |
|
|
|
|
0.001 |
| |
Negative |
487 |
(71) |
513 |
(79) |
| |
Positive |
202 |
(29) |
138 |
(21) |
| Histological grading |
|
|
|
|
<0.001 |
| |
1–2 |
374 |
(49) |
479 |
(65) |
| |
3 |
382 |
(51) |
260 |
(35) |
| Stage |
|
|
|
|
<0.001 |
| |
I–II |
459 |
(59) |
616 |
(82) |
| |
III |
322 |
(41) |
138 |
(18) |
| Nodal status |
|
|
|
|
<0.001 |
| |
Positive |
598 |
(77) |
428 |
(57) |
| |
Negative |
183 |
(23) |
326 |
(43) |
| Lymphovascular invasion |
|
|
|
|
0.23 |
| |
Negative |
96 |
(13) |
78 |
(10) |
| |
Positive |
669 |
(87) |
669 |
(90) |
| Patient age [Median (SD)] |
51.1 |
|
54.4 |
|
<0.001a
|
| |
<50 years |
371 |
(48) |
276 |
(37) |
| |
≥50 years |
410 |
(52) |
478 |
(63) |
| Surgical case-load |
|
|
|
|
<0.001 |
| |
Low |
386 |
(62) |
356 |
(74) |
| |
High |
241 |
(38) |
128 |
(26) |
| Health care insurance |
|
|
|
|
0.06 |
| |
Basic |
451 |
(64) |
399 |
(69) |
| |
Private |
259 |
(36) |
182 |
(31) |
| Income category |
|
|
|
|
0.03 |
| |
Low |
616 |
(79) |
628 |
(83) |
| |
High |
165 |
(21) |
126 |
(17) |
| Education |
|
|
|
|
0.80 |
| |
Basic |
536 |
(85) |
429 |
(86) |
| |
Tertiary |
94 |
(15) |
71 |
(14) |
| MDTC |
|
|
|
0.54 |
|
| |
No |
339 |
(48) |
285 |
(50) |
| |
Yes |
362 |
(52) |
283 |
(50) |
| Clinical research |
|
|
|
|
<0.001 |
| |
No |
401 |
(51) |
458 |
(61) |
| |
Yes |
380 |
(49) |
296 |
(39) |
| Residence |
|
|
|
|
<0.001 |
| |
Rural |
136 |
(17) |
189 |
(25) |
| |
Urban |
645 |
(83) |
565 |
(75) |
| N = number of patients, SD = standard deviation, HICT = high-intensity chemotherapy, LICHT = low-intensity chemotherapy, ER = oestrogen receptor status, MDTC = multidisciplinary tumour conference.
a
p-value (T-test) |
|
Table 2: Use of FAC-T chemotherapy in patient subgroups. |
| |
ER-positive (%)
|
|
|
|
|
|
ER-negative (%)
|
|
|
|
|
|
477/1033 (46%)
|
|
|
|
|
|
300/496 (60)
|
|
|
|
| |
|
|
N-positive |
(%) |
N-negative |
(%) |
N-positive |
(%) |
N-negative |
(%) |
| Subgroup |
Patients |
(%) |
398/746 |
(53) |
79/287 |
(28) |
197/275 |
(72) |
103/221 |
(47) |
| ≤50 years |
371/647 |
(57) |
180/282 |
(64) |
48/157 |
(31) |
91/112 |
(81) |
49/93 |
(53) |
| >50 years |
410/888 |
(46) |
218/464 |
(47) |
31/130 |
(24) |
106/163 |
(65) |
54/128 |
(42) |
| G1 or G2 |
374/853 |
(44) |
277/541 |
(51) |
41/185 |
(22) |
37/63 |
(59) |
17/61 |
(28) |
| G3 |
382/642 |
(60) |
107/190 |
(56) |
35/96 |
(37) |
153/198 |
(77) |
86/158 |
(54) |
| Stage I to II |
459/1,075 |
(43) |
185/428 |
(43) |
78/282 |
(28) |
93/144 |
(65) |
101/219 |
(46) |
| Stage III |
322/460 |
(70) |
213/318 |
(67) |
1/5 |
(20) |
104/131 |
(79) |
2/2 |
(100) |
| ER = oestrogen receptor status, N = nodal status, G = histological grading. |
Treatment adherence
In patients with both ER-positive or ER-negative BC, fewer patients in the FAC-T chemotherapy group were able to receive the full number of planned treatment cycles compared to patients in the non-FAC-T chemotherapy group (83% vs. 92%, p <0.001). Similarly, fewer patients in the FAC-T chemotherapy group were able to receive the full number of planned treatment cycles compared to patients in the non-FAC-T chemotherapy group in patients with ER-negative BC (81% vs. 94%, p <0.001). This was mainly due to a high proportion of patients receiving all 4 cycles of AC/EC chemotherapy (97% of patients in both the overall study group and in patients with ER-negative BC). In the adjusted multivariate model, affiliation to the non-FAC-T chemotherapy group was the significant predictor for the adherence to the full number of chemotherapy cycles in the overall study population (OR 2.58, 95%-CI 1.39–4.79) and in patients with ER-negative BC (OR 4.9, 95%-CI 1.5–16.3). Patient and tumour characteristics, socioeconomic and health care provider-related factors did not predict for the adherence to the full number of chemotherapy cycles. In particular, there was no evidence for elderly patients to have increased toxicity or inferior treatment adherence compared to younger patients.
Discussion
In the present population-based study, the patterns of use of different chemotherapy regimens in early BC were analysed, and tumour biology was found to be of increasing predictive significance for choosing FAC-T chemotherapy over non-FAC-T chemotherapy in patients with high-risk ER-negative BC. Chemotherapy regimens such as CMF [2] or AC/EC [4] are typically used in patients with node-negative early BC, while the newer regimens such as AC/EC followed by CMF [33], Canadian CEF [6–8], the CAF regimen [5], dose-dense cyclophosphamide, doxorubicin and paclitaxel [11–12, 15], FEC100 followed by docetaxel [14], tailored FEC [34], FEC100 [35] and TAC (docetaxel, doxorubicin, cyclophosphamide) [13] are more frequently offered to patients with node-positive BC. In our study, 72% of patients with ER-negative, node-positive BC received FAC-T chemotherapy. In 2006, Berry and colleagues retrospectively assessed the improvements in adjuvant chemotherapy in patients with ER-negative, node-positive BC compared to patients with ER-positive BC receiving adjuvant tamoxifen [36]. They compared disease-free and overall survival according to ER-status among patients enrolled in three consecutive randomised trials of chemotherapy, and found a 55% improvement of overall survival in patients with ER-negative disease receiving biweekly AC plus paclitaxel [34], compared to low-dose CAF [37]. These data suggest that the choice of chemotherapy regimens has a clinically relevant effect on the clinical outcome in patients with ER-negative BC. At the time of BC diagnosis (between 2003 and 2005), data supporting the use of more than 4 cycles of AC/EC chemotherapy [3, 38], or the addition of taxanes to anthracycline-based chemotherapy [11–13] in ER-negative BC had already been published. Accordingly, the use of FAC-T in patients with ER-negative BC was also included into the St.Gallen recommendations [39–43]. The most recent clinical practice guidelines of the National Comprehensive Cancer Network (NCCN) do not recommend grading chemotherapy according to the risk of disease recurrence, but usually include taxanes as the “preferred adjuvant regimens” [23]. In the present study, known pathological risk factors as well as patient age ≤50 years were over-represented in patients receiving FAC-T compared to those receiving non-FAC-T chemotherapy, and FAC-T chemotherapy was typically used in a risk-adapted manner in unselected patients with BC, with tumour biology becoming more important to choose FAC-T chemotherapy in ER-negative high-risk patients.
While the use of chemotherapy according to tumour risk and biology is in accordance with treatment recommendations, we found that socioeconomic and geographical factors were also important for choosing the type of chemotherapy in some cases. Interestingly, regional variability for using FAC-T or non-FAC-T chemotherapy was rather substantial, and this was independent of the patient’s disease risk profile (unselected, ER-negative BC patients). At present, the implementation of anti-HER2 targeted treatment adds another level of complexity to modern breast cancer care [44].
Schrijvers et al. reported the results of a study in 29’676 women in the UK with newly diagnosed BC [45]. Overall survival from BC was inversely related to socioeconomic deprivation, as determined by a residential district index that included pre-specified items [45]. Similar results were reported from a comparison of patients from affluent and deprived areas of Scotland [46]. In Switzerland, similar regional disparities in BC clinical outcome were reported, despite the universal access to health care in Switzerland [47], raising the question of whether heterogeneity in early BC treatment may account for different clinical outcomes. Unfortunately, there are very few data on the heterogeneity of systemic treatment in early BC patients. In the subset of 1,317 pre-menopausal ER-positive BC patients pooled from the PERCHE and TEXT studies, geography was a determinant of chemotherapy use [48]. The present study suggests that in countries with good access to health care services, there remain some imbalances in the prescribing patterns of therapies such as the type of chemotherapy used, and this may also have some impact on clinical outcome.
The results of this study should be considered with respect to the Swiss health care system, which is characterised by mandatory health insurance, universal access to modern health care services for all citizens, and by decentralisation and high fragmentation. The Swiss health care system shares several characteristics with the U.S. health care system, even more so with the planned introduction of universal health care insurance in the U.S. In fact, similar studies with regards to patient ethnicity were recently performed in the U.S. by Griggs and colleagues [49]. In the latter study, the authors found non-standard adjuvant chemotherapy regimens in 112 out of 957 women (12%) treated for early BC between March 2002 and March 2005, with African-American women being almost twice as likely to receive non-standard chemotherapy than non-African-American women [49]. In the present study, 105 women out of 1535 women (7%) received non-registered chemotherapy drugs for early BC, or chemotherapy that proved to be inferior when tested against standard regimens, a proportion that is somewhat lower than that found by Griggs and colleagues [49]. The strengths of the current study include the prospective planning of data analysis, registry-based case identification that enabled the authors to study patients in the community setting, and avoidance of systemic bias in patient accrual by central planning and structured patient inclusion. Our study is limited by its retrospective nature, missing data on the type of health care insurance, education and surgical caseload, and the fact that menopausal status had to be defined by using the age of 50 years as a cut off. In the present study, missing data cannot be considered at random, potentially resulting in some bias [50]. Our observations nevertheless are population-based and reflect clinical practice in a community setting. By including all types of providers, identification of patients through cancer registries, and achieving a large and very complete data set, potential selection bias was substantially reduced. Finally, clinical outcome data are not available at present, mainly because the follow-up time is too short for most patients.
In conclusion, tumour biology is a predominant factor for choosing FAC-T over older chemotherapy regimens in patients with ER-negative early BC, but improvements should be made to reduce the substantial regional heterogeneity. Further epidemiological studies should assess how the use of FAC-T chemotherapy is affecting clinical outcome in patients with early BC and different risk profiles.
References
1 Kataja V, Castiglione M. Primary breast cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2009;20(Suppl 4):10–4.
2 Bonadonna G, Valagussa P, Moliterni A, et al. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med. 1995;332:901–6.
3 Bonneterre J, Roche H, Kerbrat P, et al. Epirubicin increases long-term survival in adjuvant chemotherapy of patients with poor-prognosis, node-positive, early breast cancer: 10-year follow-up results of the French Adjuvant Study Group 05 randomized trial. J Clin Oncol. 2005;23:2686–93.
4 Fisher B, Brown AM, Dimitrov NV, et al. Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: results from the National Surgical Adjuvant Breast and Bowel Project B-15. J Clin Oncol. 1990;8:1483–96.
5 Hutchins LF, Green SJ, Ravdin PM, et al. Randomized, controlled trial of cyclophosphamide, methotrexate, and fluorouracil versus cyclophosphamide, doxorubicin, and fluorouracil with and without tamoxifen for high-risk, node-negative breast cancer: treatment results of Intergroup Protocol INT-0102. J Clin Oncol. 2005;23:8313–21.
6 Levine MN, Bramwell V, Pritchard K, et al. A pilot study of intensive cyclophosphamide, epirubicin and fluorouracil in patients with axillary node positive or locally advanced breast cancer. Eur J Cancer. 1992;29A:37–43.
7 Levine MN, Bramwell VH, Pritchard KI, et al. Randomized trial of intensive cyclophosphamide, epirubicin, and fluorouracil chemotherapy compared with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1998;16:2651–8.
8 Levine MN, Pritchard KI, Bramwell VH, et al. Randomized trial comparing cyclophosphamide, epirubicin, and fluorouracil with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer: update of National Cancer Institute of Canada Clinical Trials Group Trial MA5. J Clin Oncol. 2005;23:5166–70.
9 Martin M, Villar A, Sole-Calvo A. Doxorubicin in combination with fluorouracil and cyclophosphamide (i.v. FAC regimen, day 1, 21) versus methotrexate in combination with fluorouracil and cyclophosphamide (i.v. CMF regimen, day 1, 21) as adjuvant chemotherapy for operable breast cancer: a study by the GEICAM group. Ann Oncol. 2003;14:833–42.
10 Goldstein LJ, O’Neill A, Sparano JA, et al. Concurrent doxorubicin plus docetaxel is not more effective than concurrent doxorubicin plus cyclophosphamide in operable breast cancer with 0 to 3 positive axillary nodes: North American Breast Cancer Intergroup Trial E 2197. J Clin Oncol. 2008;26:4092–9.
11 Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–83.
12 Mamounas EP, Bryant J, Lembersky B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol. 2005;23:3686–96.
13 Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–13.
14 Roche H, Fumoleau P, Spielmann M, et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 Trial. J Clin Oncol. 2006;24:5664–71.
15 Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–71.
16 Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717.
17 Bria E, Nistico C, Cuppone F, et al. Benefit of taxanes as adjuvant chemotherapy for early breast cancer: pooled analysis of 15,500 patients. Cancer. 2006;106:2337–44.
18 De Laurentiis M, Cancello G, D’Agostino D, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol. 2008;26:44–53.
19 Ferguson T, Wilcken N, Vagg R, et al. Taxanes for adjuvant treatment of early breast cancer. Cochrane Database Syst Rev 2007; CD004421.
20 Francis P, Crown J, Di Leo A, et al. Adjuvant chemotherapy with sequential or concurrent anthracycline and docetaxel: Breast International Group 02-98 randomized trial. J Natl Cancer Inst. 2008;100:121–33.
21 Peto R. The worldwide overview: New results for systemic adjuvant therapies. In San Antonio Breast Cancer Symposium 2007. San Antonio: 2007.
22 Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes – dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–47.
23 National Comprehensive Cancer Network Practice Guidelines in Oncology – v.1.2010. In Jan-31-2010 Edition.
24 Carlson RW, Edge SB, Theriault RL. NCCN: Breast cancer. Cancer Control. 2001;8:54–61.
25 Eifel P, Axelson JA, Costa J, et al. National Institutes of Health Consensus Development Conference Statement: adjuvant therapy for breast cancer, November 1–3, 2000. J Natl Cancer Inst. 2001;93:979–89.
26 Curado MP, Edwards BK, Shin HR, et al. Cancer incidence in five continents, Vol. IX IARC Scientific Publications. Lyon: IARC 2007; No. 160.
27 Ess S, Joerger M, Frick H, et al. Predictors of state-of-the-art management of early breast cancer in Switzerland. Ann Oncol. 2010.
28 Ess S, Savidan A, Frick H, et al. Geographic variation in breast cancer care in Switzerland. Cancer Epidemiol. 2010;34:116–21.
29 Tomatis M, Dalmasso M, Del Mastro G, Tomatis A. QT Audit system on breast cancer treatment. CPO Piemonte: European breast cancer network- “Europe Against Cancer” Programme EUSOMA 2006. 2006.
30 McDavid K, Schymura MJ, Armstrong L, et al. Rationale and design of the National Program of Cancer Registries’ Breast, Colon, and Prostate Cancer Patterns of Care Study. Cancer Causes Control. 2004;15:1057–66.
31 Green FLC, C.C.; Fritz, A.G. AJCC Cancer Staging Atlas. New York (U.S.): John Wiley 2006.
32 Schweiz BfS. Gemeindetypologie. http://www.bfs.admin.ch/bfs/portal/de/index/regionen/11/geo/raeumliche_typologien/01.html 2000.
33 Poole CJ, Earl HM, Hiller L, et al. Epirubicin and cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy for early breast cancer. N Engl J Med. 2006;355:1851–62.
34 Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–9.
35 Bonneterre J, Roche H, Kerbrat P, et al. Long-term cardiac follow-up in relapse-free patients after six courses of fluorouracil, epirubicin, and cyclophosphamide, with either 50 or 100 mg of epirubicin, as adjuvant therapy for node-positive breast cancer: French adjuvant study group. J Clin Oncol. 2004;22:3070–9.
36 Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295:1658–67.
37 Budman DR, Berry DA, Cirrincione CT, et al. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst. 1998;90:1205–11.
38 Coombes RC, Bliss JM, Wils J, et al. Adjuvant cyclophosphamide, methotrexate, and fluorouracil versus fluorouracil, epirubicin, and cyclophosphamide chemotherapy in premenopausal women with axillary node-positive operable breast cancer: results of a randomized trial. The International Collaborative Cancer Group. J Clin Oncol. 1996;14:35–45.
39 Goldhirsch A, Glick JH, Gelber RD, et al. Meeting highlights: International Consensus Panel on the Treatment of Primary Breast Cancer. Seventh International Conference on Adjuvant Therapy of Primary Breast Cancer. J Clin Oncol. 2001;19:3817–27.
40 Goldhirsch A, Glick JH, Gelber RD, et al. Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol. 2005;16:1569–83.
41 Goldhirsch A, Ingle JN, Gelber RD, et al. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20:1319–29.
42 Goldhirsch A, Wood WC, Gelber RD, et al. Meeting highlights: updated international expert consensus on the primary therapy of early breast cancer. J Clin Oncol. 2003;21:335765.
43 Goldhirsch A, Wood WC, Gelber RD, et al. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol. 2007;18:1133–44.
44 Fang L, Barekati Z, Zhang B, et al. Targeted therapy in breast cancer: what’s new? Swiss Med Wkly. 2011;141:w13231.
45 Schrijvers CT, Mackenbach JP, Lutz JM, et al. Deprivation and survival from breast cancer. Br J Cancer. 1995;72:738–43.
46 Thomson CS, Hole DJ, Twelves CJ, et al. Prognostic factors in women with breast cancer: distribution by socioeconomic status and effect on differences in survival. J Epidemiol Community Health. 2001;55:308–15.
47 Fisch T, Pury P, Probst N, et al. Variation in survival after diagnosis of breast cancer in Switzerland. Ann Oncol. 2005;16:1882–8.
48 Regan MM, Pagani O, Walley B, et al. Premenopausal endocrine-responsive early breast cancer: who receives chemotherapy? Ann Oncol. 2008;19:1231–41.
49 Griggs JJ, Culakova E, Sorbero ME, et al. Social and racial differences in selection of breast cancer adjuvant chemotherapy regimens. J Clin Oncol. 2007;25:2522–7.
50 Altman DG, Bland JM. Missing data. BMJ. 2007;334:424.


