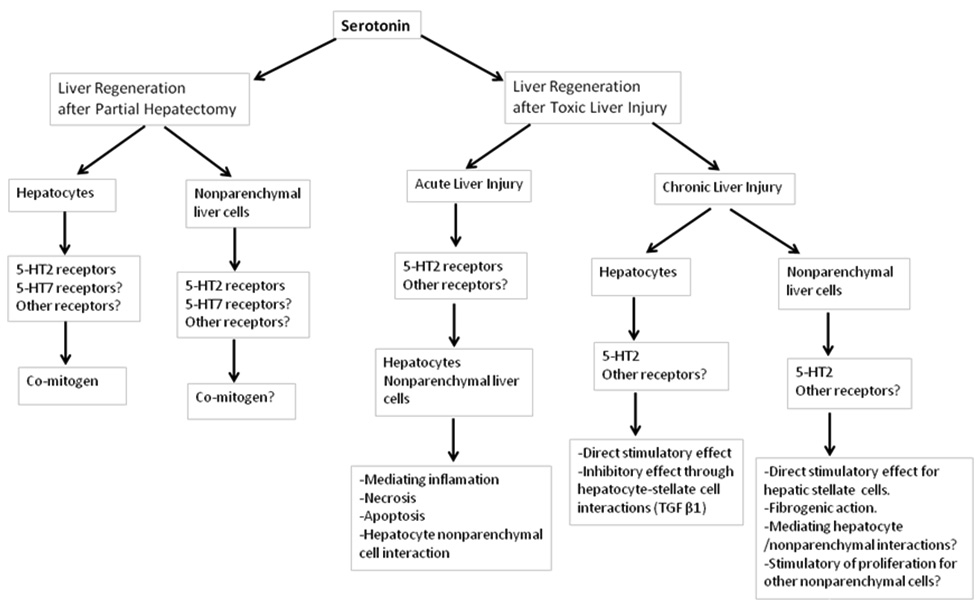
Figure 1
Schematic summary of the most important roles of serotonin in liver regeneration.
DOI: https://doi.org/10.4414/smw.2012.13548
The amazing ability of the liver to regenerate following partial resection or injury is unique, especially because the highly differentiated functions of the organ are totally maintained. Partial hepatectomy (PH) has been widely used for long as an experimental model in order to gain a deeper understanding of the underlying mechanisms of liver regeneration. The presence of numerous diverse ligands in the initiation, propagation and termination of the mitotic stimulus, such as priming factors, co-mitogens, growth factors and their suppressors, is necessary for the successful and complete restoration of hepatic mass [1, 4]. Neurotransmitters have from long attracted the scientific interest about their possible role in liver regeneration after either PH or toxic liver injury. Catecholamines are now well known comitogens for hepatocytes and their plasma levels have been shown to increase significantly within the first two hours after PH [5, 7].
Although the possible involvement of serotonin (5-hydroxytryptamine, 5HT) in liver regeneration has been hypothesised some decades ago, it was only quite recently extensively investigated in a variety of animal models. The aim of this mini-review is to briefly summarise the interesting relationship between serotonergic system and liver regeneration and provide knowledge about the recent scientific evidence on the role of serotonin in liver regeneration.
Serotonin is an evolutionarily conserved biogenic monoamine neurotransmitter with variable effects on many different target organs. It can be classified among neurotransmitters participating in appetite modulation, circadian rhythm regulation, and platelet contraction among others or may be partly considered a hormone modulating gastrointestinal tract motility [8, 9]. It is derived from the aminoacid L-tryptophan, which is hydroxylated to 5-hydroxy-L-tryptophan (5-HTP) by tryptophane hydroxylase (Tph), the rate-limiting step in serotonin biosynthesis. 5-HTP is then converted to serotonin, which can not cross the blood-brain barrier contrary to its precursor molecules L-tryptophan and 5-HTP [10, 11]. As a consequence serotonin is required to be synthesised de novo within the serotonergic neurons of the central nervous system, where it participates in the modulation of variable actions, such as mood, sleep and appetite [12, 14].
More than 90% of the total body serotonin is located in the enterochromaffin cells in the gut, where it regulates intestinal motility [15]. The secretion of serotonin from the intestinal enterochromaffin cells is followed by its platelet uptake which is dependent on the serotonin transporter (SERT). Platelets do not synthesise serotonin, but store and release it in sites of injury where it contributes to platelet recruitment and thrombus propagation [16, 17]. In the absence of an extracellular enzyme to catabolise serotonin in brain and gut, SERT has the important role in terminating its action. The enzymes monoamine oxidase and aldehyde dehydrogenase can convert serotonin to 5-hydroxyindoleacetic acid (5-HIAA) excreted in urine. The 5-HIAA urine concentration can be measured and reflects changes in whole body serotonin levels [18, 20].
Seven families of 5-HT receptors (5-HT1-7), comprising a total of 14 subtypes with structural and functional diversities, have been identified. Of these 5-HT receptors subtypes all but 5-HT3 receptor are metabotropic. 5-HT3 receptor is a ligand-gated, postsynaptically located nonspecific cation channel, which is modulating ion flux. The other 5-HT receptors are G-protein-coupled causing either hyperpolarisation or depolarisation of the cell membrane. 5-HT1A, 5-HT1B and 5-HT1D subtypes are presynaptic autoreceptors that inhibit serotonin release by a direct action of their associated G protein by opening K+ channels and hyperpolarising the cell membrane. 5-HT2A and 5-HT4 are postsynaptically located receptors, which belong to the G-protein receptor superfamily and along with 5-HT3 receptors, cause the depolarisation of the cell membrane. The expression and distribution of 5-HT receptors in different brain areas and in the periphery as well, is variable. As a paradigm, there is little evidence for expression of 5-HT2C receptors outside the central nervous system, while some others are located on the membrane of many different cellular populations, playing a pivotal role in the peripheral serotonergic neurotransmission [18, 21].
Hepatocytes are resting cells under normal conditions, but they are the first cells to divide after 60–70% PH with a peak in DNA synthesis at around 22–24h in young adult rats. Nonparenchymal liver cells, such as biliary ductal cells, Kupffer cells, stellate cells and endothelial cells divide 24h after hepatocytes [1-4, 22, 23]. Although rat liver regeneration continues up to 10 days after 60–70% PH, the major restoration of liver tissue has already been completed on the third postsurgical day. The regeneration process can be viewed as a sequence of events starting from an initial signal and comprises a priming phase, followed by a progression phase, a cell cycle phase and finally a termination signal [4, 24, 25]. The increase of portal vein pressure and liver tissue perfusion and the extracellular matrix remodeling may be considered as the initial events after PH [3, 4, 25, 26]. Shortly after, a great number of genes are activated (primed) and some of them encode transcriptional factors, essential for the propagation of the mitotic stimulus. Priming phase urge hepatocytes from quiescence to G1 phase of the cell cycle [4, 22]. Gene expression during priming phase is not capable by itself to promote liver regeneration after hepatectomy and a subsequent increase in growth factor levels is also necessary. Hepatocytes overcome the G1/S restriction point of the cell cycle only in the presence of growth factors and after this milestone they become committed to divide [22, 25, 27]. At least two intracellular signalling pathways, the MAPK and JAK-STAT, involved in liver regeneration have been identified. The MAPK (mitogen-activated protein kinase) pathway is activated after the binding of growth factors on specific cell membrane receptors leading to activation of Ras, Raf, MEK and ERK1/2 [28]. Early induction of MEK/ERK cascade is restricted to primed hepatocytes, distinguishing them from those returning to quiescence. On the other hand, the MAPK pathway activation at the late G1 phase of the cell cycle is mainly associated with cyclin D1 accumulation [29]. Expression of cyclin D1 highlights the point of G1/S transition point after which hepatocytes, independently of mitogens, can progress into the S phase of DNA synthesis [22, 29]. The activation of JAK-STAT pathway depends on the binding of cytokines to their receptors, which in turn results in the translocation of the transcription factor STAT3 into nucleus and the subsequent transcription of genes essential for the regenerative process [28].

Figure 1
Schematic summary of the most important roles of serotonin in liver regeneration.
The involvement of serotonin in the induction of hepatocyte DNA synthesis was first investigated in primary cultures of adult rat hepatocytes by Balasubramanian et al. [30], which showed that 5-HT could significantly induce hepatocyte proliferation in the presence of insulin and EGF (epidermal growth factor). On the other hand, ketanserin, a 5-HT2 receptor antagonist [31], caused a significant displacement of [3H] 5-HT in the regenerating rat liver when administered at 24h after PH, the peak point of DNA synthesis, implying an increased involvement of the hepatic 5-HT2 receptor during the regenerative response [30]. In a more recent study by Liu et al. quipazine, a selective 5-HT receptor agonist with a high affinity for the 5-HT1D, 5-HT2B and 5-HT2C receptors led to a substantial improvement of proliferation rate in L-02 cell, a human hepatocyte strain, further supporting the mitogenic activity of serotonin [32].
5-HT2 receptor has been cloned from human liver and has a great homology with that of mouse and rat liver [33]. The expression of 5-HT1A, 5-HT1B, 5-HT1D, 5-HT2A, 5-HT2B, 5-HT3A and 5-HT3B receptors has been identified in naïve mouse liver and the mRNA expression of 5-HT2A and 2B receptors, which are well known to mediate mitogenic and developmental effects, has been shown to increase after PH, while their antagonists inhibited liver regeneration [18, 34]. Our group investigated the effect of 5-HT2 blockage by ketanserin after 60–70% PH in rats and we found that its administration can arrest liver regeneration only when administrated close to the G1/S transition point implying that serotonin may be a cofactor for DNA synthesis. In the same study, we also measured the concentration of liver serotonin which started to increase around the G1/S transition point and culminated at the time of maximal hepatic proliferative activity [35]. These data were in accordance with the study by Sulaiman et al. [36] which also showed that hepatic serotonin content significantly increased during hepatocyte DNA synthesis in rats after PH. The above similar findings strongly support a possible correlation between hepatic serotonin content and the rate of cell proliferation.
The mitogenic activity of serotonin has been also confirmed in a rat model of cirrhotic liver, where splenectomy-induced thrombocytosis led to an increase of serotonin content in the damaged liver with an acceleration of liver regeneration. Nevertheless, platelet content and plasma serotonin levels were not significantly affected indicating that the serotonin increase in the damaged liver might be attributed to modulation of hepatic hemodynamics after splenectomy. The subsequent ketanserin administration nullified the beneficial effect of splenectomy though without influencing the hepatic serotonin levels [37]. The effect of thrombopoietin-induced thrombocytosis or antiplatelet-antibody-induced thrombocytopenia on the hepatic regenerative process has also been investigated in a mouse model after 70% PH, where the number of platelets seemed to strongly correlate with hepatocyte proliferative capacity [38].
The importance of serotonin has also been shown by the failure of liver regeneration after PH in tryptophan hydroxylase 1 knockout mice lacking peripheral serotonin due to the absence of the rate limiting enzyme in 5-HT biosynthesis. However, the injection of the serotonin precursor 5-HTP restored the unimpeded progression of liver regeneration [39]. In the same study by Lesurtel et al. the administration of clopidogrel, an inhibitor of platelet aggregation, prevented hepatocyte proliferation after 70% PH emphasizing the role of platelet-derived serotonin [39]. However, in a more recent study the regenerative process has not been influenced in SERT-deficient rats where 5-HT was almost completely diminished in platelets indicating that its active platelet release is not possibly essential and only very low serotonin levels may be finally required [40].
The activation of 5-HT2B receptor has been also associated with improved survival rate in mice transplanted with a small, otherwise nonviable graft by enhancing liver regeneration and hepatic microcirculation. This finding may possibly be used to develop strategies for preventing liver failure, since the small-for-size syndrome is the single most important limiting factor in liver surgery and transplantation [41].
In a recent study in our laboratory, 5-HT7 receptor blockade with competitive inhibitors greatly attenuated liver regeneration after 60–70% PH only when applied close to the G1/S transition point and this reveals an additional type of serotonin receptor mediating the proliferating action of the monoamine (work in process to be submitted for publication).
The effect of serotonin on liver regeneration has been investigated mainly regarding hepatocytes while less is known about possible proliferative effect of the monoamine on other liver cell subpopulations. Endothelial cells comprise the second most abundant cell type in the liver after hepatocytes and they have been reported to express 5-HT1D, 5-HT2B and 5-HT2C receptors at least in human umbilical veins [42, 43]. Previously published work has been controversial towards a ubiquitous mitogenic effect of serotonin on endothelial cells and this has also been confirmed in a recent study [44, 45]. At this point a possible angiogenic effect of serotonin during liver regeneration remains unknown. Finally and interestingly 5-HT7 receptor has been very recently shown to be expressed in bovine corneal endothelial cells while its possible expression and up- regulation in hepatic endothelial cells in quiescent and regenerating liver has not been investigated [46]. Serotonin has also been reported to exert a proliferative effect on cholangiocytes and to promote cholangiocarcinoma growth [47, 48] while there are no data available regarding its role as a mitogen for cholangiocytes during liver regeneration. Hepatic stellate cells (HSCs) in humans and rats express 5-HTIB, 5-HT1F, 5-HT2A, 5-HT2B and 5-HT7 receptors with increased expression of 5-HT1B, 5-HT2A and 5-HT2B upon their activation [45, 49]. Among serotonin receptors 5-HT2B receptor has been strongly associated with increased HSC proliferation and liver fibrosis [49].
The interactions between hepatocytes and nonparenchymal cells during liver regeneration and disease have been partly investigated for HSCs and the contribution of HSCs in the regulation of hepatocyte proliferation has not been established [50]. HSCs have been reported to secrete numerous factors that influence hepatocyte proliferation, such as hepatocyte growth factor (HGF), transforming growth factor β1 (TGFβ1) and interleukin-6 [50, 53] with both stimulatory and inhibitory effects. The role of serotonin as a mitogen for HSCs during liver regeneration remains hugely unknown today. The same also applies for possible interactions between hepatocytes and HSCs during the regenerative process.
Additionally, there is evidence that serotonin may also play a role in liver regeneration at the cerebral level. This observation came from Pyroja et al. which showed that the exclusively centrally located 5-HT2C receptor in the brainstem and cerebral cortex may be up-regulated during liver regeneration after PH and in hepatic neoplasia resulting in hepatocyte proliferation probably through sympathetic stimulation [54]. The above data reflect the activation of multiple mechanisms both in CNS and in the periphery.
Apart from receptor-dependent signaling, serotonin also acts through receptor-independent signaling pathways by posttranslational modification of intracellular proteins. This process constitutes serotonylation and is mediated by the enzyme transglutaminase that creates glutamyl-amide bonds by attachment of serotonin to glutamine residues of intracellular proteins [55]. Serotonylation of small GTPases mediates exocytosis of platelet alpha granules [55] and insulin release from pancreatic beta cells [56] while the same covalent modification of vascular proteins is important for vascular contraction [57]. More recently serotonylation has been found to mediate serotonin-induced proliferation and migration of pulmonary artery smooth muscle cells [58]. No data are available today in relation to the role of serotonylation during liver regeneration after partial hepatectomy or toxic liver injury.
Figure 1 summarises the known effects of serotonin on liver regeneration.
Serotonin has been recently shown to mediate the pathology of many liver diseases, such as steatohepatitis, chronic cholestasis and liver cirrhosis, although the exact mechanisms still remain unclear [18, 48, 49, 59, 60]. Serotonin signalling seems to play a pivotal role in determining the balance between regeneration and fibrogenesis in chronic liver disease and recently it has been reported that activation of 5-HT2B receptor on fibrogenic HSCs suppresses hepatocyte proliferation through augmented production of TGFβ1 [61]. At the severe end of the spectrum, 5-HT has been involved in the pathogenesis of human hepatocellular carcinoma through increased 5-HT2B expression, which seems to facilitate the survival of carcinoma cells and to inhibit autophagy [62]. There are also some interesting reports suggesting that serotonin can potentially contribute to liver tissue hypoperfusion following hepatic ischemia and reperfusion in canines raising new dilemmas about its effects on hepatic regeneration [20, 63].
In an effort to further understand the effect of serotonergic system activation in the liver we examined the effect of 5-HT2 receptor blockade in cadmium-induced acute hepatotoxicity in rats. The administration of ketanserin or ritanserin (selective 5-HT2 receptor antagonist), prior and after the administration of a sublethal cadmium dose, resulted in a remarkable reduction of inflammatory and apoptotic indices [64].
Based on the above data, the development of serotonin receptor antagonists may be potentially used as therapeutic agents for the treatment of various liver diseases.
Although the involvement of serotonin in liver regeneration has been initially suggested many years ago, it has only recently started being thoroughly studied. The results from many different experimental models have indisputably shown that the hepatocyte proliferative capacity can be substantially altered by the activation or blockade of the serotonergic system and especially the 5-HT2 receptor subfamily. Moreover, there is increasing evidence that serotonin can be potentially associated with either beneficial or detrimental effects on liver regeneration and these actions are mediated through many different receptor subtypes located either centrally or peripherally. These observations pose a plethora of questions and provide also research opportunities, necessitating further work in the coming years.
1 Riehle KJ, Dann YY, Campbell JS, Fausto N. New concepts in liver regeneration. J Gastroenterol Hepatol. 2011;26(Suppl.1):203–12.
2 Fausto N, Kampbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–53.
3 Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300.
4 Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176:2–13.
5 Cruise JL, Knechtle SJ, Bollinger RR, Kuhn C, Michalopoulos G. Alpha 1-adrenergic effects and liver regeneration. Hepatology. 1987;7:1189–94.
6 Cruise JL, Houck KA, Michalopoulos GK. Induction of DNA synthesis in cultured rat hepatocytes through stimulation of a1 adrenoreceptor by norepinephrine. Science. 1985;227:749–51.
7 Cruise JL. Alpha 1-adrenergic receptors in liver regeneration. Dig Dis Sci. 1991;36:485–8.
8 Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–12.
9 Berger M. Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–66.
10 Fuller RW, Wong DT. Serotonin uptake and serotonin uptake inhibition. Ann N Y Acad Sci. 1990;60:68–78.
11 Jonnakuty C, Gragnoli C. What do we know about serotonin? J Cell Physiol. 2008;217:301–6.
12 Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12(Suppl. 1):2–19.
13 Mendelson WB. Neurotransmitters and sleep. J Clin Psychiatry. 2001;62(Suppl.10):5–8.
14 Lam DD, Garfield AS, Marston OJ, Shaw J, Heisler LK. Brain serotonin system in the coordination of food intake and body weight. Pharmacol Biochem Behav. 2010;97:84–91.
15 Gershon MD, Tack J. The serotonin signalling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414.
16 Mercado CP, Kilic F. Molecular mechanisms of SERT in platelets: regulation of plasma serotonin levels. Mol Interv. 2010;10:231–41.
17 Angiolillo DJ, Ueno M, Goto S. Basic principles of platelet biology and clinical implications. Circ J. 2010;74:597–607.
18 Lesurtel M, Soll C, Graf R, Clavien PA. Role of serotonin in the hepato-gastrointestinal tract: an old molecule for new perspectives. Cell Mol Life Sci. 2008;65:940–52.
19 Clavien PA. Liver regeneration: a spotlight on the novel role of platelets and serotonin. Swiss Med Wkly. 2008;138:361–70.
20 Ruddell RG, Mann DA, Ramm GA. The function of serotonin within the liver. J Hepatol. 2008;48:666–75.
21 Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–152.
22 Fausto N. Liver regeneration. J Hepatol. 2000;32:19–31.
23 Michalopoulos GK, Defrances MC. Liver regeneration. Science 1997;276:60–5.
24 LaBrecque D. Liver regeneration: a picture emerges from the puzzle. Am J Gastroenterol. 1994;89:586–97.
25 Mangnall D, Bird NC, Majeed AW. The molecular physiology of liver regeneration following partial hepatectomy. Liver Int. 2003;23:124–38.
26 Marubashi S, Sakon M, Nagano H, Gotoh K, Hashimoto K, Kubota M, et al. Effect of portal hemodynamics on liver regeneration studied in a novel portohepatic shunt rat model. Surgery. 2004;136:1028–37.
27 Fausto N, Laird AD, Webber EM. Role of growth factors and cytokines in hepatic regeneration. FASEB J. 1995;9:1527–36.
28 Tian ZJ, An W. ERK1/2 contributes negative regulation to STAT3 activity in HSS-transfected HepG2 cells. Cell Res. 2004;14:141–7.
29 Talarmin H, Rescan C, Cariou S, Glaise D, Zanninelli G, Bilodeau M, et al. The mitogen-activated protein kinase kinase/extracellular signal-regulated kinase cascade activation is a key signalling pathway involved in the regulation of G1 phase progression in proliferating hepatocytes. Mol Cell Biol. 1999;19:6003–11.
30 Balasubramanian S, Paulose CS. Induction of DNA synthesis in primary cultures of rat hepatocytes by serotonin: possible involvement of serotonin S2 receptor. Hepatology. 1998;27:62–6.
31 Persson B, Heykants J, Hedner T. Clinical pharmacokinetics of ketanserin. Clin Pharmacokinet. 1991;20:263–79.
32 Liu Y, Zhang ZY. Serotonin receptor agonist quipazine promotes proliferation and apoptosis of human hepatocyte strain of L-02 strain. Hepatobiliary Pancreat Dis Int. 2009;8:278–81.
33 Bonhaus DW, Bach C, DeSouza A, Salazar FH, Matsuoka BD, Zuppan P, et al. The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors. Br J Pharmacol. 1995;115:622–8.
34 Nebiqil CG, Etienne N, Schaerlinger B, Hickel P, Launay JM, Maroteaux L. Developmentally regulated serotonin 5-HT2B receptors. Int J Dev Neurosci. 2001;19:365–72.
35 Papadimas GK, Tzirogiannis KN, Panoutsopoulos GI, Demonakou MD, Skaltsas SD, Hereti RI, et al. Effect of serotonin receptor 2 blockage on liver regeneration after partial hepatectomy in the rat liver. Liver Int. 2006;26:352–61.
36 Sulaiman P, Joseph B, Kaimal SB, Paulose CS. Decreased hepatic 5-HT1A receptors during liver regeneration and neoplasia in rats. Neurochem Res. 2008;33:444–9.
37 Nagao Y, Akahoshi T, Kamori M, Uehara H, Hashimoto N, Kinjo N, et al. Liver regeneration is promoted by increasing serotonin content in rat liver with secondary biliary cirrhosis. Hepatol Res. 2011;41:784–94.
38 Murata S, Ohkohchi N, Matsuo R, Ikeda O, Myronovych A, Hoshi R. Platelets promote liver regeneration in early period after hepatectomy in mice. World J Surg. 2007;31:808–16.
39 Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, et al. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104–7.
40 Matondo RB, Punt C, Homberg J, Toussaint MJ, Kisjes R, Korporaal SJ, et al. Deletion of the serotonin transporter in rats disturbs serotonin homeostasis without impairing liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2009;296:G963–8.
41 Tian Y, Graf R, El-Badry AM, Lesurtel M, Furrer K, Moritz W, et al. Activation of serotonin receptor-2B rescues small-for-size liver graft failure in mice. Hepatology. 2011;53:253–60.
42 Schoeffter P, Ullmer C, Gutierrez M, Weitz-Schmidt G, Lubbert H. Functional serotonin 5-HT1D receptors and 5-HT1D beta receptor mRNA expression in human umbilical vein endothelial cells. Naunyn Schmiededergs Arch Pharmacol. 1995;352:580–2.
43 Asada M, Ebihara S, Yamanda S, Niu K, Okazaki T, Sora I, et al. Depletion of serotonin and selective inhibition of 2B receptor suppressed tumor angiogenesis by inhibiting endothelial nitric oxide synthase and extracellular signal-regulated kinase ½ phosphorylation. Neoplasia. 2009;11:408–17.
44 Ruiz-Perez MV, Sanchez-Jimenez F, Quesada AR, Medina MA. A re-evaluation of the mitogenic effect of serotonin on vascular endothelial cells. J Biol Regul Homeost Agents. 2011;25:13–20.
45 Lesurtel M, Soll C, Humar B, Clavien PA. Serotonin: A double-edged sword for the liver? Surgeon. 2011;ahead of print.
46 Grueb M, Rohrbach JM, Schlote T, Mielke J. Serotonin (5-HT7) receptor-stimulated activation of cAMP-PKA pathway in bovine corneal epithelial and endothelial cells. Ophthalmic Res. 2012;48:22–7.
47 Frampton GA, Li H, Ramirez J, Mohamad A, Demorrow S. Biogenic amines serotonin and dopamine regulate cholangiocyte hyperplastic and neoplastic growth. World J Gastrointest Pathopsysiol. 2010;1:63–8.
48 Marzioni M, Glaser S, Francis H, Marucci L, Benedetti A, Alvaro D, et.al. Autocrine/paracrine regulation of the growth of the biliary tree by the neuroendocrine hormone serotonin. Gastroenterology. 2005;128:121–37.
49 Ruddell RG, Oakley F, Hussain Z, Yeung I, Bryan-Lluka LJ, Ramm GA, et al. A role for serotonin (5-HT) in hepatic stellate cell function and liver fibrosis. Am J Pathol. 2006;169:861–76.
50 Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Psysiol Rev. 2008;88:125–72.
51 Wallace K, Burt AD, Wright MC. Liver Fibrosis. Biochem J. 2008;411:1–18.
52 Marshall A, Rushbrook S, Davies SE, Morris LS, Scott IS, Vowler SL, et al. Relation between hepatocyte G1 arrest, impaired hepatic regeneration, and fibrosis in chronic hepatitis C virus infection. Gastroenterology. 2005;128:33–42.
53 Malik R, Selden C, Hodgson H. The role of non-parenchymal cells in liver growth. Semin Cell Dev Biol. 2002;13:425–31.
54 Pyroja S, Joseph B, Paulose CS. Increased 5-HT2C receptor binding in the brain stem and cerebral cortex during liver regeneration and hepatic neoplasia in rats. J Neurol Sci. 2007;254:3–8.
55 Walther DJ, Peter JU, Winter S, Holtje M, Paulmann N, Grohmann M, et al. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alha-granule release. Cell. 2003;115:851–62.
56 Paulmann N, Grohmann M, Voigt JP, Bert B, Vowinckel J, Bader M, et al. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol. 2009;7:e1000229.
57 Guilluy C, Eddahibi S, Agard C, Guignabert C, Izikki M, Tu L, et al. RhoA and Rho kinase activation in human pulmonary hypertension: role of 5-HT signaling. Am J Respir Crit Care Med. 2009;179:1151–8.
58 Liu Y, Wei L, Laskin DL, Fanburg BL. Role of protein transamidation in serotonin-induced proliferation and migration of pulmonary artery smooth muscle cells. Am J Respir Cell Mol Biol. 2011;44:548–55.
59 Nocito A, Dahm F, Jochum W, Jang JH, Georgiev P, Bader M, et al. Serotonin mediates oxidative stress and mitochondrial toxicity in a murine model of nonalcoholic steatoheptitis. Gastroenterology. 2007;133:608–18.
60 Beaudry P, Hadengue A, Callebert J, Gaudin C, Soliman H, Moreau R, et al. Blood and plasma 5-hydroxytryptamine levels in patients with cirrhosis. Hepatology. 1994;20:800–3.
61 Ebrahimkhani MR, Oakley F, Murphy LB, Mann J, Moles A, Perugorria MJ, et al. Stimulating healthy tissue regeneration by targeting the 5-HT2B receptor in chronic liver disease. Nat Med. 2011;17:1668–73.
62 Soll C, Jang JH, Riener MO, Moritz W, Wild PJ, Graf R, et al. Serotonin promotes tumor growth in human hepatocellular cancer. Hepatology. 2010;51:1244–54.
63 Murata R, Hamada N, Nakamura N, Kobayashi A, Fukueda M, Taira A, et al. Serotonin activity and liver dysfunction following hepatic ischemia and reperfusion. In Vivo. 2003;17:567–72.
64 Tzirogiannis KN, Demonakou MD, Papadimas GK, Skaltsas SD, Manta GA, Kourentzi KT, et al. Effect of 5-HT2 receptor blockade on cadmium-induced acute toxicity. Dig Dis Sci. 2007;52:2351–8.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.