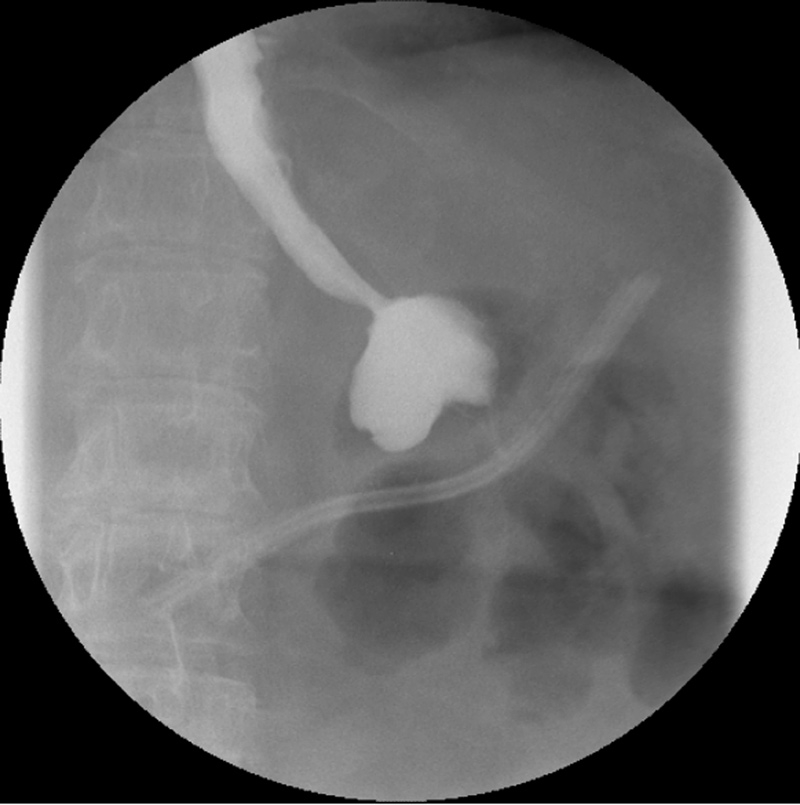
Figure 1
Upper Gastrointestinal series showing a stomal obstruction at the level of the gastrojejunal anastomosis.
DOI: https://doi.org/10.4414/smw.2012.13556
While morbid obesity remains a health problem in the developed countries, the number of laparoscopic Roux-en-Y Gastric Bypass (RYGB) continues to increase significantly. Between 2004 and 2007, there was a 125% growth in the number of laparoscopic RYGB performed in the United States [1]. Currently, the laparoscopic RYGB is considered by many as the gold standard bariatric procedure [2–4].
Despite the recognised benefits of a laparoscopic approach (reduction of hospital stay, postoperative pain, and complications when compared to open procedures) [5], laparoscopic RYGB remains a difficult operation, associated with potentially severe complications [6]. One of the most serious is gastrointestinal leak [7]. In most centers, the risk of anastomotic leak or obstruction has been the rationale for obtaining an Upper Gastrointestinal (UGI) contrast studies following RYGB. However, so far, the role of routine UGI remains at least controversial [6–12]. While potential advantages of this strategy is early identification and management of anastomotic complications, routine UGI involves many disadvantages as additional cost, discomfort for the patient, risk of irradiation, risk of aspiration pneumonia, and risk of prolonged hospitalisation in case of false positive results [9].
Since the introduction of robotics in the armamentarium of bariatric surgery, several groups have reported a reduced rate of anastomotic complications [13–15]. However, to the best of our knowledge, there is no data concerning the validity of UGI after a robotic gastrojejunal (GJ) and jejunojenunal (JJ) anastomosis.
The aim of the study was to assess the cost of routine UGI and its validity in checking for postoperative complications following robot-assisted RYGB at a single institution.
Between July 2006 and December 2010, 167 robot-assisted RYGB procedures were performed using the same technique at our institution. Data were entered prospectively into a dedicated bariatric database and retrospectively reviewed.
Patients included in this study met the NIH consensus criteria [16] for bariatric surgery and fulfilled the institutional guidelines of medically supervised weight loss and psychological clearance.
All the patients that met the criteria for bariatric surgery were eligible for a robotic approach. There were no selection criteria specific for robotics and the exclusion criteria were the same as for laparoscopy (anesthesiological contra-indications, evident hostile abdomen). During the study period, 210 laparoscopic RYGB were performed (55.7%). However, there is a clear trend in favour of robotics, with more than 80% of RYGB performed robotically since the last two years. The choice of the approach was mainly based on the availability of the robotic team and system.
As part of the preoperative workup, each patient underwent a medical and surgical evaluation, along with a routine upper endoscopy for evaluation of esophageal and stomach anatomy, hiatal hernia and reflux disorder.
Patients did not receive routinely preoperative anticoagulation prophylaxis.
Queries on patient demographics, operative variables and complications were performed. Age, gender, preoperative weight and BMI, intraoperative and postoperative complications (30-day morbidity), and readmission within 30 days of surgery were also evaluated. Patients were initially followed up in the outpatient clinic.
Our technique for robot-assisted RYGB included a laparoscopic cholecystectomy and a laparoscopic creation of the small gastric pouch (around 20–25 ml) in all cases. Then, the da Vinci Surgical System (Intuitive, Sunnyvale, CA) was docked, using a 5-port setting. The GJ was a single-layer running hand-sewn anastomosis. The JJ consisted in a side-to-side running hand-sewn anastomosis. The GJ was routinely tested for leak with insufflations and simultaneous occlusion of distal jejunum. A closed-suction drain was placed posterior to the GJ in all cases.
Postoperative care was standardised according to a clinical care pathway. All patients remained Nihil Per Os (NPO) until the second postoperative day when an UGI was obtained with gastrografin (Bayer AG, Zürich, Switzerland) at a concentration of 370 mg/ml. The UGI was performed under fluoroscopy in order to evaluate for anastomotic integrity and to exclude any stenosis. The patient was required to swallow between 40 and 60 ml while standing. Images were obtained in anteroposterior, right and left oblique projections, at the level of the gastrojejnual anastomosis. Then 15 minutes later, an additional anteroposterior film was taken to assess emptying of the pouch, passage of contrast through the jejunum, reflux into the duodenum and possible delayed leak. Several attending radiologists interpreted all 167 studies, in cooperation with the different operative surgeons.
The cost of this test to the patient was CHF 560.– (= USD 580.– on 10 January 2011).
Leaks were defined as evidence of extravasation of contrast material on the UGI or abdominal computed tomography scan or by identification of enteric spill in the drain or at the time of laparotomy [7].
In cases of normal exam, patients were started on a post gastric bypass diet, and the drain was removed 72 hours later.
Once able to tolerate the diet well and meet the discharge criteria, patients were discharged home and followed up with subsequent visits to the outpatient clinic.
A 30-day follow-up was available for the entire cohort.
During the study period, 123 women (73.7%) and 44 men (26.3%) underwent a robot-assisted RYGB. The mean age was 43 years (range: 19–69 years) and the mean preoperative weight and BMI were 122.8 kg (range: 80–191.7 kg) and 44 kg/m2 (range: 30.9–60.8 kg/m2) respectively. The patient with a BMI of 30.9 kg/m2 underwent a robot-assisted RYGB after a previous laparoscopic adjustable gastric banding. Patient demographics are summarised in table 1.

Figure 1
Upper Gastrointestinal series showing a stomal obstruction at the level of the gastrojejunal anastomosis.
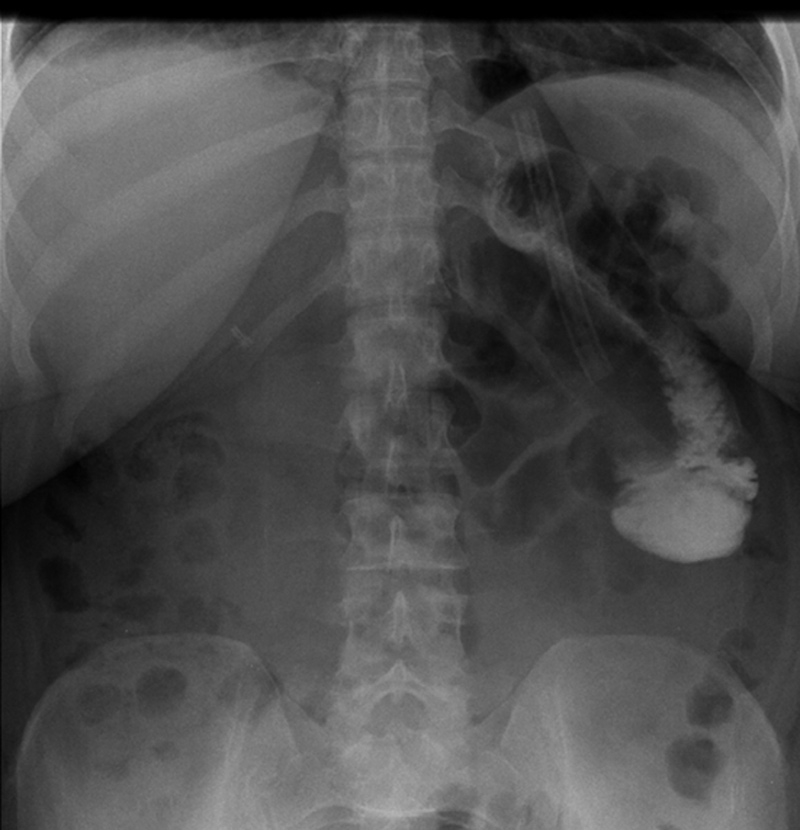
Figure 2
X-ray showing a stenosis at the level of the jejunojejunal anastomosis.
The mean operative time was 295.2 minutes (range: 150–600). The procedure that lasted 600 minutes was the first robot-assisted RYGB we performed at our institution.
There was one intra-operative complication (0.6%): a misfiring of the stapler leading to a conversion. There was another conversion due to massive adhesions (table 2).
There were a total of 24 postoperative complications (30-day morbidity: 14.4%). Among those, two patients required a reoperation: one for an internal hernia and one for an intra-peritoneal hemorrhage (bleeding from the stapled line). The later stayed at the hospital for 24 days.
Concerning the other complications, there were seven pulmonary embolisms and two deep venous thromboses, three neurological complications (two leg paresthesias and one arm dysesthesia), three hemorrhages (one who required reoperation as discussed above and the two remaining necessitating transfusions), two bacteriemias, and one atelectasia. Two patients were readmitted to the hospital: one for a wound infection requiring antibiotics and one for abdominal pain after a large meal. Of note, there was no statistical difference between the operative time of patients that experienced pulmonary embolism or deep venous thrombosis and patients without any thromboembolic complications (335.6 vs. 293 minutes; p = 0.19).
There was no intra or postoperative mortality.
The mean length of stay was 7.2 days (range: 3–24 days).
All patients underwent a routine postoperative UGI that was well tolerated. There was no leak.
In two patients, the results showed a stoma obstruction due to a postoperative edema at the level of the GJ anastomosis (1.2%) (fig. 1). The post gastric bypass diet was delayed and the UGI was performed again at postoperative day 4 with resolution of the edema. One patient presented an anastomotic stenosis at the level of the JJ anastomosis with delayed emptying (0.6%) (fig. 2). The patient was treated conservatively with success as well. Of note, the patients who presented an abnormal UGI study were all symptomatic with early nausea and vomiting.
The remaining UGI procedures showed no abnormalities and all of the readmitted patients had an initial normal UGI. The total cost for 167 UGI procedures was CHF 93,520 (= USD 96,886).
| Table 1: Patients demographics. | |
| Patients | 167 |
| Gender Men Women | 44 (26.3%) 123 (73.7%) |
| Mean age ± SD [range] | 43 ± 10.8 [19–69] |
| Mean initial BMI in kg/m2 ± SD [range] | 44 ± 4.9 [30.9–60.8] |
| Mean initial weight in kg ± SD [range] | 122.8 ± 20.7 [80–191.7] |
| SD: Standard Deviation. BMI: Body Mass Index. | |
| Table 2: Perioperative results. | |
| Procedures | 167 |
| Mean operating time in minute ± SD [range] | 295.2 ± 96 [150–600] |
| Intra-operative complication | 1 (0.6%) |
| Conversion | 2 (1.2%) |
| Postoperative complications Pulmonary embolism Neurological complications Hemorrhage GJ anastomosis edema Bacteriemia Deep venous thrombosis JJ anastomosis stenosis Internal hernia Wound infection Abdominal pain Atelectasia | 24 (14.4%) 7 (4.2%) 3 (1.8%) 3 (1.8%) 2 (1.2%) 2 (1.2%) 2 (1.2%) 1 (0.6%) 1 (0.6%) 1 (0.6%) 1 (0.6%) 1 (0.6%) |
| Reoperation | 2 (1.2%) |
| Mean length of stay in days ± SD [range] | 7.2 ± 2.5 [3–24] |
| SD: Standard Deviation. GJ: gastrojejunal. JJ: jejunojejunal. | |
Currently, one-third of the US population is obese [17]. As more data are accumulated, it is clear that medically supervised weight loss is inferior to surgery for long-term sustained results [18].
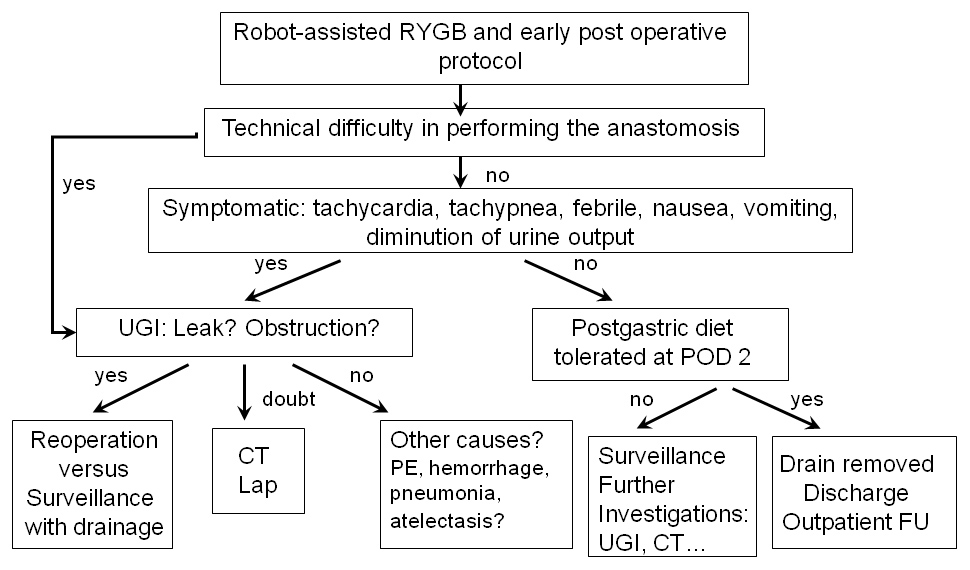
Figure 3
Decisional algorithm.
FU: Follow Up. UGI: Upper Gastrointestinal series. CT: Computer Tomography. RYGB: Roux-en-Y Gastric Bypass. Lap: exploratory Laparoscopy. POD: Post-Operative Day. PE: Pulmonary Embolism.
Today, laparoscopic RYGB is seen as the gold standard in the United States [2–4]. Still, despite this popularity, the laparoscopic approach is associated with specific and severe anastomotic complications. As a result of the potential severity of these complications, routine postoperative UGI with gastrografin has become a common strategy used by many centres. Different groups [6–12], however, have studied the role of routine UGI and have found controversial results, bringing into question the appropriateness of its use.
Moreover, in the last decade, the introduction of robotics has been seen as a potential evolution to overcome the natural limitations of laparoscopy. Even advanced procedures have been reported with encouraging results [19–22].
In the bariatric field, many groups have reported their experience with robotic RYGB [13–15, 23–27]. The real advantages continue to be under debate. For example, the operative time, as reported herein, is relatively longer than for laparoscopy. In the present series including the very first cases, the mean operative time was 295 minutes. While recently we have reported a short learning curve associated with the robotic approach and a shorter operative time as well (223 minutes) [27], it remains true that this approach is still longer than laparoscopy. Overall our recent results compare favourably to the robotic literature [13, 15, 23, 25, 26].
On the other hand, some groups have clearly reported a diminution of anastomotic complications following robotic RYGB when compared to laparoscopy [13–15]. In a large and recent study, Snyder et al. [15] compared 320 robot-assisted RYGB to 356 laparoscopic RYGB. They found a significant reduction of anastomotic leak following robot-assisted approach (0% vs. 1.7% after a laparoscopic approach; p = 0.05). In addition, Ayloo et al. [13] compared 45 laparoscopic to 90 robot-assisted RYGB. While not statistically significant, they reported a diminution of marginal ulcer and GJ stenosis following robotic approach. Finally, the interest of robotics in bariatric surgery is obvious in performing a hand-sewn anastomosis that reproduces the open technique.
The most serious complication following RYGB is anastomotic leak with an incidence reported somewhere in the range of 0.58% and 4.4% [6–10]. Hamilton et al. [7], in a series of 210 patients, noted an incidence of GJ leak of 4.3%. More recently, Doraiswamy et al. [9] reported a leak rate of 0.58% after 516 laparosocpic RYGB. Besides, the incidence of leak following a robotic approach is reported somewhere in the range of 0% and 1.7% [13, 15, 23–27].
It is clear from these large series that the incidence of GJ leak is low, although it can have devastating results. This has been the rational for obtaining routine UGI following the procedure, to identify and curtail the risk of leak. How-ever, UGI can sometimes fail to detect postoperative GJ leaks, as it was reported by Hamilton et al. [7] with 7 cases of false negative UGI (sensitivity of 22%). In 4 cases, a computed tomography (CT) finally gave the diagnosis of GJ leak. More recently, Doraiswamy et al. [9] showed a UGI sensitivity of 33% and a positive predictive value of only 11.1% for the detection of leak. On the other hand, Serafini et al [8] showed a sensitivity of 66% of UGI to detect a GJ leak. Of note, 75% of their RYGB were performed by an open approach. Finally, in our series of 167 robot-assisted RYGB, we have not noticed any gastrointestinal leaks.
Another indication for UGI following RYGB is to rule out a partial or complete obstruction, although reported incidence rates are relatively low, with reported rates in between 0% and 7.2% [6, 9, 13]. Sims et al. [6] identified a rate of 6% for delayed emptying and similar results were reported by others [9]. Moreover, Sims et al. [6] reported 7 patients with complete bowel obstruction (3.5%), while only 3 of them were diagnosed by UGI.
Patients can present with symptoms of vomiting or nausea and epigastric pain due to stoma edema. Most of the stoma obstructions are treated conservatively with intravenous hydration and usually resolve spontaneously [6]. In our series, we reported two patients with GJ edema (1.2%), treated conservatively with success.
Like others [7, 9–12], we have shown that routine UGI is of limited value. Indeed, in the present series, this exam diagnosed three anastomotic complications (edema and stenosis) in symptomatic patients. Lyass et al. [10] performed radiologic studies only in presence of symptoms (10% of patients). Interestingly, all the asymptomatic patients (n = 327) did not undergo any radiologic study and no complications developed in these patients.
Moreover, the patients undergoing UGI are exposed to radiation, as well as the discomfort and added costs. In our study, the total cost was almost US$ 100,000 for the 167 patients, or US$ 580 per patient. While this economical evaluation remains superficial and takes into account only the radiological study and not the overall cost, it remains clear that the price of a hypothetical leak is very high. Yet, as reported recently [28], we have shown that the use of the robotic technology is cost effective when compared to open and laparoscopy by avoiding anastomotic complications and reducing the stapler use.
Finally, as reported by others [6], the interest of UGI for an early radiological detection of a leak can result in a significant shorter hospital stay when compared to a clinical leak (7.7 days vs. 40.2 days for a clinical leak, p <0.03). We are unable to confirm these findings, since we had no leak following robotic anastomoses. Thus, the money saving is important in a specialised centre where the leak rate is very low. Yet, the robotic charges are important as well, and should be taken into consideration.
We believe that in experienced centres, it is not necessary to perform routine UGI unless clinical signs of a leak exist or if technical difficulties were encountered when performing the anastomosis. Hamilton et al. [7] have also shown that reliance should be placed on clinical signs such as the presence of tachypnea or respiratory distress, diminution of urine output and tachycardia (with heart rate exceeding 120 beats per minute). In their series, UGI detected only 22% of the leak, however with 100% of specificity. In fact, patients with severe tachycardia and respiratory distress had a 20% chance of harbouring a leak [7]. Besides, others reported fever, vomiting and leukocytosis at postoperative day 1 as additional clinical parameters associated to a leak [9, 11].
Of note, at our institution, the hospital stay was clearly longer than those previously reported [7, 9], reflecting probably the difference in health system between Europe and North America. This longer hospital stay may allow a longer clinical surveillance as well.
Thus, in the era of robotics, where the risk of anastomotic leak seems low and maybe lower than after a laparoscopic approach, it appears clear that this type of examination should be selectively proposed to symptomatic patients. In cases of doubt, a prompt CT scan of the abdomen with oral and intravenous contrast or an exploratory laparoscopy should be performed. To address this, we have developed a decisional algorithm integrating these findings (fig. 3).
In most centres, the risk of anastomotic leak has been the rationale for obtaining an UGI following RYGB. However, in the robotic era, it is clear from this study that the incidence of acute anastomotic complications is very low, although they can have devastating results.
The authors show this exam to be expensive and of limited value in an experienced centre and propose a decisional algorithm to determine when its use could be appropriate in symptomatic patients. This algorithm is currently under evaluation.
1 Hinojosa MW, Varela JE, Parikh D, Smith BR, Nguyen XM, Nguyen NT. National trends in use and outcome of laparoscopic adjustable gastric banding. Surg Obes Relat Dis. 2009;5(2):150–5.
2 Schauer PR, Ikramuddin S. Laparoscopic surgery for morbid obesity. Surg Clin North Am. 2001;81(5):1145–79.
3 Schauer PR, Ikramuddin S, Gourash W, Ramanathan R, Luketich J. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann Surg. 2000;232(4):515–29.
4 Buchwald H, Williams SE. Bariatric surgery worldwide 2003. Obes Surg. 2004;14(9):1157–64.
5 Nguyen NT, Goldman C, Rosenquist CJ, Arango A, Cole CJ, Lee SJ, et al. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Ann Surg. 2001;234(3):279–89; discussion 89–91.
6 Sims TL, Mullican MA, Hamilton EC, Provost DA, Jones DB. Routine upper gastrointestinal Gastrografin swallow after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2003;13(1):66–72.
7 Hamilton EC, Sims TL, Hamilton TT, Mullican MA, Jones DB, Provost DA. Clinical predictors of leak after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Surg Endosc. 2003;17(5):679–84.
8 Serafini F, Anderson W, Ghassemi P, Poklepovic J, Murr MM. The utility of contrast studies and drains in the management of patients after Roux-en-Y gastric bypass. Obes Surg. 2002;12(1):34–8.
9 Doraiswamy A, Rasmussen JJ, Pierce J, Fuller W, Ali MR. The utility of routine postoperative upper GI series following laparoscopic gastric bypass. Surg Endosc. 2007;21(12):2159–62.
10 Lyass S, Khalili TM, Cunneen S, Fujita F, Otsuka K, Chopra R, et al. Radiological studies after laparoscopic Roux-en-Y gastric bypass: routine or selective? Am Surg. 2004;70(10):918–21.
11 Singh R, Fisher BL. Sensitivity and specificity of postoperative upper GI series following gastric bypass. Obes Surg. 2003;13(1):73–5.
12 Katasani VG, Leeth RR, Tishler DS, Leath TD, Roy BP, Canon CL, et al. Water-soluble upper GI based on clinical findings is reliable to detect anastomotic leaks after laparoscopic gastric bypass. Am Surg. 2005;71(11):916–8; discussion 8–9.
13 Ayloo SM, Addeo P, Buchs NC, Shah G, Giulianotti PC. Robot-assisted versus Laparoscopic Roux-en-Y Gastric Bypass: Is There a Difference in Outcomes? World J Surg. 2011;35(3):637–42.
14 Snyder BE, Wilson T, Scaborough T, Yu S, Wilson EB. Lowering gastrointestinal leak rates: a comparative analysis of robotic and laparoscopic gastric bypass. J Robotic Surg. 2008;2:159–63.
15 Snyder BE, Wilson T, Leong BY, Klein C, Wilson EB. Robotic-assisted Roux-en-Y Gastric bypass: minimizing morbidity and mortality. Obes Surg. 2010;20(3):265–70.
16 Hubbard VS, Hall WH. Gastrointestinal Surgery for Severe Obesity. Obes Surg. 1991;1(3):257–65.
17 Ogden CL, Carroll MD. Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1976–1980 through 2007–2008. National Center for Health Statistics. 2010: Available at http://www.cdc.gov/nchs/fastats/overwt.htm. Accessed September 2010.
18 Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–52.
19 Buchs NC, Addeo P, Bianco FM, Elli EF, Ayloo S, Giulianotti PC. Robotic palliation for unresectable pancreatic cancer and distal cholangiocarcinoma. Int J Med Robot. 2011;7(1):60–5.
20 Buchs NC, Addeo P, Bianco FM, Gangemi A, Ayloo SM, Giulianotti PC. Outcomes of robot-assisted pancreaticoduodenectomy in patients older than 70 years: a comparative study. World J Surg. 2010;34(9):2109–14.
21 Giulianotti PC, Coratti A, Sbrana F, Addeo P, Bianco FM, Buchs NC, et al. Robotic liver surgery: results for 70 resections. Surgery. 2010;149(1):29–39.
22 Buchs NC, Addeo P, Bianco FM, Ayloo S, Benedetti E, Giulianotti PC. Robotic versus open pancreaticoduodenectomy: a comparative study at a single institution. World J Surg. 2011;35(12):2739–46.
23 Hubens G, Balliu L, Ruppert M, Gypen B, Van Tu T, Vaneerdeweg W. Roux-en-Y gastric bypass procedure performed with the da Vinci robot system: is it worth it? Surg Endosc. 2008;22(7):1690–6.
24 Moser F, Horgan S. Robotically assisted bariatric surgery. Am J Surg. 2004;188(4A Suppl):38S–44S.
25 Yu SC, Clapp BL, Lee MJ, Albrecht WC, Scarborough TK, Wilson EB. Robotic assistance provides excellent outcomes during the learning curve for laparoscopic Roux-en-Y gastric bypass: results from 100 robotic-assisted gastric bypasses. Am J Surg. 2006;192(6):746–9.
26 Scozzari G, Rebecchi F, Millo P, Rocchietto S, Allieta R, Morino M. Robot-assisted gastrojejunal anastomosis does not improve the results of the laparoscopic Roux-en-Y gastric bypass. Surg Endosc. 2011;25(2):597–603.
27 Buchs NC, Pugin F, Bucher P, Hagen ME, Chassot G, Koutny-Fong P, et al. Learning curve for robot-assisted Roux-en-Y gastric bypass. Surg Endosc. 2011; Nov 2.
28 Hagen ME, Pugin F, Chassot G, Huber O, Buchs N, Iranmanesh P, et al. Reducing cost of surgery by avoiding complications: the model of robotic Roux-en-Y gastric bypass. Obes Surg. 2012;22(1):52–61.
Funding / potential competing interests: Monika Hagen has a financial relationship with Intuitive Surgical. No other potential conflict of interest relevant to this article was reported.