Knocking at the door of the unborn child: engineered nanoparticles at the human placental barrier
DOI: https://doi.org/10.4414/smw.2012.13559
Tina
Buerki-Thurnherr, Ursula
von Mandach, Peter
Wick
Summary
Exposure of pregnant women and their unborn children to engineered nanoparticles (NPs) is not yet of major public concern. However, this may soon change in light of the ever-increasing production of NPs and the continuous appearance of novel NP-containing consumer products. However, NPs may not only pose risks to exposed individuals; they offer major potential for the development of novel therapeutic strategies to treat specifically either the mother or the developing foetus. Hence there is every reason to explore the transplacental transfer of engineered NPs in more detail, and to find answers to the vast number of open questions in this fascinating field of research.
Should we worry about the new visitors at the placental door? – Introduction
For many years, the placenta has been perceived as an impenetrable barrier for any pharmacological or toxic agent between the mother and the foetus, a perfectly secure door closed to all unknown visitors. But isn’t every door prone to being opened at some time or another? Indeed, since the discovery of thalidomide-induced birth defects in the 1960’s, many additional studies have indicated that the placental door is leakier to many chemical substances and environmental pollutants than was previously anticipated. For instance, exposure to alcohol, tobacco, methylmercury, lead or polychlorinated biphenyls (PCBs) has been shown to cause deleterious functional, cognitive or reproductive defects to the foetus [1]. Nevertheless, the placental barrier is indispensable as it mediates the exchange of nutrients and metabolic waste products, performs vital metabolic functions and secretes hormones that maintain pregnancy.
But these days there is a new visitor knocking at the placental door. With the growing use of nanotechnology, the placenta is likely to come into contact with novel nanoparticles, either accidentally through exposure to these materials, or intentionally in the case of potential nanomedical applications. Should we worry about the new guest, considering that the placenta is already regularly dealing with a vast amount of exogenous toxins, particles and chemicals? Concerns arise from the exceptional properties associated with these novel materials. Due to their small size and high surface area, NPs acquire new physicochemical characteristics not displayed by the corresponding bulk materials such as catalytic activity, conductivity or mechanical properties. Although these novel characteristics drive the development of numerous promising applications in many fields of technology and medicine, they may also have unforeseen effects if particles come into contact with cells and tissues of the human body. In fact, nanoparticles have a relatively marked propensity to cross cell membranes. Biological barriers such as the blood-brain barrier that are hardly amenable to larger particles or drugs can be penetrated by certain nanomaterials [2]. Therefore it is of major importance to understand whether nanoparticles interfere with cellular functions and have adverse effects in biological tissues. Apparently these tiny particles comprise a very mixed crowd of good guys, bad guys and everything in between. To give a few examples, superparamagnetic iron oxide nanoparticles (SPIONs) are in general classified as biocompatible, and some SPIONs are already approved for clinical application as MRI contrast agents [3]. In contrast, there is ample evidence to suggest that inhalation of carbon nanotubes may provoke pulmonary inflammation, oxidative stress, and long-term pathological effects such as granulomas, fibrosis and wall thickening [4]. But it is only marginally understood why some particles are more toxic than others, as current knowledge of the mechanisms underlying their adverse effects is very limited. Reverting to the placenta, research on transplacental transfer of nanomaterials and their effects on the placenta and the growing foetus is still very much in its infancy. However, studies in this area should be intensified, not only due to the aforementioned toxic potential of certain nanoparticles in other organ systems, but also in the light of epidemiological evidence that prenatal exposure to environmental fine particles (<2.5 µm in diameter) and ultrafine particles (<0.1 µm in diameter) adversely affects foetal health and development. As such, increased perinatal mortality, pre-term birth and low birth weight have been shown to correlate with high particulate matter exposure during pregnancy [5–8]. In this regard it is evident now that the growing foetus is particularly vulnerable to foreign particles even at doses that do not have adverse effects in other tissues. Nevertheless, toxicity concerns are only one side of the coin and it is questionable whether sufficiently large numbers of particles would reach the placenta in an environmental exposure setting. Another, perhaps more relevant motive for strengthening transplacental transfer studies is to exploit the potential of nanoparticles for novel drug delivery strategies to specifically target the mother, the placenta or the foetus. Currently, drug intake during pregnancy is more commonplace than is generally realised, despite the high concomitant risk of deleterious effects on the growing foetus [9, 10]. The fact that nanoparticles can be tailored to allow efficient drug loading, specific targeting and controlled drug release may help to lower the requisite therapeutic doses and to prevent off-target side effects. Moreover, nanoparticle-based therapy concepts may also help to overcome shortcomings in the treatment of foetal diseases or placental complications. But there is still a long way to go before we see whether nanoparticles fulfill our expectations, and we must first acquire a basic understanding of the behaviour of these particles at the placental barrier.
What are possible keys to passing through the placental door? – Potential uptake mechanisms
Knowing that the placenta is not only permeable to specific endogenous molecules but also lets pass a number of environmental substances or drugs, we should now address the question whether nanoparticles may be taken up by some of the already known uptake routes. However, before discussing possible uptake mechanisms, we first examine the architecture of the placental door, in particular the cellular barriers potentially encountered by our small visitors. The human placenta is of the hemochorial type, in which the foetal tissue is in direct contact with the maternal blood. The placental barrier separating maternal and foetal circulation consists of the syncytiotrophoblast, cytotrophoblast and the foetal capillary endothelium (fig. 1). It decreases in thickness throughout gestation due to thinning of the syncytiotrophoblast and spreading of the cytotrophoblastic layer. Consequently, transfer of substances across the placenta is most efficient in the last trimester when substantial amounts of nutrients are required to sustain rapid foetal growth.
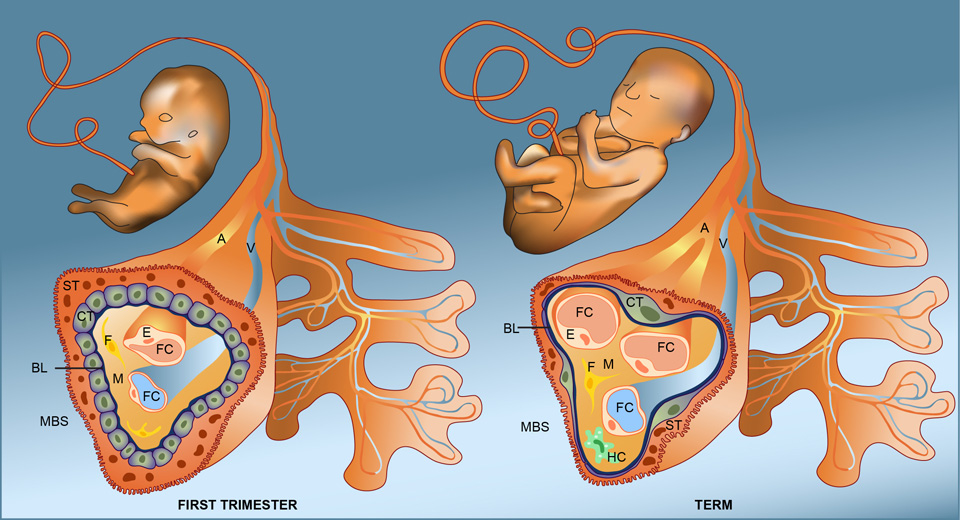
Figure 1
Structure of the placenta barrier in the first trimester and at term.
Illustration of a foetal villus in the first trimester and at term. In early gestation, the placental barrier separating the foetal (FBS) and maternal bloodstream (MBS) consists of a syncytium of syncytiotrophoblasts (ST), a monolayer of cytotrophoblasts (CT) and the foetal capillary endothelium (E). In between, the basal lamina (BL) and various cells of the mesenchyme (M) including fibroblasts (F) and Hofbauer cells (HC) may further restrict the transfer of NPs across the placenta. At term, the placental barrier is much thinner due to thinning of the syncytiotrophoblast and the spreading of the cytotrophoblastic layer. A: foetal artery; FC: foetal capillary, V: foetal vein.
So far only very few studies have investigated whether nanoparticles pass the human placenta, and there is virtually no information on the transplacental uptake mechanisms involved. Several mechanisms are already known for the placental exchange of endogenous substances including passive diffusion, facilitated diffusion, active transport, endocytotic pathways and putative transtrophoblastic channels [11, 12]. Passive diffusion is the predominant transfer mechanism for most small substances (<500 Da) and pharmacologically active compounds. It is not energy-dependent and is not saturable. However, passive transfer is profoundly influenced by the concentration gradient, the properties of the placenta and the physicochemical characteristics of the substance. If the transport of a compound down its concentration gradient is mediated by a specific membrane exchanger protein, it is called facilitated diffusion. This process is passive, saturable and does not require any input of energy. Only a few exogenous compounds have been suggested as being transported across the placental barrier by facilitated diffusion, and chiefly endogenous substances such as glucose, amino acids, nucleosides and metabolites are exchanged by this transfer route. In the case of active transport, energy is required to achieve transport against a concentration gradient. This mechanism is mediated by particular protein pumps of the syncytiotrophoblasts, most of which function in the maternal direction for the excretion of xenobiotics and toxic metabolites. The ATP-binding cassette (ABC) family of active efflux pumps comprises the known major placental efflux transporters. However, active transporters are not strictly selective for their substrate and nonphysiological compounds with structural similarity have the potential to be recognised as well. Whether NPs will cross the placental barrier by one of the aforementioned processes is probably dependent on their size, hydrophobicity, polarity and protein binding, inter alia. However, to our opinion, most NPs will be too large for passive or facilitated diffusion, particularly considering their propensity to form small agglomerates in biological fluids. Nevertheless, we do not completely exclude passive uptake mechanisms for transplacental transfer of NPs, as there is microscopic and mechanistic evidence for such uptake routes for cetain NPs in other cell types [13]. In addition, NP uptake by active transporters is also questionable as similarity to other endogenous substances is unlikely. Rather, different endocytotic pathways may be involved in the potential transplacental transfer of certain nanomaterials. Endocytosis is an active process that is employed by cells for the uptake of large, polar molecules that cannot pass through the hydrophobic membrane of a cell. It comprises clathrin-mediated endocytosis, caveolae, macropinocytosis and phagocytosis. In all cases, molecules either absorb to cell membranes or bind to specific receptors (receptor-mediated endocytosis), followed by the invagination of cell membranes and formation of intracellular vesicles. Expected size restriction for the uptake is 120 nm for clathrin-medicated endocytosis, 60 nm for caveolae and up to 1 µm for macropinocytosis respectively [14]. Indeed, syncytial membranes express caveolin-1 [15] and are richly endowed with clathrin [16, 17]. Once internalised, endocytotic vesicles can fuse with lysosomes, recycle to the site of entry or translocate to the opposite polar membrane to release their content. Our assumption of an endocytotic uptake mechanism for larger NPs or small NP agglomerates at the placental barrier is based on the fact that endocytotic vesicles exhibit an appropriate size range and that in many cell types and tissues such NPs often prevail in membrane-bound vesicles in the cytoplasm, indicating an active uptake process [18]. Interestingly, NPs may even be actively directed towards receptor-mediated endocytotic uptake by functionalising their surface with selected ligands [19, 20]. In the placenta, receptor-mediated uptake is, inter alia, employed by immune globulins (IgG) that bind to Fc receptors on the apical membrane of the syncytiotrophoblast. NPs coated with IgG have already been used in other circumstances, such as for nanomedical applications, and it would be interesting to know whether such functionalised particles have the potential to cross the placenta in a similar endocytotic process as is described for the endogenous IgG molecules. Finally, transtrophoblastic channels of some 20 nm diameter, spanning from maternal blood across the placental barrier to the fetal blood stream, have been proposed as an exchange route for small molecules between the mother and foetus [21–23]. However, their existence is still very much under discussion and there is no broad acceptance that these channels are contiguous and functional in intact placentas. Only under elevated foetal hydrostatic pressure was transport of water and small water-soluble molecules observed, and – notably – exclusively from the foetal-to-maternal direction [21, 24, 25]. Strong evidence against a contiguous transtrophoblastic channel system in normal placenta is also provided by the lack of transfer of numerous small water-soluble molecules, considering that a 20 nm wide pore would easily accommodate molecules of up to 50,000 Da. In conclusion, we propose that if Mr. Small and Little Miss Tiny are able to cross the placental door, they will most likely use one of the various endocytotic pathways described for endogenous small molecules. But we should also be prepared for the eventuality that they could just as well manage to find another, as yet unknown entry route.
Which spyholes are available to observe the entrance? – Model systems to study transplacental transfer
It is evident that translocation studies cannot be performed in the most perfect model system, namely in pregnant women, for obvious ethical reasons. Consequently, various alternative models have been developed including human in vitro and ex vivo systems as well as in vivo transfer studies in rodents. Here we present a brief overview of some of the most common model systems to address the transfer of substances across the placenta (for a comprehensive review see [26, 27]).

Figure 2
Key components of a placental perfusion experimental setup.
The middle picture shows a perfusion system with a perfusion chamber in a 37% water bath (white square). Other relevant components include a) gas mixture used for equilibration of the maternal and foetal circulation (maternal: 95% air, 5% CO2; foetal: 95% N2, 5% CO2); b) peristaltic pump allowing a specific adjustment of the flow rate; c) oxygenator with a bubble trap; d) online monitoring of pressure and temperature in the foetal circulation; e) foetal and maternal reservoir in water bath (37 °C); f) maternal side of a clamped placenta showing a perfused cotyledon with three blunt-tipped needles; g) foetal side of a clamped placenta with cannulated vessels; h) pressure and temperature sensor in a heating chamber; i) flow meter.
In vitro models using human cell cultures (primary cytotrophoblasts or choriocarcinoma cell lines), or isolated plasma membrane vesicles from human/animal placenta, are widely used to study transplacental transfer of a variety of drugs and compounds. The most popular example are BeWo monolayer cells, a choriocarcinoma derived placental cell line that strongly resembles cytotrophoblastic cells. The b30 subclone can be grown on permeable membranes in bicameral chambers to form confluent cell layers, enabling rates to be determined of both uptake into the cells from the apical surface and efflux from the basolateral membrane. BeWo cell monolayers were recently used to identify peptide ligands that facilitate placental transcytosis of viral particles across this cell culture model of the human trophoblast barrier [28]. Although in vitro models allow for the investigation of specific mechanisms of transfer, such as the type of transport (active transport, passive diffusion, transporter proteins or enzymes) they lack anatomical integrity and blood flow. Thus it remains to be demonstrated in future whether these in vitro models will be suitable to address the transfer of NPs across the placenta.
First indications that nanosized materials may cross the placental tissue did not come from such in vitro models but were obtained from a study in pregnant rats showing that gold NPs were transferred to the embryos 24 hours after intravenous injection [29]. However, in rats or mice three trophoblast layers are present between maternal blood and fetal blood capillary, whereas in humans a single syncytiotrophoblast layer arises from the fusion of cytotrophoblast cells and forms a true syncytium with no lateral cell membranes [30]. Hence, animal data cannot simply be extrapolated to humans because the placenta is the most species-specific mammalian organ [31]. As a consequence, various human model systems have been developed trying to mimic the situation in pregnant women as closely as possible in order to circumvent species-dependent differences. In vivo studies evaluating the transplacental kinetics of xenobiotics in humans include sampling of umbilical cord blood as well as of maternal blood at the time of delivery. Calculation of the cord blood (C) to maternal blood (M) concentration ratio (C:M) can provide information on how much of a substance did cross the placenta in vivo. However, it is difficult to determine accurately the transplacental transfer kinetics of a particular substance by this method, because measurements are taken at only one time point and typically in a small number of mother–infant pairs. This is problematic, as there is often wide variability between pairs due to sample contamination, timing of sample collection, sampling site, interindividual differences, and duration of exposure. Moreover, maternal venous blood concentrations do not reflect those presented to the placenta and may depend on the tissues drained by the selected vein if an arteriovenous gradient exists [32]. Yet, sampling of cord blood is probably only a useful approach to study of the passage of drugs that are anyway administered during pregnancy to treat the mother or the foetus but not for NPs with unknown toxicity. Hence the human placenta perfusion model provides a better surrogate for study of transplacental transport of NPs [33–35]. Essentially, the foetal artery and vein of an intact cotyledon are cannulated and the placental tissue is placed in a perfusion chamber (fig. 2). The maternal side is perfused by introducing three blunt metal cannulas into the intervillous space by penetration of the decidual plate. Furthermore, a venous drain connected to a peristaltic pump removes the perfusate from the chamber and returns it to the maternal reservoir. Foetal and maternal cannulas are connected to two separate perfusion circuits, and peristaltic pumps hold the foetal circuit flow at a rate of 6 mL/min and the maternal circuit at 12 mL/min. Human placental perfusion models provide information on inter aliatransplacental transfer, placental metabolism, acute toxicity, the potential role of transporters and foetal exposure. Although this approach is very close to the in vivo situation, a major limitation concerns the use of a full-term placenta that does not allow estimation of the transfer in the first trimester when the foetus is most vulnerable. In principle, first term placentae can be obtained from elective surgical terminations of pregnancy, but these tissues are very fragile and difficult to perfuse. Moreover, it is unclear whether perfusions of such placental tissues represent the early exposure of healthy pregnant women, as termination of pregnancy is often due to a pathological situation (e.g. insufficient placental function). Another constraint is that the perfusion time is limited to approximately 4–8 hours, which is sufficient to study bidirectional transport and short-term effects but is too short to investigate chronic effects. Nevertheless, it has recently been confirmed that placental perfusion is a valid model to predict placental drug transfer at term reliably when adjusting for extra parameters [32].
Do we already know something about the new guests? – Current studies on placental permeability to ENPs
In the past few years, studies on the effects of environmental and engineered nanomaterials in animals or human placenta model systems have started to emerge (table 1). For an overview on current understanding of the consequences of prenatal NP exposure for the placenta and foetal integrity we suggest a review of Menezes et al. as well as two very recent original articles of Yamashita et al. and Pietroiusti et al. that have not yet been covered by any review [11, 36, 37]. Focusing on the transplacental transfer, it appears that most nanoparticles tested so far were detected in the foetal circulation or tissues. This strongly suggests that the placenta does not provide a tight barrier for nanoparticle entry to the foetus similar to what has already been discovered for other xenobiotics or drugs. In rodents, transplacental passage has been reported for Quantum dots (Qdots) [38], TiO2 NPs [37, 39], SiO2 NPs [37], C60 fullerenes [40], polystyrene beads (PS) [41] and gold NPs [42]. Among these nanoparticles, only PS beads and gold NPs have been studied in a human placenta perfusion model system [43, 44]. While PS beads were able to cross the human placenta in a size-dependent manner [44], gold NPs were retained in the placenta’s trophoblastic cell layer [43]. An explanation why gold NPs apparently pass the rodent but not the human placenta might be the use of different gold NPs, the different experimental setup or, more probably, the fairly dissimilar structure of human and rodent placenta. Or it might simply be a technical issue as only trace amounts of gold NPs (0.0006% of 1.4 nm gold NPs and 0.00005% of 18 nm gold NPs) were measured in foetal mouse tissue [42] and the detection limit of the method used in the human placenta model was estimated at 0.13–0.2% of particles [43]. In our opinion, reliable detection of NPs in the foetal tissues or circulation and their quantification is in general one of the main challenges in transplacental transfer studies, especially under realistic exposure scenarios where transfer rates are expected to be very low. Besides the lack of sensitivity, detection often relies on radioactive or fluorescent labelling of NPs bearing the risk of an unspecific release of the label. Indeed, there are several studies where the placental passage of NPs is unclear either because the detection systems used lacked sensitivity [36, 45] or because no method existed at all to identify the particles unambiguously (C60 fullerenes in [36], DE in [46, 47]). Nevertheless, from the few studies that allowed NP tracking, it appears that transplacental transfer is not solely dependent on the size of particles [42, 44] but also on their physicochemical characteristics and coating [38]. Thus our advice to Mr. Little: if you want to enter make sure you are wearing the right coat.
|
Table 1: Summary of studies on NP transfer across the placenta. |
|
NP type
|
NP size (nm) / modification
|
Dose / route of administration
|
NP application / length of exposure
|
Model system
|
Major outcomes
|
Reference
|
| Gold |
5 and 30 / radiolabeled |
0.02 mg / iv |
GD 19 / 24 h |
Rat |
Transplacental transfer rates of 0.018% for 5 nm and 0.005% for 30 nm NP |
[47] |
| Gold |
1.4 and 18 / radiolabeled and TPPTS |
N.s. / iv |
3rd trimester / 24 h |
Rat |
Size-dependent uptake in placenta (0.03% of 1.4 nm and 0.0002% of 18 nm NP) and fetus (0.006% of 1.4 nm and 0.00005% of 18 nm NP) |
[41] |
| Gold |
10 / PEG
15 / PEG
10 and 15 / PEG |
9.1*109 NP / ml
9.1*109 NP / ml
3.6*1010 NP / ml |
6 h perfusion
6, 24 and 48 h in vitro BeWo |
Human |
No NP transfer observed, particles accumulate in the trophoblastic cell layer
Uptake and retention of NP in BeWo cells |
[42] |
| TiO2
|
25-70 |
0.1 mg / sc |
GD 3, 7,10 and 14 / P4 and 6 weeks |
Mouse |
NP cross the placenta and can damage the genital and cranial nervous system |
[38] |
| TiO2
|
21 / modified with Al, Si and Zr and coated with polyalcohols |
40 mg/m3; 1h / day / inhalation |
GD 8-18 / P2 and P23-24 |
Mouse |
Offspring displays neurobehavioral alterations but unclear if effect is direct or indirect; transplacental transfer of NPs not investigated |
[44] |
| TiO2 and
SiO2 (nSP) |
35
70, 300 and 1000 / nSP70 carboxylic or amine |
0.8 mg (nSP70 also at 0.4 mg) / iv |
GD 16 and 17 / GD 18 |
Mouse |
TiO2 and 70 nm SiO2 cause pregnancy complications but not larger sNPs or modified sNPs; TiO2 and 70 nm SiO2 are present in the placenta, fetal liver and fetal brain |
[36] |
| Silicon nanovectors |
519, 834 and 1000 |
1.2*109 NP/ml/ iv |
GD 20 / 4 h |
Rat |
Transplacental transfer of the particles is size dependent (particles > 800 nm do not cross) |
[48] |
| Polystyrene
beads (PS) |
20, 100, 500/ fluorescent, carboxylic;
200 /fluorescent, amine |
from 0.5%w stock: 0.6 µl (PS 20, 100), 8 µl (PS 500), 1.25 µl (PS 200) /
injection via extraembryonic tissue |
GD 7.5 / 12 h |
Rat |
Modification affects transplacental transfer: Amine-PS 200 pass extraembryonic tissue while carboxy-PS bigger than 100 nm accumulate in the extraembryonic tissue |
[40] |
| Polystyrene
beads (PS) |
50, 80, 240, 500 / fluorescent |
25 µg/ml |
6h perfusion |
Human |
Size-dependent transplacental transfer (35% of PS 50, 30% of PS 80, 9% of PS 240 and 1% of PS 500) |
[43] |
| Quantum-dots |
1.7, 2.6 and 3.2 / pristine or coated with MPA, PEG and SiO2
|
20, 50, 86, 125 µg / iv |
GD 20-22 / P0 |
Mouse |
Size-dependent transfer of particles across the placenta; coating of particles reduced the transfer |
[37] |
| Fullerenes |
Average size < 10 |
0.3 mg/kg / iv |
GD 15 / 24 and 48 h |
Rat |
Particles cross the placenta and are transmitted to the offspring |
[39] |
| Single-walled carbon nanotubes |
850 x 2.37 / pristine; 760 x 1.58 / oxidized;
370 x 1.8 / ultra-oxidized |
10 ng to 30 µg / injection into retrobulbar plexus
0.1-100 µg/ml |
GD 5.5 / 10 d
EST (NIH3T3 and mES) / 10 d |
Mouse |
Low doses of particles affect embryonic development; effects more pronounced for oxidized particles EST predict in vivo data, identifying oxidized particles as the more toxic compound |
[35] |
| CoCr |
29.5 |
0.12 mg / iv
0.04 mg/ml |
GD 9.5 or 12.5 / 7 d
In vitro transwell co-cultures (BeWo + fibroblasts)/ 24 h |
Mouse
Human |
DNA damage of CoCr NPs across bilayered but not monolayered barriers
Indirect DNA damage to fibroblasts without passage of NPs is dependent on thickness of the BeWo barrier |
[49]
[51] |
| EST: embryonic stem cell test; mES: mouse embryonic stem cells; GD: gestation day; iv: intravenous; MPA: 3-mercaptopropionic acid; NP: nanoparticles; N.s.: not stated; P: postnatal day; PEG: polyethylene glycol; sc: subcutaneous; TPPTS: sulfonated triphenylphosphine |
What else do we have to know to prepare for their visit? – Open questions remaining
Along the previous lines, the reader may already have come up with some questions regarding the placental passage of NPs. Here we will add some of our own reflections that may likewise help to stimulate future research in this exciting field.
– Is the ability to cross the placental barrier an inherent property of all NPs? So far, it appeared that basically all types of NPs tested were capable of crossing the placental barrier even if simply in trace amounts. But as the number of studies on transplacental transfer is so small, more types of nanomaterials need to be tested, in particular those with a high exposure risk or of potential medical use. If NPs pass the human placenta, it will be of major importance to know more about the degree of NP-passage as well as the transfer rates, as the particle dose often determines the biological effects. In this regard the available knowledge is scanty, indicating that the percentage of particles in foetal tissues may be anywhere between trace amounts as measured for gold NPs (<1%) [42, 48] or significant doses in the case of 80 and 50 nm PS beads (30–35%) [44]. However, these numbers must be interpreted with care, taking into consideration the differences in the routes of administration and the methods used to quantify the particles. For future studies it will also be important to take into account the testing of realistic doses to obtain meaningful results. These values may be rather low for environmental exposure scenarios, but high for nanoparticles with medical applications.
– What are the key characteristics of NPs that determine their transplacental transfer? Particle size appears to be an important factor as several studies have shown that the transfer efficiency is higher the smaller the particles are [38, 42, 44, 49]. In addition, particle coating appears to play another significant role in their uptake [37, 38].
– Are adverse effects on embryonic development induced directly by translocated particles, or are they an indirect consequence of altered placental permeability or functionality – or even due to the release of mediators in the maternal tissue? Indirect effects have already been proposed by several groups, but clear evidence is not yet available [36, 37]. Nevertheless, a small series of articles demonstrating indirect toxicity of NPs across various in vitro biological barriers seems to further corroborate this hypothesis [50–52]. Interestingly, indirect toxicity was only observed in multilayered barriers (including a late gestation BeWo placenta model) but not in monolayered barriers (including an early gestation BeWo placenta model) [52].
– What are the effects of prenatal NP exposure on the unborn child? Due to ethical reasons, data on the embryotoxicity of NPs have been obtained from experiments on pregnant rodents. These studies have demonstrated that prenatal exposure to carbon nanotubes, PS beads or small TiO2 NPs correlates with foetal complications [36, 37, 39, 41]. A feature of note is that the validated mouse embryonic stem cell test (EST) was able to predict in vivo toxicity, suggesting that it can be used to replace in vivo testing in mice [36].
Finding answers to all of these questions will be a fascinating challenge. Currently available model systems and biological tests may have to be adapted for the testing of nanomaterials, as it is well known that NPs have many ways of interfering with various test procedures. In addition, entirely new placenta models and detection systems may need to be developed, requiring a multidisciplinary effort including experts from the material, chemical, medical and biological fields. As such, it would be of great interest to devise a novel advanced in vitro model of the human placenta for high-throughput testing of many different conditions. This would not only allow the rapid pre-screening of a large variety of nanomaterials, but would also enable more systematic studies on the uptake or toxicity mechanisms. In this direction, simple models such as a monolayer of BeWo cytotrophoblast cells in a 2D-culture dish or transwell insert are already described, but they may not reflect the in vivo situation very closely. A novel approach may comprise the use of primary cells directly isolated from placental tissue in combination with a more in vivo-like setup such as a 3D-placental microtissue or a perfused transwell system. To study the transfer of nanoparticles primary cytotrophoblasts that spontaneously form a syncytium without lateral separations may be better suited to representing the placenta than BeWo cells that are very difficult to differentiate. Moreover, as translocation of nanoparticles occurs in a dynamic surrounding, we assume that a perfused transwell approach or a microfluidic system will provide a significantly improved model as compared to a static transwell system. To obtain such a perfused transwell model, co-cultures of placental cells could be obtained on a transwell insert under static conditions similar to other multi-type cell models, such as an efficiently working triple-cell culture system of the lung [53, 54]. To test nanoparticle translocation, these inserts could then be placed in a perfusion chamber that allows mimicking of the maternal and foetal circulation. For mechanistic studies, 3D-microtissues of primary co-cultures or mixed primary cells and cell lines may provide a good in
vivo-like model system suited to high-throughput testing. Such organotypic 3D microtissue spheres with tissue-like structure and functions can be obtained by gravity-enforced self-assembly of cells in hanging drops [55]. Ultimately it will be of major importance to show that such models do indeed represent an improvement on current in vitro tests, how well they are suited to the study of nanoparticles and to what extent they correlate with the ex vivoperfusion system.
References
1 Lanphear BP, CV Vorhees, DC Bellinger. Protecting children from environmental toxins. PLoS Med. 2005;2(3):e61.
2 Koffie RM, et al. Nanoparticles enhance brain delivery of blood–brain barrier-impermeable probes for in vivo optical and magnetic resonance imaging. Proceedings of the National Academy of Sciences, 2011.
3 Kim BY, JT Rutka, WC Chan. Current concepts: Nanomedicine. N Engl J Med. 2010;363(25):2434–43.
4 Shvedova AA, et al. Mechanisms of pulmonary toxicity and medical applications of carbon nanotubes: Two faces of Janus? Pharmacology & Therapeutics. 2009;121(2):192–204.
5 Bobak M. Outdoor air pollution, low birth weight, and prematurity. Environ Health Perspect. 2000;108(2):173–6.
6 Huynh M, et al. Relationships between air pollution and preterm birth in California. Paediatr Perinat Epidemiol. 2006;20(6):454–61.
7 Salvi S. Health effects of ambient air pollution in children. Paediatr Respir Rev. 2007;8(4):275–80.
8 Woodruff TJ, LA Darrow, JD Parker. Air pollution and postneonatal infant mortality in the United States, 1999-2002. Environ Health Perspect. 2008;116(1):110–5.
9 Andrade SE, et al. Prescription drug use in pregnancy. Am J Obstet Gynecol. 2004;191(2):398–407.
10 Gagne JJ, et al. Prescription drug use during pregnancy: a population-based study in Regione Emilia-Romagna, Italy. Eur J Clin Pharmacol. 2008;64(11):1125–32.
11 Menezes V, A Malek, JA Keelan. Nanoparticulate drug delivery in pregnancy: placental passage and fetal exposure. Curr Pharm Biotechnol. 2011;12(5):731–42.
12 Syme MR, JW Paxton, JA Keelan. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet. 2004;43(8):487–514.
13 Geiser M, et al. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ Health Perspect. 2005;113(11):1555–60.
14 Conner SD, SL Schmid. Regulated portals of entry into the cell. Nature. 2003;422(6927):37–44.
15 Lee WK, JK Choi, SH Cha. Co-localization and interaction of human organic anion transporter 4 with caveolin-1 in primary cultured human placental trophoblasts. Exp Mol Med. 2008;40(5):505–13.
16 Lambot N, et al. Evidence for a clathrin-mediated recycling of albumin in human term placenta. Biol Reprod. 2006;75(1):90–7.
17 Ockleford CD, A Whyte. Differeniated regions of human placental cell surface associated with exchange of materials between maternal and foetal blood: coated vesicles. J Cell Sci. 1977;25:293–312.
18 Zhao F, et al. Cellular Uptake, Intracellular Trafficking, and Cytotoxicity of Nanomaterials. Small. 2011;7(10):1322–37.
19 Jiang W, et al. Nanoparticle-mediated cellular response is size-dependent. Nat Nano. 2008;3(3):145–50.
20 Zhang L, et al. Receptor-mediated cellular uptake of nanoparticles: A switchable delivery system. Small. 2011;7(11):1538–41.
21 Kertschanska S, G Kosanke, P Kaufmann. Pressure dependence of so-called transtrophoblastic channels during fetal perfusion of human placental villi. Microsc Res Tech. 1997;38(1-2):52–62.
22 Kertschanska S, et al. Distensible transtrophoblastic channels in the rat placenta. Placenta. 2000;21(7):670–7.
23 van der Aa EM, et al. Mechanisms of drug transfer across the human placenta. Pharm World Sci. 1998;20(4):139–48.
24 Stulc J, Stulcova B. Asymmetrical transfer of inert hydrophilic solutes across rat placenta. Am J Physiol. 1993;265(3 Pt 2):R670–5.
25 Stulc J, Stulcova B. Effect of NaCl load administered to the fetus on the bidirectional movement of 51Cr-EDTA across rat placenta. Am J Physiol. 1996;270(5 Pt 2):R984–9.
26 Myren M, et al. The human placenta – an alternative for studying foetal exposure. Toxicol In Vitro. 2007;21(7):1332–40.
27 Prouillac C, Lecoeur S. The role of the placenta in fetal exposure to xenobiotics: importance of membrane transporters and human models for transfer studies. Drug Metab Dispos. 2010;38(10):1623–35.
28 Basha S, Vaidhyanathan S, Pauletti GM. Selection of peptide ligands for human placental transcytosis systems using in vitro phage display. Methods Mol Biol. 2011;716:141–56.
29 Semmler-Behnke M, et al. Biodistribution of 1.4- and 18-nm gold particles in rats. Small. 2008;4(12):2108–11.
30 Enders AC, Blankenship TN. Comparative placental structure. Adv Drug Deliv Rev. 1999;38(1):3–15.
31 Ala-Kokko TI, Myllynen P, Vähäkangas K. Ex vivo perfusion of the human placental cotyledon: implications for anesthetic pharmacology. International Journal of Obstetric Anesthesia. 2000;9(1):26–38.
32 Hutson JR, et al. The human placental perfusion model: a systematic review and development of a model to predict in vivo transfer of therapeutic drugs. Clin Pharmacol Ther. 2011;90(1):67–76.
33 Malek A, et al. The impact of cocaine and heroin on the placental transfer of methadone. Reprod Biol Endocrinol. 2009;7:61.
34 Panigel M, Pascaud M, Brun JL. Radioangiographic study of circulation in the villi and intervillous space of isolated human placental cotyledon kept viable by perfusion. J Physiol. (Paris) 1967;59(1 Suppl):277.
35 Schneider H, Panigel M, Dancis J. Transfer across the perfused human placenta of antipyrine, sodium and leucine. Am J Obstet Gynecol. 1972;114(6):822–8.
36 Pietroiusti A, et al. Low doses of pristine and oxidized single-wall carbon nanotubes affect mammalian embryonic development. ACS Nano. 2011;5(6):4624–33.
37 Yamashita K, et al. Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat Nanotechnol. 2011;6(5):321–8.
38 Chu M, et al. Transfer of quantum dots from pregnant mice to pups across the placental barrier. Small. 2010;6(5):670–8.
39 Takeda K, et al. Nanoparticles transferred from pregnant mice to their offspring can damage the genital and cranial nerve systems. J Health Sci. 2009;55:95–102.
40 Sumner SC, et al. Distribution of carbon-14 labeled C60 ([14C]C60) in the pregnant and in the lactating dam and the effect of C60 exposure on the biochemical profile of urine. J Appl Toxicol. 2010;30(4):354–60.
41 Tian F, et al. Surface modification and size dependence in particle translocation during early embryonic development. Inhal Toxicol. 2009;21(Suppl 1):92–6.
42 Semmler-Behnke M, et al. Uptake of 1.4 nm versus 18 nm gold nanoparticles in secondary target organs is size dependent in control and pregnant rats after intratracheal or intravenous application. In: EuroNanoForum 2007 – Nanotechnology in Industrial Applications. 2007. Düsseldorf, Germany.
43 Myllynen PK, et al. Kinetics of gold nanoparticles in the human placenta. Reprod Toxicol. 2008;26(2):130–7.
44 Wick P, et al. Barrier capacity of human placenta for nanosized materials. Environ Health Perspect. 2010;118(3):432–6.
45 Hougaard KS, et al. Effects of prenatal exposure to surface-coated nanosized titanium dioxide (UV-Titan). A study in mice. Part Fibre Toxicol. 2010;7:16.
46 Fujimoto A, et al. Diesel exhaust affects immunological action in the placentas of mice. Environ Toxicol. 2005;20(4):431–40.
47 Sugamata M, et al. Maternal diesel exhaust exposure damages newborn murine brains. J Health Sci. 2006;52:82–4.
48 Takahashi S, Matsuoka O. Cross placental transfer of 198Au-colloid in near term rats. J Radiat Res. (Tokyo), 1981;22(2):242–9.
49 Refuerzo JS, et al. Size of the nanovectors determines the transplacental passage in pregnancy: study in rats. Am J Obstet Gynecol. 2011;204(6):546 e5-9.
50 Bhabra G, et al. Nanoparticles can cause DNA damage across a cellular barrier. Nat Nanotechnol. 2009;4(12):876–83.
51 Parry MC, et al. Thresholds for indirect DNA damage across cellular barriers for orthopaedic biomaterials. Biomaterials. 2010;31(16):4477–83.
52 Sood A, et al. Signalling of DNA damage and cytokines across cell barriers exposed to nanoparticles depends on barrier thickness. Nat Nanotechnol. 2011.
53 Lehmann AD, et al. An in vitro triple cell co-culture model with primary cells mimicking the human alveolar epithelial barrier. Eur J Pharm Biopharm. 2011;77(3):398–406.
54 Rothen-Rutishauser BM, Kiama SG, Gehr P. A three-dimensional cellular model of the human respiratory tract to study the interaction with particles. Am J Respir Cell Mol Biol. 2005;32(4):p. 281–9.
55 Kelm JM, Fussenegger M. Microscale tissue engineering using gravity-enforced cell assembly. Trends in Biotechnology. 2004;22(4):195–202.

