Impact of adherence to GOLD guidelines on symptom prevalence, lung function decline and exacerbation rate in a Swiss COPD cohort
DOI: https://doi.org/10.4414/smw.2012.13567
Anja
Jochmann, Andreas
Scherr, Dirk Christian
Jochmann, David
Miedinger, Salome
Schafroth Török, Prashant N.
Chhajed, Michael
Tamm, Jörg Daniel
Leuppi
Summary
PRINCIPLES: The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines aim to optimise chronic obstructive pulmonary disease (COPD) diagnosis and treatment.
However, little is known about the extent to which general practitioners’ (GP) adherence to GOLD guidelines improves patient outcomes.
METHODS: In this questionnaire-based study, COPD patients were screened and enrolled; exacerbation history was recorded, and demographic, spirometric and management data were collected for 12 months. Spirometry was performed at least every 6 months according to American Thoracic Society guidelines. Based on these data, patients were grouped into GOLD COPD severity classifications. Data were expressed as the difference between baseline and month 12.
RESULTS: Among 139 GPs, 454 patients were analysed regarding baseline and 12 month data. There was no significant change in distribution of GOLD COPD severity grades, lung function or guideline adherence. Chronic cough and sputum production were significantly reduced (p <0.001; p <0.020), as was exacerbation rate (p = 0.041). Factors associated with exacerbations were male sex, asthma and cerebrovascular insult as a co-morbidity. Exacerbation rate was significantly reduced in patients treated with combination therapy (long-acting β2-agonist (LABA)+ inhaled corticosteroids (ICS); p = 0.0178) and long-acting anticholinergics (LAAC; p = 0.0011). Patients treated per guidelines had no advantage in lung function, estimation of symptom prevalence or, most importantly, exacerbation rate.
CONCLUSIONS: While there was no improvement in adherence to GOLD guidelines, disease severity was not affected detrimentally, suggesting that guideline adherence does not seem to impact symptom prevalence, exacerbation rate or lung function decline after one year of follow up.
List of abbreviations
COPD chronic obstructive pulmonary disease
GOLD Global Initiative for Chronic Obstructive Lung Disease
GP general practitioner
FMH Swiss Medical Association
FEV1 forced expiratory volume in 1 second
FVC forced vital capacity
SD standard deviation
MRC Medical Research Council
ICS inhaled corticosteroid
LAAC long-acting anticholinergic
LABA long-acting β2-agonist
SABA short-acting β2-agonist
SAAC short-acting anticholinergic
Combination therapy inhaled corticosteroid + long-acting β2-agonist
Introduction
Chronic obstructive pulmonary disease (COPD) is a highly prevalent condition characterised by airflow limitation that is not fully reversible, and causes significant morbidity and mortality [1]. In Switzerland prevalence of COPD was found to be around 9% by the SAPALDIA study group with an incidence of 1.3%/year in adults [2].
Whilst guidelines are essential for the standardisation of disease, management and clinical practice often differs from proposed treatment recommendations [3]. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines aim to give advice on how to optimise COPD prevention, diagnosis and treatment [1].
In the case of asthma, some studies suggest that guidelines may improve adherence to evidence-based treatment and reduce financial burden [4, 5]. This situation may be similar for COPD. Nevertheless, there is an ongoing debate regarding whether treatment that conforms to guideline recommendations improves disease outcomes. Akinbami et al., for example, could not identify a clear benefit for children with asthma after adherence to authoritative guidelines for 16 years [6].
Little is currently known regarding the extent to which GOLD guidelines improve care and outcome in COPD patients [7]. Therefore, we designed a 24-month questionnaire-based study, performed in Switzerland, to assess the standard of diagnosis and treatment of COPD in primary care practice.
We previously analysed baseline data from our study, from which we made three main observations [8]. Firstly, misdiagnosis of COPD was a frequent event; application of the GOLD criteria following central lung function evaluation revealed 22% of the study population to be “non-COPD” sufferers. Secondly, non-pharmacological treatment is under-prescribed in general practice with only 4% of COPD patients receiving pulmonary rehabilitation. Thirdly, 47% of patients received treatment that differed from the recommendations of the GOLD guidelines.
The objective of the current analysis was to evaluate whether there was a significant change in symptom prevalence, lung function decline and exacerbation rate in relation to prescription patterns of pharmacological treatments, mostly inhaled corticosteroids (ICS) and long-acting β2-agonist (LABA)/ long-acting anticholinergic (LAAC), which adhered to GOLD recommendations in the previous 12 months. Furthermore, factors associated with exacerbation rate, which is one of the most important reasons for impaired quality of life in COPD patients, were analysed.
Methods
Initially, 139 general practitioners (GPs) from a total number of 3512 GPs listed on the register provided by the Swiss Medical Association (FMH) [9] participated in the study.
The study was approved by the ethics committee for each canton and all patients provided written documentation of informed consent. The trial commenced in January 2007 and extended over a period of 36 months, during which time the diagnosis, treatment and clinical course of COPD were analysed. Patients were seen by their GPs at 3-monthly intervals; symptoms according to the patients’ subjective estimation, co-morbidities and treatment were recorded. Furthermore, GPs performed lung function testing via spirometry at least every 6 months. The 6 month data was not analysed separately since follow up for exacerbation rate, for example, was too short. Data collected by the GPs are summarised in table 1. Interpretation of spirometry data was performed according to the criteria of the GOLD committee [10].
Spirometry
Spirometry was performed as described previously [8]. Of note, investigators used the same type of spirometer with automated quality feedback (EasyOne™; Medizintechnik AG, Zürich, Switzerland). GPs had been instructed by sales representatives of the company on how to perform spirometry according to the American Thoracic Society (ATS) guidelines [11]. The following lung function data were collected: forced expiratory volume in 1 second (FEV1) in litres; FEV1 percentage of reference value, forced vital capacity (FVC) in litres; FVC percentage of reference value; FEV1/FVC (%) (Tiffeneau index). For predicted values reference values by the SAPALDIA group (Swiss study on air pollution and Lung Diseases in Adults) were used [2].
Assessment of severity of COPD
Severity of COPD was assessed centrally by the investigators as in our baseline analysis of this study [8]. Spirometry data provided by the GPs were interpreted according to the criteria of the GOLD committee [10]. According to the GOLD criteria, airway obstruction was defined as having a forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) <70% [1]. Severity was classified as follows:
| Stage 1 (Mild): |
FEV1/FVC <0.70
FEV1 ≥80% predicted |
| Stage II (Moderate): |
FEV1/FVC <0.70
50% ≤ FEV1 <80% predicted |
| Stage III (Severe): |
FEV1/FVC <0.70
30% ≤ FEV1 <50% predicted |
| Stage IV (Very Severe): |
FEV1/FVC <0.70
FEV1 <30% predicted or FEV1 <50% predicted plus chronic respiratory failure. |
Comparison of prescribed medication with recommendations of GOLD
The use of bronchodilators and ICS currently received by patients with assumed COPD was compared with that recommended by the GOLD guidelines according to the GOLD severity of COPD grades [12]. COPD severity grades were assigned centrally following confirmation of COPD through spirometry. GOLD recommends prescription of short-acting bronchodilators for all severities of COPD, if required. Long-acting bronchodilators should be given to those with COPD GOLD stage II or worse, if required. Similarly, prescription of ICS (whether alone or in combination with a long-acting bronchodilator) should be reserved for GOLD stage III and IV, if the patient experiences repeated exacerbations. Data were analysed according to appropriate prescription of long-acting bronchodilators and ICS recommended by the GOLD guidelines in same way it was done in our analysis of the baseline data [8]. Non-conformity was defined as prescription of long-acting bronchodilators and ICS to GOLD severity stages other than recommended. Since prescription of ICS for co-morbid asthma could confound the analysis for GOLD stages I and II, data were also filtered considering use of ICS as acceptable for these patients. Patients with known asthma were therefore not counted as non-conformity to the GOLD guidelines.
Statistical analysis
Continuous variables were expressed as means (± standard deviation [SD]) and categorical variables were shown as relative frequencies and percentages. Comparisons were made using cross-tabulation for categorical data and summary statistics for metric data. To visualise categorical variables, mosaic plots were displayed. A mosaic plot is a graphical display that allows examination of the relationship among two or more categorical variables. The mosaic plot starts as a square with length one. The square is divided first into horizontal bars whose widths are proportional to the probabilities associated with the first categorical variable. Then each bar is split vertically into bars that are proportional to the conditional probabilities of the second categorical variable. Additional splits can be made if wanted using a third, fourth variable [13]. To predict exacerbations from potential risk factors, logistic regression was performed for each parameter separately. Time, age and gender were also included in each regression model. Hence results were adjusted for these parameters. A potential interaction between TIME and these factors were also included in these models. Non-significant interactions were deleted from the models.
As the patients were controlled at two time points a generalised mixed effect model (GLMM) approach was applied (details are described by Ripley [14]).
Odds ratios with corresponding 95% confidence intervals (CI) were estimated from the GLMM. In the case of an ordinal predictor, odds ratios were expressed increasing the predictor unit. A p-value <0.05 was considered as significant.
This study was exploratory therefore p-values are not adjusted for multiple comparisons.
All analyses were done using R version 2.8.1by R Foundation Bell Laboratories [15].
|
Table 1: Data requested. |
| Demographic data |
Surgery ID; patient ID; date of visit; patient initials; age group; gender; height; ethnic group; agricultural worker; current smokers |
| Symptoms |
Sputum; cough; MRC dyspnoea score 1–5* |
| Exacerbations |
Increased sputum production; discoloured sputum; shortness of breath |
| Treatment of exacerbation in preceding 3 months |
Antibiotics; systemic steroids |
| Spirometry |
FVC (L); FVC (% of reference value); FEV1 (L); FEV1 (% of reference value); FEV1/FVC (% – Tiffeneau-Index) |
|
Treatment
|
|
| Non-pharmacological treatment |
Physical exercise (minimum twice a week); pulmonary rehabilitation |
| Pharmacological treatment |
Short-acting β2-agonists; short-acting anticholinergics; long-acting β2-agonists; long-acting anticholinergics; inhaled steroids
Combination: long-acting β2-agonists and inhaled steroids; methylxanthines; N-acetyl-cysteine; systemic long-term steroids |
| Vaccinations |
Influenza; pneumococcus |
| Co-morbidities |
Asthma; coronary heart disease; congestive heart failure; hypertension; peripheral arterial occlusive disease; cerebral vascular insult; diabetes; cancer; lung cancer |
| Working ability |
Not restricted; retired; reduced (if reduced, by what percentage? Assessed by patient or GP?) |
| Death |
If a patient died, was it related to COPD? |
| Lost to follow-up |
|
| Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GP, general practitioner; COPD, chronic obstructive pulmonary disease.
*The Medical Research Council defines breathlessness as follows: Grade 1: Not troubled by breathlessness except on strenuous exercise. Grade 2: Short of breath when walking up a slight hill. Grade 3: Walks slower than contemporaries on level ground because of breathlessness. Grade 4: Stop for breath after 100 yards or after a few minutes on level ground. Grade 5: Too breathless to leave the house or breathless when dressing [30] |
Results
Demographic data and lung function characteristics
A total number of 828 patients were screened by GPs. Before the start of the study, 29 GPs subsequently withdrew due to time constraints, which resulted in the exclusion of 213 screened patients. After a baseline visit, 111 included patients dropped out due to co-morbidities and nursing home admissions, for example. Data was missing for 50 patients after the baseline visit. Therefore, 454 patients in total met the criteria for the per protocol population. For these patients, spirometry and symptom severity data were collected at baseline, six months and at 12 months. Patient demographic data and lung function characteristics are shown in table 2. Over 12 months, there was no significant change in the distribution of COPD severity grades that were centrally assigned according to GOLD classification. About half of all patients (59%) were considered to have GOLD stage II or III disease. The number of patients who did not have COPD according to spirometric criteria increased from 22% to 24%. About 6% of patients had discontinued smoking by 12 months. There was no significant decline in lung function after 12 months (fig. 1).
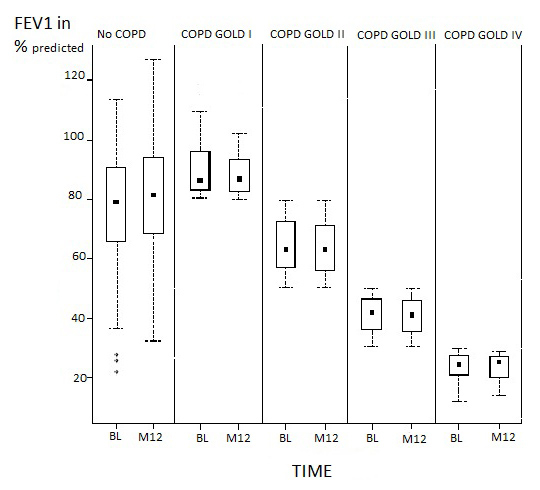
Figure 1
Trend of FEV1 over 12 months.
Comparison of average FEV1 predicted in different COPD groups at BL and after 12 months. Abbreviations: FEV1, forced expiratory volume in 1 second; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease.

Figure 2
Prevalence of dyspnoea, after 12 months.
Comparison of prevalence of dyspnoea at BL and after 12 months. No significant reduction after 12 months p = 0.24420. Abbreviations: COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; BL, baseline; M12, month 12.

Figure 3
Non-conformity to GOLD guidelines.
Comparison of guideline aberrant treated patients at BL and after 12 months. Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; BL, baseline; M12, month 12.
Symptom severity
The change of symptoms is illustrated in figure 2 for dyspnoea. Compared with baseline, symptom severity as described by the patients at 12 months decreased. There were fewer patients suffering from sputum production in all COPD groups compared with baseline (p = 0.002, OR = 0.68 CI = 0.53–0.86). Chronic cough was also significantly reduced in all patient groups according to the subjective feeling of the patients (p<0.001, OR = 0.39, CI = 0.31–0.51). The incidence of dyspnoea, however, varied with GOLD stage (p= 0.244). For GOLD stage I, the percentage of patients presenting with Medical Research Council (MRC) grade 1 dyspnoea increased whilst the percentage of patients with MRC grade 2 dyspnoea decreased. The reverse was observed for GOLD stage IV, where there was an increase in the percentage of patients with MRC grade 4 dyspnoea and a decrease in the percentage of patients with MRC grade 2 dyspnoea.
Adherence to GOLD Guidelines
The pharmacotherapy prescribed by primary care physicians was compared to the GOLD treatment guidelines. This comparison focused primarily on the prescription of long-acting bronchodilators and inhaled corticosteroids (ICS). A greater discrepancy between GOLD guidelines and prescribing practice was observed for GOLD stage I and II disease. Only 56% of GOLD stage I patients and 36% of GOLD stage II patients received pharmacotherapy that conformed to the treatment guidelines (fig. 3). Guideline adherence increased for GOLD stages III and IV compared with baseline. Of the GOLD stage III patients, 69% patients and 82% of GOLD stage IV patients received treatment adherent to the GOLD guidelines, which represented an increase of 7–9% from baseline. Overall, there was no statistically significant change in guideline adherence compared to baseline (fig. 3).
Treatment
Whilst 24% of patients claimed to exercise regularly at baseline, this percentage dropped to 22% at 12 months. Similarly, 4% of patients were prescribed pulmonary rehabilitation at study outset compared with 2% after 12 months. The prescription pattern of rescue medication did not change significantly over time: approximately 25% of patients used short-acting β2-agonists. The administration of long-acting anticholinergics (LAAC) increased significantly in all GOLD stages (p= 0.002); an additional 13% in GOLD stage 1; 8% in stage II; 5% in stage III; and 7% in stage IV inhaled LAAC. ICS prescriptions did not change significantly (p= 0.394). In patients with GOLD stage I disease ICS prescriptions increased by 9%, but decreased slightly in patients of all other GOLD stages. Combination therapy (long-acting β2-agonists [LABA] and ICS) was administered to more than 50% of patients assigned to GOLD stages III and IV, and to more than 30% of GOLD stage I patients. There was no significant change in total use. At 12 months, three patients more than at the baseline (BL) visit received systemic steroids as basic therapy in stable disease. Basic pharmacological treatment is shown in table 3.
Exacerbations
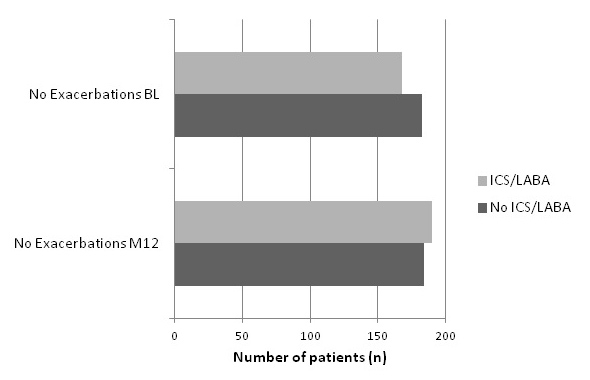
In the 3 months prior to the 12-month visit, 17% of patients had experienced an exacerbation requiring pharmacological treatment. Compared with baseline, the exacerbation rate decreased from 23% to 17% (p = 0.041, OR = 0.7 CI 0.50–0.98). Despite this reduction in exacerbation rate, a higher percentage of patients required treatment with antibiotics or systemic steroids at 12 months. The percentage of patients hospitalised for exacerbations varied between severity groups. Factors associated with exacerbations were treatment with ICS, combination therapy, LABA or systemic steroids in stable disease. Proving the correctness of this data, cough, sputum and dyspnoea were also significantly associated. Potential risk factors seem to be male sex, asthma and cerebrovascular insult as a co-morbidity, demonstrated in table 4. Exacerbations were significantly reduced in the group of patients treated with LAAC (p = 0.0011, OR = 0.37 CI: 0.21–0.67) and combination therapy (p = 0.018, OR = 0.39 CI: 0.39–0.71). These relationships are displayed in fig. 4a and b.
|
Table 2: Demographic data. |
| |
No-COPD
|
COPD
GOLD I
|
COPD
GOLD II
|
COPD
GOLD III
|
COPD
GOLD IV
|
Sum (%)
|
| Subjects, n (%), BL |
100 (22) |
43 (9) |
164 (36) |
119 (26) |
28 (6) |
454 (100) |
| Subjects, n (%), M12 |
111 (24) |
36 (8) |
151 (33) |
118 (26) |
38 (8) |
454 (100) |
| Male, BL |
65 (65) |
24 (56) |
102 (62) |
88 (74) |
21 (75) |
300 (66) |
| Female, BL |
35 (35) |
19 (44) |
62 (38) |
31 (26) |
7 (25) |
154 (34) |
| Age, years ± SD, BL |
66 ± 13.04 |
66 ± 10.63 |
69 ± 12.18 |
68 ± 9.85 |
67 ± 12.99 |
67 ± 11.81 |
| Height (cm) ± SD, BL |
169 ± 9.14 |
167 ± 8.45 |
167 ± 8.10 |
169 ± 7.57 |
170 ± 10.24 |
168.11 ± 8.42 |
| Current smokers, n (%), BL |
36 (36) |
24 (56) |
68 (42) |
48 (41) |
11 (39) |
187 (41) |
| Current smokers, n (%), M12 |
40 (36) |
10 (28) |
55 (37) |
41 (35) |
11 (30) |
157 (35) |
| Pack-years ± SD, BL |
37 ± 13.19 |
37 ± 10.85 |
40 ± 12.57 |
40 ± 10.14 |
39 ± 16.30 |
39.05 ± 12.07 |
| (% of COPD group), Abbreviations: COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; BL: baseline visit; M12: month 12; SD: standard deviation. |
|
Table 3: Basic pharmacological treatment. |
|
Basic Medication
|
No-COPD
n in % of group
|
COPD
GOLD I
n in % of group
|
COPD
GOLD II
n in % of group
|
COPD
GOLD III
n in % of group
|
COPD
GOLD IV
n in % of group
|
Sum
N in %
|
| LABA, BL |
16 |
19 |
18 |
24 |
25 |
19 |
| LABA, M12 |
14 |
19 |
15 |
23 |
21 |
17 |
| LAAC, BL |
24 |
12 |
29 |
51 |
54 |
33 |
| LAAC, M12 |
40 |
25 |
37 |
56 |
61 |
44 |
| ICS, BL |
12 |
2 |
12 |
21 |
25 |
14 |
| ICS, M12 |
9 |
11 |
10 |
16 |
21 |
12 |
| ICS+LABA, BL |
50 |
35 |
52 |
56 |
68 |
52 |
| ICS+LABA, M12 |
41 |
36 |
55 |
55 |
66 |
51 |
| Syst. steroid, BL |
6 |
2 |
2 |
10 |
4 |
5 |
| Syst. Steroid, M12 |
5 |
6 |
2 |
8 |
18 |
6 |
| (in % of COPD group or related to per protocol population)
Abbreviations: COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; LABA, long-acting β-agonist; LAAC, Long-acting anticholinergics, ICS, inhaled corticosteroids; M12, month 12, Syst. steroid: systemic steroids |
|
Table 4: Predicting factors for exacerbations. |
| |
OR (Risk)
|
95% CI
|
p-value
|
| Sex |
1.87 |
1.22–2.86 |
0.00397
|
| Smoking |
0.92 |
0.61–1.39 |
0.70292 |
| ICS |
1.82 |
1.14–2.91 |
0.01287
|
| ICS/LABA BL |
1.78 |
1.22–2.59 |
0.00284
|
| LAAC |
1.05 |
0.72–1.53 |
0.81384 |
| LABA |
2.16 |
1.41–3.32 |
0.00049
|
| Asthma |
2.09 |
1.24–3.51 |
0.00564
|
| CVI |
3.66 |
1.60–8.34 |
0.00223
|
| Guidelines |
1.00 |
0.66–1.52 |
0.99533 |
| Heart failure |
1.46 |
0.84–2.52 |
0.18141 |
| Hypertension |
1.28 |
0.81–2.02 |
0.28213 |
| Systemic steroids |
2.75 |
1.38–5.47 |
0.00414
|
| Abbreviations: OR: Odd’s Ratio; CI: Confidence Interval; LABA, long-acting β-agonist; LAAC, Long-acting anticholinergics, ICS, inhaled corticosteroids; CVI: cerebrovascular insult |
Discussion
This study has three main findings:
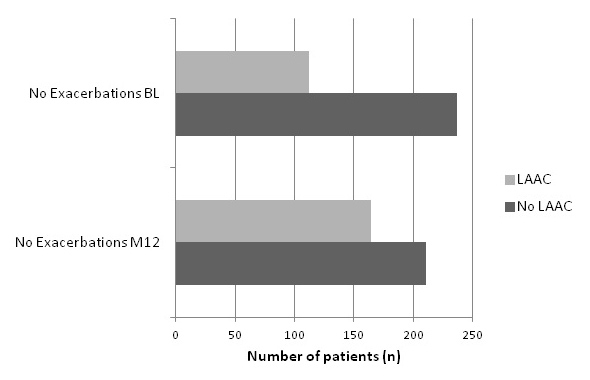
Figure 4
Influence of a.) combination therapy b.) LAAC on exacerbation rate.
a.) Proportion of patients inhaling combined therapy or not at BL visit in relation to their exacerbations compared to patients inhaling combined therapy at M12 in relation to their exacerbations: (Significant Increase of exacerbation free patients in the group inhaling combined therapy: p = 0.018, OR = 0.39 CI: 0.39–0.71).
b.) Proportion of patients inhaling LAAC or not at BL visit in relation to their exacerbations compared to patients inhaling LAAC at M12 in relation to their exacerbations: (significant Increase of exacerbation free patients in the group inhaling LAAC: p = 0.0011, OR = 0.37 CI: 0.21–0.67).
– Adherence to guidelines had no significant impact on lung function decline, symptom prevalence or exacerbation rate after one year of follow up
– Potential risk factors for exacerbations appear to be male sex, asthma and cerebrovascular insult as a co-morbidity
– Exacerbations were significantly reduced in the group of patients treated with combination therapy and LAAC
We sought to assess the extent that primary care physicians adhered to GOLD treatment guidelines over the first 12 months of follow up. Guidelines serve to standardise disease management with the aim of improving patient care [4]. The benefits of treatment for COPD are as follows: reduction in the number of exacerbations; delay in loss of lung function; improvement in quality of life. Treatment algorithms are tailored to GOLD stages with the aim of improving outcomes in patients according to the severity of their disease. Long-acting bronchodilators are recommended and have been shown to improve outcomes in GOLD stage II and more severe disease, with the addition of ICS in patients with stage III or IV disease who have repeated COPD exacerbations [1, 16].
Evaluating the prescription patterns of pharmacological and non-pharmacological treatments: at 12 months, the number of patients prescribed pulmonary rehabilitation had halved, whilst there was no significant change in patients performing physical exercise.
In contrast, there was an increase in the prescription of ICS in GOLD stage I patients and a slight decrease in the other stages. Despite GOLD recommendations, approximately one third of GOLD I and II patients received combination therapy (ICS + LABA). The prescription of LAAC in patients of all GOLD severity stages increased.
After one year of follow up, guideline adherence had not changed significantly:
An inappropriate treatment for their stage of disease was found in 44% of all COPD patients after one year of follow up compared to 47% at baseline (patients with asthma on inhaled steroids excluded). These observations are in line with a Swiss study from 2005 claiming over-use of inhaled steroids in mild COPD patients and a recent study from the United States reporting only 55% of patients with COPD were treated according to guideline recommendations [17, 18]. The potential risks of over-prescibing ICS in mild COPD and under-prescribing ICS, LAAC and LABA in severe COPD patients have been discussed previously [8].
Patients included in this study were seen at a three months interval by their GPs who performed spirometric testing at least every six months. There was no significant decline in FEV1 seen after one year of follow up. Patients treated adherent to GOLD guidelines had no advantage considering loss of FEV1 after one year. The greatest decrease of FEV1 was seen in COPD GOLD I patients who lost 100 ml FEV1 (1% of predicted FEV1) during the first year. COPD GOLD III patients lost 40 ml FEV1 in one year. GOLD II and IV patients had no decline in FEV1. Our findings are comparable to the UPLIFT trial which investigated the effect of tiotropium versus standard therapy on lung function decline in COPD patients and found a mean decrease of 42 ml per year in the placebo group and 40ml in the tiotropium group [19]. Decline in FEV1 is not as meaningful an endpoint as exacerbation rate since the follow up of only one year is rather short compared to the TORCH study with four years of follow up to see a significant difference in this relatively small number of patients.
Prevalence of sputum (p = 0.02) and cough (p <0.001) decreased significantly in the subjective estimation of all patients, dyspnoea varied between the different severity stages. Exacerbation rate showed a significant decrease over all COPD stages (p = 0.041).
After 12 months of follow up there was no significant difference in lung function decline, symptom frequency or exacerbation rate between the guideline adherent and not adherent patients. We did not measure quality of life per se. However assuming that this parameter is directly dependent on symptom frequency [20] there appeared to be no difference between patients treated according to guidelines versus patients not treated according to guidelines.
Compared with baseline, the overall exacerbation rate had fallen significantly. Factors associated with exacerbations were treatment with ICS, combination therapy, LABA or systemic steroids in stable disease.
This interrelation might be due to the fact that patients suffering from repeated exacerbations were treated with this medication due to recurrent infections. Several studies have shown a reduction in exacerbation rate and improvement of symptoms for treatment with ICS, LABA or combination therapy [19, 21, 22]. Despite not being the GOLD standard for treatment of mild to moderate COPD, an epidemiologic Canadian study found that 56% of mild COPD patients were also treated with inhaled steroids [23].
On the other hand it remains unclear whether some cases of pneumonia were mistaken for exacerbations. Inconsistent to the GOLD guidelines, 47% of GOLD I and 65% of GOLD II patients received inhaled steroids. The TORCH investigators reported an increased rate of pneumonia in patients treated with fluticason or combination therapy of salmeterol and fluticason [21]. Relating to pneumonia as an adverse event, in the TORCH study Halpin et al. criticised the lack of predefined diagnostic standards [24].
In order to control correctness of this factor analysis we also correlated cough, sputum and dyspnoea with exacerbations confirming a significant association for each characteristic. Male sex, asthma and cerebrovascular insult as a co-morbidity were significantly associated with a higher exacerbation rate and may be potential risk factors for developing exacerbations. This association will have to be proven in larger studies and a longer follow up term since one year is a rather short period in a chronic disease such as COPD to assess its course of exacerbations. A recent French study compared acute exacerbations in men and women and stated that significantly more women with exacerbations reported asthma as a co-morbidity [25]. Faganello et al. only found older age and low peripheral oxygen saturation to be risk factors for exacerbations when analysing patients staged by BODE index and GOLD recommendations, and thus concluded that other not detected variables seem to be predictors for exacerbations [26]. Patients suffering from severe co-morbidities, like a CVI, seem to be at a higher risk for developing an exacerbation. As no definite data on this point is available, further studies to identify clear risk factors for exacerbations are needed.
Exacerbations were significantly reduced in patients inhaling LAAC and combination therapy adjusted for age, gender and FEV1. The percentage of patients suffering from an exacerbation at BL visit in patients taking LAAC showed a remarkable reduction of 9% after one year of follow up. The effect of tiotropium (LAAC) on exacerbation rate was studied in the UPLIFT trial. A reduction of 14% in exacerbation rate could be seen after 4 years of tiotropium vs. placebo [19] being rather close to reduction of 9% after one year in patients treated with LAAC in our trial. A recent US study with 7376 COPD patients came to the same conclusion of tiotropium being superior to salmeterol in preventing exacerbations [16].
The TORCH trial compared salmeterol plus fluticason versus placebo, salmeterol alone and fluticason alone and found a significant reduction of exacerbations in comparison to the placebo group but also compared to the single components [21]. This corresponds to our findings of combination therapy (ICS + LABA) reducing exacerbation rate significantly more than LABA or ICS alone.
The current study does have the following limitations: a relatively small patient population was investigated, especially in GOLD stage I and IV patients; the short follow-up period; and the unknown quality of spirometric data provided by the primary care physicians. Whilst we are aware that a larger patient population and longer study duration would have yielded higher quality data, we believe that our trial has generated meaningful results. Nevertheless, it is unknown whether the 213 patients, who had to be excluded from the study since 29 GPs resigned their participation before the start, might have been treated less closely to the GOLD guidelines. It can only be speculated upon whether these GPs might have been less adherent and that their conformity to guidelines is even worse in reality. This also applies to the 161 drop outs whose guideline conformity in the course of the study cannot be assessed.
We could not verify the quality of spirometric data, nevertheless, we consider the spirometric measurements to be of a good standard, as the size of different COPD severity groups did not change significantly. Furthermore, a study conducted using Easy One™ spirometers in general practice in Switzerland found 60% of measurements to be of acceptable quality [27] whilst Miedinger et al. reported spirometry to be superior to questionnaires for the diagnosis of COPD in primary care [28]. The percentage of patients misdiagnosed in this study was 24%, which is comparable to other international studies [29].
Conclusion
Although guideline adherence did not affect COPD outcomes, the treatment prescribed, especially LAAC and combination therapy, improved patients’ estimation of symptoms and exacerbation rate. Overall, the data from this analysis suggest that adherence to GOLD guidelines does not have a perceivable impact on symptom prevalence, exacerbation rate or lung function decline after one year of follow up. Male sex, asthma and severe co-morbidities as a cerebrovascular insult could be associated with a risk for frequent exacerbations. The outcome of this analysis demonstrates an interesting trend, even if only over a 12-months period. Analysis of the 24-month data as well as future larger studies of a longer duration will provide further information about the effect of guideline adherence on the clinical course of COPD.
Acknowledgements: Participating cantons were: Aargau, Appenzell-Ausserrhoden Basel Stadt, Basel Land, Bern, Fribourg, Glarus, Graubünden, Jura, Luzern, Neuchatel, Nidwalden, Obwalden, St. Gallen, Schaffhausen, Schwyz, Solothurn, Thurgau, Uri, Wallis, Zug, Zurich. The authors would like to thank Natalie Dennis and Dr.Yamini Khirwadkar from PAREXEL for helping to edit the manuscript; this assistance was funded by Boehringer Ingelheim (Switzerland) GmbH.
References
1 GOLD. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. http://www.goldcopd.com. 2010:1–91.
2 Brandli O, Schindler C, Kunzli N, Keller R, Perruchoud AP. Lung function in healthy never smoking adults: reference values and lower limits of normal of a Swiss population. Thorax. 1996;51(3):277–83.
3 Miravitlles M. Guidelines versus clinical practice in the treatment of chronic obstructive pulmonary disease. Eur Respir J. 2002;20(1):243–4.
4 Bailey R, Weingarten S, Lewis M, Mohsenifar Z. Impact of clinical pathways and practice guidelines on the management of acute exacerbations of bronchial asthma. Chest. 1998;113(1):28–33.
5 Roche N, Lepage T, Bourcereau J, Terrioux P. Guidelines versus clinical practice in the treatment of chronic obstructive pulmonary disease. Eur Respir J. 2001;18(6):903–8.
6 Akinbami LJ, Schoendorf KC. Trends in childhood asthma: prevalence, health care utilization, and mortality. Pediatrics. 2002;110(2 Pt 1):315–22.
7 Pauwels RA. National and international guidelines for COPD: the need for evidence. Chest. 2000;117(2 Suppl):20S–2S.
8 Jochmann A, Neubauer F, Miedinger D, Schafroth S, Tamm M, Leuppi JD. General practitioner’s adherence to the COPD GOLD guidelines: baseline data of the Swiss COPD Cohort Study. Swiss Med Wkly. 2010 Apr 21.
9 Statistik Bf. N. Anzahl und Dichte der berufstätigen Ärzte und Zahnärzte. Available from: <http://www.bfs.admin.ch/bfs/portal/de/index/themen/14/03/03/key/01.html>2007.
10 Pauwels RA, Buist AS, Ma P, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summary. Respir Care. 2001;46(8):798–825.
11 Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–36.
12 Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–55.
13 Chen C-hH, Wolfgang; Unwin, Antony, eds. Handbook of data visualization. Springer Handbooks of Computational Statistics. Springer. 2008.
14 Ripley. WNVaBD. Modern Applied Statistics with S-PLUS (Third Edition) Springer, New York. 1999:(xi) + 501 pages.
15 Team RDC. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria 2008.
16 Vogelmeier C, Hederer B, Glaab T, Schmidt H, Rutten-van Molken MP, Beeh KM, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364(12):1093–103.
17 Mularski RA, Asch SM, Shrank WH, Kerr EA, Setodji CM, Adams JL, et al. The quality of obstructive lung disease care for adults in the United States as measured by adherence to recommended processes. Chest. 2006;130(6):1844–50.
18 Fritsch K, Jacot ML, Klarer A, Wick F, Bruggmann P, Krause M, et al. Adherence to the Swiss guidelines for management of COPD: experience of a Swiss teaching hospital. Swiss Med Wkly. 2005;135(7-8):116–21.
19 Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–54.
20 Blinderman CD, Homel P, Billings JA, Tennstedt S, Portenoy RK. Symptom distress and quality of life in patients with advanced chronic obstructive pulmonary disease. J Pain Symptom Manage. 2009;38(1):115–23.
21 Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–89.
22 Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320(7245):1297–303.
23 Bourbeau J, Sebaldt RJ, Day A, Bouchard J, Kaplan A, Hernandez P, et al. Practice patterns in the management of chronic obstructive pulmonary disease in primary practice: the CAGE study. Can Respir J. 2008;15(1):13–9.
24 Halpin DM, Gray J, Edwards SJ, Morais J, Singh D. Budesonide/formoterol vs. salmeterol/fluticasone in COPD: a systematic review and adjusted indirect comparison of pneumonia in randomised controlled trials. Int J Clin Pract. 2011;65(7):764–74.
25 Moncelly L, Maurer C, Roche N, Zureik M, Chavaillon JM, Quieffin J, et al. Acute COPD exacerbations in women: EABPCO-CPHG study by the College of general hospital pneumologists. Rev Pneumol Clin. 2010;66(2):107–19.
26 Faganello MM, Tanni SE, Sanchez FF, Pelegrino NR, Lucheta PA, Godoy I. BODE index and GOLD staging as predictors of 1-year exacerbation risk in chronic obstructive pulmonary disease. Am J Med Sci. 2010;339(1):10–4.
27 Leuppi JD, Miedinger D, Chhajed PN, Buess C, Schafroth S, Bucher HC, et al. Quality of Spirometry in Primary Care for Case Finding of Airway Obstruction in Smokers. Respiration. 2009 Sep 26.
28 Miedinger D, Linz A, Praehauser C, Chhajed PN, Buess C, Schafroth Torok S, et al. Patient-reported respiratory symptoms and pre-bronchodilator airflow limitation among smokers in Switzerland. Prim Care Respir J. 2010;19(2):163–9.
29 Chavez PC, Shokar NK. Diagnosis and management of chronic obstructive pulmonary disease (COPD) in a primary care clinic. COPD. 2009;6(6):446–51.
30 Wedzicha JA, Calverley PM, Seemungal TA, Hagan G, Ansari Z, Stockley RA. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177(1):19–26.