Continuous 24 hour intraocular pressure monitoring for glaucoma with a contact lens sensor – time for a paradigm change
DOI: https://doi.org/10.4414/smw.2012.13545
Kaweh
Mansouri, Robert N
Weinreb
Summary
Glaucoma is the main cause of irreversible blindness and intraocular pressure (IOP) is its only modifiable risk factor. The importance of robust lowering of IOP for prevention of glaucoma onset and progression is well established. Although IOP is a dynamic parameter with individual circadian rhythms, current management usually relies on single IOP measurements during regular clinic hours performed a few times a year. Recent technological advances have provided clinicians with tools for continuous IOP monitoring during a 24 hour period in an ambulatory setting. There are two approaches being investigated. The first is permanent IOP monitoring through an implantable sensor and the other is temporary monitoring through a contact lens sensor. In this article, we discuss the shortcomings of the current gold standard for tonometry (Goldmann Applanation Tonometry) and the current experience with the first commercially available continuous 24 hour IOP monitoring technology (SENSIMED Triggerfish®); a telemetric contact lens sensor produced by a Swiss start-up company (Sensimed AG, Lausanne, Switzerland). Recent studies suggest that 24 hour continuous monitoring of IOP can be integrated into clinical practice and have the potential to contribute to the reduction of glaucoma-related vision loss.
Background
Glaucoma is the leading cause of irreversible blindness worldwide with nearly 70 million people affected by it [1]. It is a chronic neurodegenerative disease characterised by progressive thinning of the retinal nerve fibre layer (RNFL) and of the neuroretinal rim within the optic nerve head (ONH) [2]. Glaucoma represents a spectrum of different disease processes that have a common endpoint, which is the destruction of retinal ganglion cell axons and resulting visual impairment. Primary open-angle glaucoma (POAG) is the most common form in populations of European descent [1]. It is frequently a slowly progressive disease and usually evolves without any symptoms in its early and moderate stages [2]. The insidious nature of the disease poses a serious challenge for its diagnosis and management. Despite its importance as a major public health problem, a survey of Swiss households showed that only 25% of the population could identify glaucoma as an eye disease [3].
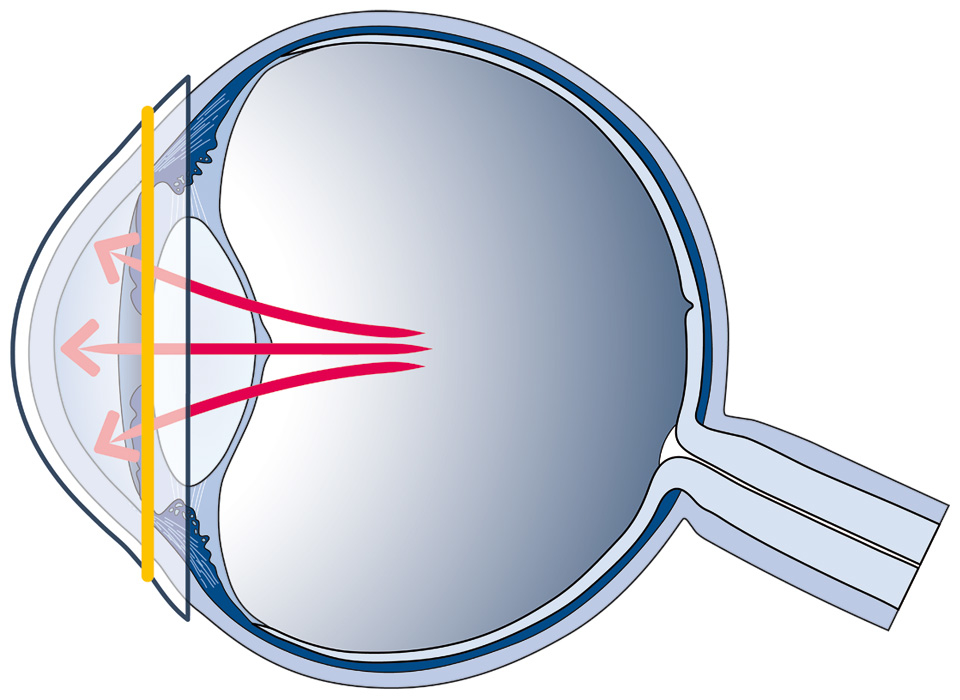
Figure 1
Principle behind the SENSIMED Triggerfish. Circumferential fluctuations in the area of the corneo-scleral junction (represented by a yellow line), directly correlated to fluctuations in intraocular pressure, are measured by a highly-sensitive strain gauge embedded in a soft contact lens.

Figure 2
Schematic set-up of the SENSIMED Triggerfish contact lens sensor. Data from the contact lens sensor are wirelessly transferred to a loop antenna based in a soft patch worn around the patient's eye. From here a cable is connected to a portable recorder, worn around the patient’s waist.
The understanding of the underlying disease mechanisms for glaucoma is incomplete. Historically, it was assumed that elevated intraocular pressure (IOP), defined as an IOP above 21 mm Hg, was the hallmark of glaucoma. A substantial number of patients who are diagnosed with glaucoma, however, have an IOP below this threshold, while many individuals with an IOP above 21 mm Hg never develop the disease, giving birth to potentially misleading terms such as “normal-tension glaucoma” and “ocular hypertension”, respectively [4]. While IOP is no longer considered to be a defining feature of glaucoma, it is nonetheless considered, together with age, to be one of two independent risk factors and the only modifiable one [2]. Large-scale, prospective, randomised studies have convincingly shown the efficacy of IOP-lowering therapy to reduce the risk of incident glaucoma and progressive visual field loss [5–7]. Results from the pivotal Early Manifest Glaucoma Trial suggest that even a 1 mm Hg increase in IOP is associated with an 11% increase in the hazard ratio for the progression of glaucoma [8]. Therefore, measuring IOP accurately is essential in the management of glaucoma.
Goldmann Applanation Tonometry (GAT), named after the eponymous Swiss ophthalmologist, is ubiquitously used for measuring IOP [9]. This technique was described over half a century ago and has remained unchanged ever since, placing it on a select and small list of diagnostic techniques within the medical field that have remained “gold standard” for such a long period of time. In GAT measurements, the cornea is flattened over a defined area (3.06 mm in diameter) and the required applanation pressure is used to estimate IOP based on the Imbert-Fick “law”, which assumes the eyeball to be a perfect sphere and the cornea a perfectly thin, elastic and flexible membrane. The Imbert-Fick “law” is not a physical law and none of its assumptions are true: the cornea is not an ideal thin membrane and exerts some resistance to the applanation. The surface tear film exerts additional resistance to the tonometer tip [10]. Furthermore, despite its pivotal role in glaucoma care, GAT is influenced by several ocular factors, such as central corneal thickness (CCT), corneal biomechanical properties and scleral rigidity [11, 12].
The most significant shortcoming of GAT, however, is the static nature of its measurements, which represent a 1–2 second snapshot of an individual’s IOP, taken in the upright position. Yet, IOP is a dynamic parameter with distinct circadian rhythms that vary between individuals. There is evidence that single IOP measurements during regular clinic hours fail to reflect the true range of an individual’s IOP. Studies that evaluate 24 hour IOP in glaucoma patients find that two-thirds of them exhibit their highest IOP values outside regular clinic hours, most frequently during the nocturnal/sleep period [13, 14]. It is noteworthy that in one such 24 hour IOP study, the availability of IOP data throughout the 24 hour period lead to an immediate change of therapy in 36% of patients [13]. Furthermore, IOP fluctuations of as much as 4–5 mm Hg in healthy individuals, and substantially higher in some glaucoma patients, are a common finding [14–16]. Despite the lack of a consensus, some studies have suggested that fluctuations of IOP, representing deviations from a physiological steady state, are an important contributor to the risk for glaucoma progression [17, 18].
Currently, the most common method for studying glaucoma patients’ 24 hour IOP rhythm is through a diurnal tension curve (DTC), which represents multiple IOP readings at different time points during clinic hours, or hospitalisation in a sleep laboratory. The former only provides daytime IOP values, while the latter is cumbersome, expensive and requires waking patients during the nocturnal/sleep period, potentially introducing stress-related artefacts [19]. Consequently, less than 1% of glaucoma patients undergo a DTC or sleep laboratory monitoring in European countries. Different approaches to self-tonometry by the patient have been proposed in recent years [20]. These techniques, however, seem to be inaccurate and technically challenging for many elderly glaucoma patients. Most importantly, they do not provide IOP measurements during the period when the patient is asleep, even though most individuals spend one third of their day asleep. Studies performed under the monitored and controlled environment of sleep laboratories have shown that the IOP of most untreated and treated glaucoma patients is at its highest during the nocturnal/sleep period with the patient in the supine body position [14, 21]. Due to the unavailability of IOP-measuring techniques that do not disturb the normal sleep cycle, our understanding of the significance of nocturnal IOP rhythms in relation to the pathophysiology of glaucoma remains insufficient [22].
In short, while patients with high blood pressure or diabetes have had access to continuous 24 hour blood pressure monitoring or self-monitoring of blood glucose for several decades, glaucoma patients, until now, have had to rely on outdated technology for infrequent monitoring of the only treatable risk factor for their disease.
Introduction of continuous 24 hour IOP monitoring into clinical practice
Despite efforts to develop a reliable and practical method for continuous 24 hour IOP monitoring which are now several decades old [23–25], only recently has a viable product become available. The SENSIMED Triggerfish® contact lens sensor (Sensimed, Lausanne, Switzerland) was approved by European regulatory authorities (Class IIa device CE-mark) in 2009 and has been introduced into the clinical management of glaucoma patients in select tertiary centres [26]. The device is based on a novel approach, in which changes in corneal curvature and circumference are assumed to correspond to changes in IOP [27]. The relationship between these changes and IOP changes has been validated in vitro by Leonardi et al. [27, 28]. The SENSIMED Triggerfish® is a contact lens sensor (CLS), whose key element consits of two platinum-titanium sensing-resistive strain gauges capable of recording circumferential changes in the area of the corneo-scleral junction (fig. 1). A microprocessor embedded in the contact lens sends an output signal proportional to changes of the strain gauges. Wireless power and data transfer are achieved using a patched, peri-orbital antenna and a cable that is connected to a portable recorder, containing the battery that powers the device. The portable unit is worn around the patient’s waist (fig. 2–3). Silicone was chosen for the contact lens material because of its excellent oxygen permeability and minimal water absorption (0.2% in weight), making it insensitive to the hydration level at the ocular surface. To render silicone hydrophilic and thus achieve proper fitting conditions of the lens on the eye, the contact lens surface is treated with oxygen plasma. The disposable contact lens exists in 3 different base curves with a thickness of about 585 μm at the centre and 260 μm at the border, a thickness already used in some commercial refractive silicone lenses.

Figure 3
The SENSIMED Triggerfish contact lens sensor.
The device can record IOP fluctuations in real-life situations for up to 24 hours and remains active during undisturbed sleep. Approximately 300 data points are acquired during a 30 second period, every 5 minutes, providing a total of 288 measurements over a 24 hour period. Recorded profiles are visualised graphically on a computer interface (fig. 4).
Safety and functionality
Being integrated in a contact lens, this device may be subject to side effects known to occur with standard contact lenses used for vision correction. Two prospective clinical studies investigated the safety and tolerability of the CLS in healthy subjects and glaucoma patients [26, 29]. In both series, the main adverse effects were innocuous superficial corneal staining and conjunctival hyperemia, occurring in about half of studied individuals. Three cases (out of a total of 25 tested eyes) of uncomplicated and reversible corneal erosion were also observed. The comfort score for the contact lens sensor was high and did not decrease significantly during the 24 hour period [29]. At the time of these studies, only one contact lens size was available. Of note, despite all of 15 patients in the initial cohort of glaucoma patients being non-contact lens wearers, all but one patient could finish the entire 24 hour monitoring period [26]. These studies attest to the safety and comfort of the device. Device malfunction occurred in 1 of the 15 patients and no other device-related adverse events were reported.

Figure 4
Example of the computer interface of a 24-hour continuous IOP monitoring session. Middle row shows the 288 datapoints, each corresponding to the median of 30 seconds of recordings that were repeated every 5 minutes. Close-up windows show the detailed output signal during a 30 seconds period. Towards the end of the nocturnal period (zoom A), the patient is still asleep and no eye blinks are seen. The signal represents the ocular pulse amplitude corresponding to IOP changes with the cardiac cycle. In zoom B, the patient is gradually awakening with occasional eye blinks.
Since the output signal of the CLS is dependent on changes occurring at the corneo-scleral junction, non-IOP-related changes in corneal shape and thickness may potentially affect the device output. Corneal swelling is a physiologic phenomenon that occurs when less oxygen reaches the cornea through the closed eyelids during sleep, leading to accumulation of lactate and fluid inside the cornea as a result of osmotic shift [30]. Normal 24 hour changes in biomechanical properties of the cornea, however, were not found to have a significant effect on 24 hour change in IOP in young healthy subjects but may play a role in older glaucoma patients [31, 32]. Normal corneal swelling may be emphasised by the contact lens acting as an additional barrier to oxygen delivery. Klink et al. have investigated the effect of overnight SENSIMED Triggerfish® CLS wear on corneal thickness and the possible influence of swelling on device output in 20 glaucoma patients (Personal communication, 2011). Although they found a statistically significant increase in CCT from baseline in the study eye but not the fellow eye, the difference between the two groups was not statistically significant. Their findings are consistent with our experience and we believe that there is probably minimal to no effect of overnight CCT changes on the device output. Further analysis of ongoing clinical trials will provide more conclusive evidence on this issue.
Clinical impact
The transition from single measurements of IOP during clinic hours to continuous 24 hour IOP monitoring represents a paradigm change in the diagnosis and management of glaucoma. Generations of ophthalmologists have had to rely on single GAT IOP measurements for formulating a treatment target and evaluation of the treatment response. The translation into practice of the wealth of data (288 IOP data-points instead of a single one) provided by the CLS poses a challenge for the clinician. This is compounded by the fact that the current generation of this technology does not display the output signal in mm Hg but in arbitrary units that are proportional to the electric signal generated by the contact lens-embedded strain gauge. Also, as the CLS only provides indirect measurements of IOP through changes in corneal curvature, more research is needed to validate it with other tonometry techniques and to assess the effect of other potential confounders such as corneal and scleral rigidity.
Mansouri and Shaarawy have shown how information obtained through 24 hour IOP monitoring can impact the management of glaucoma patients [26]. In their study, a series of 15 glaucoma patients with worsening disease despite IOPs that seemed to be controlled on GAT during clinic hours underwent 24 hour IOP monitoring with the CLS. They found that in 69% of patients the highest IOP values were recorded during the nocturnal/sleep period and that 80% had prolonged IOP peaks (with 75% occurring outside clinic hours). These findings lead to treatment changes in 73% of patients, ranging from changes in timing of anti-glaucomatous drops to addition of drops, laser and surgery. It is possible that earlier 24 hour IOP monitoring in these patients would have lead to a more timely detection of insufficient IOP control and therefore prevention of glaucoma progression.
Continuous 24 hour IOP monitoring – outlook for the future
Currently, the SENSIMED Triggerfish®CLS is the only commercially available device that has been shown to be able to provide 24 hour IOP data in a clinical setting. In future, we expect other manufacturers with different approaches (such as the implantable permanent IOP recorder produced by ImplanData GmbH, Germany) to enter the market and provide complementary and alternative approaches to glaucoma management. We strongly believe that integration of 24 hour continuous IOP monitoring into routine clinical practice will improve diagnosis and management of glaucoma in multiple ways:
– Early detection.The availability of information for the entire 24 hour period should improve detection of unfavourable IOP patterns and therefore, earlier identification of this risk factor for glaucoma onset and progression.
– Individualised therapy. Clinicians can now study how a particular anti-glaucomatous eye drop or intervention modifies the IOP rhythm throughout the day instead of relying on statistical averages of its effect in a given population. This should facilitate early selection of the appropriate treatment plans.
– Prevention of progression. The glaucoma community is recognising the need to move beyond simple IOP reduction to more qualitative IOP modulation in order to prevent the progression of glaucoma [33]. With the advent of telemetric devices for 24 hour continuous IOP monitoring, improved treatment algorithms based on the timing and frequency/amplitude of IOP fluctuations can be formulated in lieu of current strategies, some of which can resemble “trial and error”.
– Alternative interventions. Having studied in excess of two hundred 24 hour telemetry recordings, we have observed that certain patients present extreme IOP values as a reaction to physical or emotional stress. This subset of glaucoma patients may be amenable to behavioural changes as an adjunct to their routine glaucoma therapy.
– Improved adherence.Glaucoma is a complex disease and even long-time glaucoma patients do not have sufficient knowledge of their disease [34]. In chronic diseases, particularly insidious ones such as glaucoma, lack of understanding of the disease process reduces adherence to its treatment [35]. We believe that the ability to show uncontrolled IOP patterns to patients through the computer interface and the impact of anti-glaucomatous therapy on these may be an effective intervention to improve adherence to treatment.
In conclusion, continuous 24 hour IOP monitoring is now a reality for glaucoma patients. Before the vast amount of information obtained through 24 hour IOP monitoring can be translated into the wider clinical practice, a better understanding of the effect of IOP fluctuations and nocturnal changes of IOP on the risk of disease progression is necessary.
References
1 Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–51.
2 Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363(9422):1711–20.
3 Mansouri K, Orgul S, Meier-Gibbons F, Mermoud A. Awareness about glaucoma and related eye health attitudes in Switzerland: a survey of the general public. Ophthalmologica. 2006;220(2):101–8.
4 Sommer A. Ocular hypertension and normal-tension glaucoma: time for banishment and burial. Arch Ophthalmol. 2011;129(6):785–7.
5 The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998;126(4):498–505.
6 The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration.The AGIS Investigators. Am J Ophthalmol. 2000;130(4):429–40.
7 Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–13; discussion 829–30.
8 Bengtsson B, Leske MC, Hyman L, Heijl A. Fluctuation of intraocular pressure and glaucoma progression in the early manifest glaucoma trial. Ophthalmology. 2007;114(2):205–9.
9 Goldmann H. [Not Available]. Bull Mem Soc Fr Ophtalmol. 1954;67:474–7; discussion, 7–8.
10 Whitacre MM, Stein R. Sources of error with use of Goldmann-type tonometers. Surv Ophthalmol. 1993;38(1):1–30.
11 Ehlers N, Bramsen T, Sperling S. Applanation tonometry and central corneal thickness. Acta Ophthalmol (Copenh). 1975;53(1):34–43.
12 Liu J, Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysis. J Cataract Refract Surg. 2005;31(1):146–55.
13 Barkana Y, Anis S, Liebmann J, Tello C, Ritch R. Clinical utility of intraocular pressure monitoring outside of normal office hours in patients with glaucoma. Arch Ophthalmol. 2006;124(6):793–7.
14 Liu JH, Zhang X, Kripke DF, Weinreb RN. Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci. 2003;44(4):1586–90.
15 Liu JH, Kripke DF, Twa MD, Hoffman RE, Mansberger SL, Rex KM, et al. Twenty-four-hour pattern of intraocular pressure in the aging population. Invest Ophthalmol Vis Sci. 1999;40(12):2912–7.
16 Konstas AG, Mantziris DA, Stewart WC. Diurnal intraocular pressure in untreated exfoliation and primary open-angle glaucoma. Arch Ophthalmol. 1997;115(2):182–5.
17 Mansouri K, Orguel S, Mermoud A, Haefliger I, Flammer J, Ravinet E, et al. Quality of diurnal intraocular pressure control in primary open-angle patients treated with latanoprost compared with surgically treated glaucoma patients: a prospective trial. Br J Ophthalmol. 2008;92(3):332–6.
18 Mansouri K, Medeiros FA, Weinreb RN. Letter to the editor: 24-hour versus daytime intraocular pressure phasing in the management of patients with treated glaucoma. Br J Ophthalmol. 2011 Jan 26.
19 Liu JH, Weinreb RN. Monitoring intraocular pressure for 24 h. Br J Ophthalmol. 2011;95(5):599–600.
20 Liang SY, Lee GA, Shields D. Self-tonometry in glaucoma management – past, present and future. Surv Ophthalmol. 2009;54(4):450–62.
21 Liu JH, Medeiros FA, Slight JR, Weinreb RN. Diurnal and nocturnal effects of brimonidine monotherapy on intraocular pressure. Ophthalmology. 2010 Jul 20.
22 Weinreb RN, Liu JH. Nocturnal rhythms of intraocular pressure. Arch Ophthalmol. 2006;124(2):269–70.
23 Maurice DM. A recording tonometer. Br J Ophthalmol. 1958;42(6):321–35.
24 Schnell CR, Debon C, Percicot CL. Measurement of intraocular pressure by telemetry in conscious, unrestrained rabbits. Invest Ophthalmol Vis Sci. 1996;37(6):958–65.
25 Akaishi T, Ishida N, Shimazaki A, Hara H, Kuwayama Y. Continuous monitoring of circadian variations in intraocular pressure by telemetry system throughout a 12-week treatment with timolol maleate in rabbits. J Ocul Pharmacol Ther. 2005;21(6):436–44.
26 Mansouri K, Shaarawy T. Continuous intraocular pressure monitoring with a wireless ocular telemetry sensor: initial clinical experience in patients with open angle glaucoma. Br J Ophthalmol. 2011;95(5):627–9.
27 Leonardi M, Leuenberger P, Bertrand D, Bertsch A, Renaud P. First steps toward noninvasive intraocular pressure monitoring with a sensing contact lens. Invest Ophthalmol Vis Sci. 2004;45(9):3113–7.
28 Leonardi M, Pitchon EM, Bertsch A, Renaud P, Mermoud A. Wireless contact lens sensor for intraocular pressure monitoring: assessment on enucleated pig eyes. Acta Ophthalmol. 2009;87(4):433–7.
29 De Smedt S, Mermoud A, Schnyder C. 24-hour intraocular pressure fluctuation monitoring using an ocular telemetry sensor: tolerability and functionality in healthy subjects. J Glaucoma. 2011 May 19.
30 du Toit R, Vega JA, Fonn D, Simpson T. Diurnal variation of corneal sensitivity and thickness. Cornea. 2003;22(3):205–9.
31 Kida T, Liu JH, Weinreb RN. Effect of 24-hour corneal biomechanical changes on intraocular pressure measurement. Invest Ophthalmol Vis Sci. 2006;47(10):4422–6.
32 Kida T, Liu JH, Weinreb RN. Effects of aging on corneal biomechanical properties and their impact on 24-hour measurement of intraocular pressure. Am J Ophthalmol. 2008;146(4):567–72.
33 Caprioli J, Varma R. Intraocular pressure: modulation as treatment for glaucoma. Am J Ophthalmol. 2011;152(3):340-4 e2.
34 Mansouri K, Iliev ME, Rohrer K, Shaarawy T. Compliance and knowledge about glaucoma in patients at tertiary glaucoma units. Int Ophthalmol. 2011 Oct 7.
35 Mansouri K, Shaarawy T. Will improvement of knowledge lead to improvement of compliance with glaucoma medication? Acta Ophthalmol. 2009;87(4):468–9; author reply 9–71.



