Current status and future challenges of deep brain stimulation in Switzerland
DOI: https://doi.org/10.4414/smw.2012.13570
Markus
Christen, Sabine
Müller
Summary
QUESTIONS UNDER STUDY: Deep brain stimulation (DBS) has become a standard therapy for some forms of severe movement disorders and is investigated for other neurological and psychiatric disorders, although many scientific, clinical and ethical issues are still open. We analyse how the Swiss DBS community addresses these problematic issues and future challenges.
METHODS: We have performed a survey among Swiss DBS centres and a Delphi study with representatives of all centres and further stakeholders related to the topic.
RESULTS: The current DBS infrastructure in Switzerland consists of seven facilities. About 850–1,050 patients have received a DBS system in Switzerland for various indications since its advent in 1976. Critical issues like patient selection and dealing with side effects are in accordance with international standards. There are indications of a conservative referral practice in Switzerland for DBS interventions, but the data available do not allow verifying or refuting this point.
CONCLUSIONS: Issues to investigate further are whether or not there is an unmet medical need with respect to DBS, long-term medical and psychosocial sequelae of the intervention, conditions for enhancing the (research) collaboration of Swiss DBS centers, and the effect of the recent decision to reduce the number of DBS centres to 4 (resp. possibly 3) on the potential of this therapeutic approach.
Introduction
For several decades, chronic neurostimulation for therapeutic purposes was part of the options, neurosurgeons and neurologists could offer to their patients [1]. Among these options is deep brain stimulation (DBS), a technique that can be traced back to the early 1950’s [2] (see fig. 1). Swiss researchers have been actively involved in DBS development. If the fraction of DBS papers compared to all neuroscience publications per country is taken as a measure for research activity, Switzerland is among the 10 most productive countries in DBS research [3]. Already in the 1940’s the Swiss neurophysiologist Walter R. Hess, who performed stimulation research in cats, spoke encouragingly about using this technique in patients [4, chapter 2]. The neurosurgeon, Jean Siegfried started DBS in pain patients in 1976 (Jean Siegfried, personal communication, see methods section) and performed in 1982 his first DBS intervention for movement disorders in a dyskinesia patient [5, 6].

Figure 1
A schematic overview of the emergence of DBS summarising three historical reviews about the history of deep brain stimulation [1, 2, 4: chapter 2] demonstrates that almost all modern applications of DBS had precursors reaching back up to the 1950’s. The stereotactic frame introduced by Ernst Spiegel and Henry Wycis in 1947 set the technological precondition for precisely addressing targets deep in the brain. First, psychiatric applications have been discussed and implemented – but they also had the longest delay, probably due to the critique the experiments of José Delgado, Robert Heath and others have raised at that time. Pain (beginning in the late 1950’s, with a first culmination in the 1970’s; beside others: Mazars and Hosobuchi) and epilepsy (beginning in the late 1970; beside others: Cooper) experienced a first appreciation as diseases potentially suitable for DBS both before today’s re-emergence. DBS for addressing movement disorders had been experimentally approached already in the early 1960’s (beside others: Alberts and Albe-Fessard) and were, although on low profile, an ongoing issue within early DBS research before its modern re-emergence marked by the paper of Benabid et al. [38]. The chart also shows the dates of official approvals of Medtronic DBS-systems either by the European CE mark or by the Food and Drug Administration (FDA) of the United States (data: Regina Strasser, personal communication; HDE: Human Device Excemption) and the founding dates of Swiss DBS centres (compare with table 1).
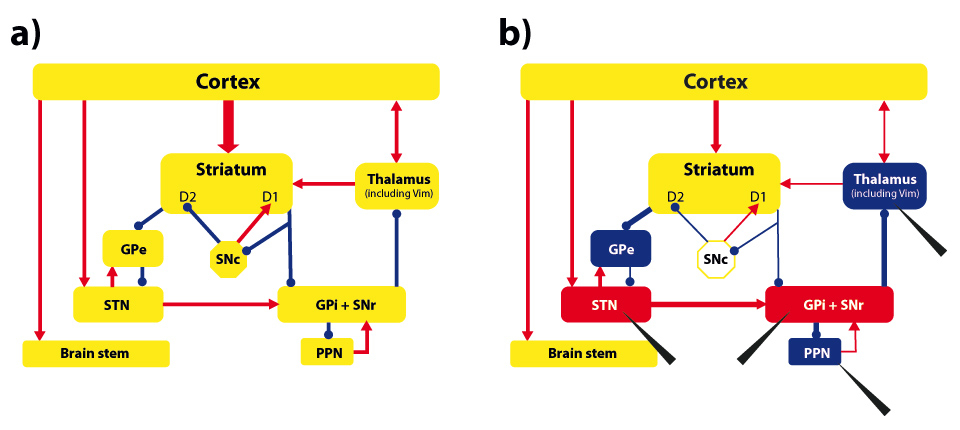
Figure 2
A simplified scheme of the basal-ganglia and thalamo-cortical network responsible for movement control (based on [39, 40]). Part a) shows the functional network, where red lines stand for excitatory and blue lines for inhibitory connections. Part b) shows the dysfunctional network resulting from a loss of dopaminergic neurons in the Substantia nigra pars compacta(SNc). Network nodes in red color indicate hyper-activation, nodes in blue color hypo-activation. D1/D2: sub-populations of neurons in the striatum; GPe: Globus pallidus externus;STN: Nucleus subthalamicus;GPi: Globus pallidus internus;SNr: Substantia nigra pars reticulate;PPN: Nucleus pedunculopontinus; Vim: ventral intermediate part of Thalamus (Vim). Black arrowheads indicate potential DBS targets in which quadripolar electrodes are stereotactically implanted. The electrodes are connected to a pulse generator (usually placed under the skin in the subclavicular or abdominal area) that controls several parameters of the chronically applied electrical stimulation (frequency, pulse width, and amplitude). The intervention is usually made in the alert patient, as his active cooperation is required while the electrodes are positioned for evaluating target accuracy and stimulation benefit.
Since 2000, both the application of DBS and its appreciation in the literature have grown remarkably [7]. Medtronic – the leading DBS supplier – estimates that in January 2011 about 85,000 patients worldwide have obtained a DBS intervention (Regina Strasser, personal communication; see methods section). Similar estimations account for 75,000 patients in spring 2010 [7] and 35,000 for late 2006 [8]. Because these numbers are based on sales statistics (some sold DBS devices are replacements), they provide an upper bound of 10’000 interventions per year. US-data indicate that 2,500 to 3,000 patients obtain annually a DBS system for Parkinson’s disease (PD) and essential tremor (ET) in 200 to 250 US-centres [9], other data indicate even more (approx. 4,000) interventions per year [10]. Extrapolating these numbers to the “top-10” countries in DBS research (which host approx. 430 centres; [3]) provides a lower bound of 5,000 to 7,500 interventions per year globally. This conforms to other estimations of the annual accrual of new patients per year worldwide (8,000–10,000; [11]). These numbers are likely to increase, since DBS is assessed for various novel neurological and psychiatric indications, and because stimulation in established indications at an earlier time may be more beneficial for the patient [12], which decreases rejection probability due to exclusion criteria. Up to date, DBS has been approved in PD, ET, dystonia, epilepsy, and obsessive-compulsive disorder. Current DBS research includes refractory depression, Tourette syndrome, hypertension, Alzheimer’s disease, minimally conscious state, obesity, memory impairment, severe (auto-) aggressiveness, and drug addiction [13].
Economic data confirm the increasing appreciation of deep brain stimulation. Currently, DBS involves about 1/6 of the neuromodulation devices market with an estimated global volume of 3 billion USD in 2010 [4, chapter 1]. Although this market only represents 1% of the worldwide sales of the global medical devices industry (300 billion USD in 2011, [14]), it is forecasted to grow at a Compound Annual Growth Rate of 26.3 percent over the period of 2010–2014 [15]. Based on Medtronic’s sales, the annual growth rate for DBS alone is 22% [16], which supports this estimation. The DBS market leader Medtronic Inc. (Minneapolis, USA) provides all necessary equipment for DBS interventions (electrodes, wires, stimulators), although in the broader field of stereotactic and functional surgical procedures several other companies are active [17]. St. Jude Medical has brought an approved DBS system (for PD) to the market and at least one additional company (Boston Scientific) will enter the DBS market in the next future. As Medtronic started in 1997 the Swiss Manufacturing Operations in Tolochenaz in the canton of Vaud, where all Medtronic DBS systems are produced, Switzerland is currently the most important global supplier for the DBS community.
Deep brain stimulation relied on experiences made in ablative surgery [4]. In movement disorders, such interventions were the only option available for addressing severe symptoms before Levodopa and other medication were available. Movement disorders are still the most common indications for DBS, in which subthalamic structures like the Nucleus subthalamicus (STN), the Globus pallidus internus (GPi), the ventral intermediate part of Thalamus (Vim), or the Nucleus pedunculopontinus (PPN) are targeted (see fig. 2). As DBS involves surgical intervention into the brain, it usually becomes an option for a patient, when medication is not any more sufficient to control the symptoms or has unbearable side effects like fluctuations in movement quality. Compared to ablative surgery, DBS has the advantage of being adjustable and reversible.
Whereas the beneficial effects of DBS on motor functions are well established [18], its cognitive, affective and behavioural sequelae are increasingly discussed [7, 19, 20]. The evaluation of these sequelae is nontrivial, as they may result from three causes: surgery, stimulation, or drug reduction. Furthermore, in the case of PD, one has to take into account that similar effects may result both from disease progression as well as from medication. The spectrum of cognitive, affective and behavioral side effects of DBS is broad, but they are often transient or can be managed through the adaptation of the stimulation parameters or by psychotropic drugs. Some effects seem paradoxical: Affective and social problems, especially in relationships and work, may occur in spite of a good clinical outcome. Sometimes the changes are evaluated positively by the patients, but negatively by their social surrounding, in particular if these changes involve novelty seeking, risk willingness, or sexual drive. Finally, DBS initialises a long-lasting relationship between the patient and medical specialists of several disciplines. These factors make the ethical evaluation of DBS interventions challenging.
With respect to DBS in Switzerland, an additional point is important: The decision-making body of the inter-cantonal agreement on highly specialised medicine has recently decided (21 June 2011, [21]) to concentrate DBS in four centres (Bern, Lausanne, St. Gallen, and Zurich; the latter two might have to merge their activities in future according to a concentration concept to be elaborated). These centres have to include all disciplines necessary for DBS interventions, have to meet a minimal case load of 20 per year, and have to establish a network with other clinics for patient selection and long-term care. Furthermore, it demands building up a registry that includes all aspects of process and results quality and to document and coordinate research activities and teaching. The decision is limited in time until 2014 and will then be re-evaluated by the HSM project office.
Given this increasing appreciation of DBS as therapeutic strategy for various indications, the medical and ethical complexity of the intervention, and the recent decision to manage the development of the DBS infrastructure in Switzerland, we address in this contribution the following questions:
1) How many DBS centres are currently active in Switzerland, how many patients do they operate on per year and for which indications, and how many patients have been treated in Switzerland so far? Are these numbers in accordance with the actual need, or is the referral practice too conservative or too liberal?
2) How do the Swiss DBS centers deal with critical issues like patient selection, sequelae, ensuring the quality of the intervention, and alternatives to DBS?
Methods
For question one, we used a standardised questionnaire that provides information on the numbers of interventions, offered and planned indications, brain targets, disciplines involved in assessment, and the start of the activity of the centre. For question two, we utilised an interview scheme that included the issues patient selection, dealing with side effects, the quality of the intervention, and alternatives to DBS. We interviewed active members of the DBS centres that represent a core discipline involved in DBS (neurology, neurosurgery, or both).
From mid-2010 to mid-2011, the following representatives of all Swiss DBS centres were interviewed by the first author face-to-face for approximately 60 to 120 minutes with exception of the representative of Geneva, whose centre just recently started its activity and where the interview was performed by phone (in brackets: discipline, affiliation, and date of interview): Ronald Bauer (neurosurgery, Cantonal Hospital St. Gallen; 8 July 2011), Christian Baumann (neurology, University Hospital Zurich; 27 April 2011), Stefan Hägele-Link (neurology, Cantonal Hospital St. Gallen; 8 July 2011), Thomas Mindermann (neurosurgery, Klinik im Park Zurich; 3 May 2011), Pierre Pollak (neurology, University Hospital Geneva; 14 July 2011), Claudio Pollo (neurosurgery, University Hospital Lausanne; 4 August 2011), Alexander Stibal (neurosurgery, Inselspital Bern; 5 May 2011), Oguzkan Sürücü (neurosurgery, University Hospital Zurich; 27 April 2011) and Ethan Taub (neurosurgery, University Hospital Basel; 26 August 2010). The interviews were performed using a Delphi methodology, i.e., the interview partners were invited to assess and comment the results that emerged out of the interrogation [22]. In this phase, we have included comments from Michael Schüpbach (neurology, Inselspital Bern). Based on the interviews, the questionnaire was completed by us and sent to all centres for review and correction so that the state of affairs by end of 2011 could be determined. All centres with the exception of the Klinik im Park have provided data.
To complement this research, the first author also interviewed the following persons: Hans-Peter Ludin (neurology, former chairman of the research board of the Swiss Parkinson Association; 5 July 2010), Adrian Merlo (neurosurgery, president Swiss Society for Neurosurgery; 26 August 2010), Jean Siegfried (neurosurgery, former physician at the University Hospital and the Klinik im Park Zurich; 23 August 2010), and Regina Strasser (Product Manager Medtronic Switzerland; 12 July 2010). We also contacted the project office with respect to planning highly specialised medicine in Switzerland (HSM) on 24 September 2011. Our goal was to obtain the report “Neurochirurgie in der Schweiz” dated from 3 May (to which the decision [21] on concentrating DBS in Switzerland referred) for having a basis of comparison of our own data. This report has not been disclosed to us due to confidentiality reasons. We then have posed some formal questions that have been answered on 27 October 2011 by the HSM project secretary. Those were partly incorporated in this contribution and have been crosschecked by the project secretary on 23 November 2011.
Results
Quantitative survey
First, we present the data obtained by the questionnaire (table 1): Switzerland has 6 DBS facilities based in public hospitals (5 of them in university hospitals) and one in a private hospital. A private clinic in Kreuzlingen (Eastern part of Switzerland) also used to perform DBS interventions, but is no longer active in this field (Christoph Hamburger, personal communication, 18 April 2011). Four centres in public hospitals started their activity not before 2007, reflecting the global growth of the number of DBS interventions in the last few years. Switzerland has one centre per 1.12 m inhabitants, compared to one center per 1.70 m inhabitants in Germany (48 facilities currently offer DBS in Germany; [3]). The implementation of the concentration decision will raise the inhabitant-per-center-ratio to 1.97 m inhabitants per centre (4 centres) resp. 2.63 inhabitants per centre (3 centres).
|
Table 1: An overview of Swiss DBS centers which are currently operative. The column “start of current activity” refers to the year in which the first patient has been operated at the center. The data reflect the situation by end of 2011. |
|
Location
|
Start of current activity
|
Current indications
|
Planned indications
|
# Patients / year
|
Potential # patients / year
|
Total # of patients operated so far
|
| Basel, University Hospital |
2007 |
PD, ET, dystonia, MS tremor |
|
~12 |
~24 |
31 |
| Bern, University Hospital |
1996 |
PD, ET and other forms of tremor (MS, rubral), chronic pain, dystonia, OCD, automutilation, Tourette |
Addiction, epilepsy, headache, refractory depression |
~20 |
40–60 |
~250 |
| Geneva, University Hospital |
2011 |
PD, ET, dystonia, epilepsy, MS tremor, Tourette |
Addiction, anorexia, obesity, OCD, refractory depression |
~15 |
~25 |
7* |
| Lausanne, University Hospital |
~1995 |
PD, ET, chronic pain, dystonia, epilepsy |
Refractory depression |
10–15 |
~50 |
~200 |
| St. Gallen, Cantonal Hospital |
2007 |
PD, ET, chronic pain |
|
3–5 |
~20 |
18 |
| Zurich, University Hospital |
2009 |
PD, ET, dystonia, epilepsy, OCD |
Chronic pain, refractory depression, Tourette |
~15 |
20–25 |
31 |
| 1976–1989 |
|
|
|
|
119 [24] |
| Zurich, Klinik im Park |
1989 |
PD, ET, chronic pain |
|
N/A |
N/A |
200–400 |
| * In Geneva, DBS surgery started in April 2011.
PD: Parkinson’s disease; ET: essential tremor; OCD: obsessive-compulsive disorders. |
The current mean number of annual interventions is approximately 80 patients per year. This number is lower, but in the same order as the data of the HSM project office (approximately 100 cases per year based on a survey made in 2010). The numbers of annual interventions per centre lie between 3–5 (St. Gallen) and ~20 (Bern). Only one centre (Bern) matches the minimal case load of 20 interventions per year; that is requested by the concentration decision [21] and that is considered as minimum for a training institution in DBS [23]. The current infrastructure would allow operations on up to 200 patients per year. Whether the infrastructure is also sufficient for long-term clinical follow-up (e.g., with respect to experts in neurology) is, according to the experts, less clear, as additional qualified personnel in various fields may be required. The cumulative number of patients that obtained DBS in Switzerland is in the order of 650, including 119 patients that were operated on at the University Hospital Zurich from 1976 to 1989 [24]. As no data from the Klinik im Park were available, we took the implantations by Siegfried performed in the pioneer phase (13 years) as a lower bound and estimate (linear scaling) that at least 200 patients from 1989 to 2011 were operated on in this facility. Approximately 400 patients have been supervised by the “Klinik im Park” since the early 1990s (Thomas Mindermann, personal communication); this number can be taken as an upper bound. Therefore, the total number of patients who have obtained DBS in Switzerland since its advent in the late 1970s (i.e., including DBS for pain) is about 850 to 1,050 patients.
In order to judge whether these numbers reflect the current need or indicate either a conservative or a liberal referral practice, the following information is required: data about prevalence and incidence of the diseases which can be treated by DBS, and the ratio of patients per disease for which DBS is the best option. We investigated this point only for PD, the major indication for DBS. Epidemiologic studies on PD show large variations in incidence and prevalence estimations across countries [25], but reliable data for Switzerland is missing (also according to the experts interrogated). It can be estimated that the number of patients that develop PD in Switzerland per year is between 1,000 to 2,000, which is validated by German sources that estimate that 10,000 to 20,000 people develop PD per year [26, 27], whereas the German population is 10 times larger than the Swiss population. Reliable data on the number of PD patients who qualify for DBS are also missing. A survey of Italian DBS centres referring to the selection procedure almost 10 years ago (2002–2003) estimates that 1.6% to 4.5% of PD patients are eligible to STN-DBS [28]. But it has been criticised that this study may considerably underestimate the ratio of PD patients who qualify for DBS [29]. In particular, the ratio would be higher, if patients would be referred earlier to DBS [12], which seems to be a trend in various centres [30]. The experts consulted by us estimate the ratio of PD patients suitable for DBS by 10–20%. Based on latter estimation and the prevalence estimations, 100 to 400 patients per year for PD alone may qualify for DBS in Switzerland. Since the actual number of 80–100 DBS patients per year includes other conditions (e.g., dystonia, ET, pain), the assignment practice in Switzerland seems to be conservative, which is also the consensus opinion of the DBS experts interviewed.
The Swiss DBS centres offer interventions for all indications that are currently approved for DBS (fig. 1, lower part), although not every centre offers all of them. Treatment of PD and ET is most common and available in all centres. There is some disagreement regarding the optimal target between the experts: whereas several centres (e.g., Lausanne) prefer the STN in most cases, other centres more often target also the GPi (in Bern in approximately 40% of the cases) because of the larger probability of side-effects in STN-DBS [31]. DBS interventions in pain, epilepsy, and psychiatric indications (OCD, Tourette) are only offered in selected centres, some are currently assessing to offer DBS for refractory depression and other novel indications.
Qualitative interviews
As indicated in the introduction, there are various clinical and ethical issues associated with DBS. Our interviews confirmed that the Swiss DBS centres are aware of them: All centres have established interdisciplinary teams for assessing potential patients. These teams always include neurologists, neurosurgeons, psychiatrists, and neuropsychologists. Some centres also routinely include social workers, care and rehabilitation specialists, physiotherapists, occupational therapists and speech therapists. Family members or other reference persons are always part of the selection process. This process includes several (usually 2) stages, although the practice of selection differs, e.g. the procedure may require up to 7 days in hospital (St. Gallen). Patients suitable for DBS may wait up to 4 months for surgery. In the majority of cases, the stimulation electrodes are placed bilaterally, and the surgery (including placement of the stimulator) is performed in one single session – although variability in the detailed procedure due to individual patient’s constitution is high.
The issue of unwanted side effects is well acknowledged and a central issue in the selection process. This includes both surgery-related adverse events and the impact on cognition, mood and behaviour. Several options for minimising the risk of side effects are used, e.g., selecting the GPi as the target, especially in older patients, or intensifying post-surgery supervision. Some experts observed in rare cases “personality changes” of DBS patients that gave rise to conflicting interpretations of the result (e.g., between the patient and family members). In Bern, potential candidates are explicitly directed to this issue and patients are informed that in case of conflicting interpretations, the doctor will have the right to change stimulation settings. The experts also confirmed to some degree the phenomenon of a “satisfaction gap” [32] that may be present in particular in PD patients. There is some disagreement between the experts regarding the amount of the benefit of DBS for individual patients. Some suspect that the dissatisfaction rate may be higher than reported in the literature, although no quantitative evidence has been provided to support this argument.
There is no consensus among the experts which minimal number of cases operated per year within a centre is necessary to ensure the quality of DBS interventions. Some emphasised that a minimal number is necessary to keep the quality of treatment at a high level. Others referred to the training and experience of the experts involved as being the more decisive parameter and considered the fixation of a minimal case load as arbitrary to some degree. It has also been argued that too many cases may interfere with building up a close relation to the patient, which is a necessary condition for successful treatment. With respect to training, there is a consensus that DBS centres should include professionals with long-term experience. For being a qualified surgeon, a minimum of 200 DBS surgeries has been mentioned, which is in accordance with the current training chart in movement disorders surgery [23]. The qualification of the experts involved is a decisive element in order to setup a DBS centre; e.g., for the avoidance of hardware complications, for which the experience of the surgical team is the main determinant [33], or with respect to optimal DBS programming by the neurologist [34]. Obtaining and maintaining qualified personnel is not an easy task and so far has had a significant impact on centre activity. In Bern, where the surgeon left the institution temporarily in 2003, patients were unwilling to move to other centres, as long as the possibility to obtain surgery locally remained. This is supported by the (mostly) localised patient catchment areas of the Swiss DBS centres that have been reported to us.
Finally, regarding the issue of alternatives to DBS, some disagreement between the experts is detected as well, in particular regarding lesion procedures. Although no expert suggested abolishing ablations completely, they disagreed to what extent they might be a suitable option for some patients. The majority, however, was skeptical with respect to the risks associated with lesion procedures (e.g., no reversibility).
Discussion
Our study revealed several issues that require further attention: First, we have obtained expert opinion that the referral practice in Switzerland for DBS interventions may be conservative, i.e., some patients do not get the optimal treatment. However, this point needs further backing by more solid data. In particular, one should investigate whether this finding results from a justified skepticism regarding possible adverse effects of DBS, or whether it reflects lack of knowledge or prejudice in the referring stakeholders and/or patients. Research on this issue should complement the current international discussion with respect to the optimal time window and criteria for referring patients to a DBS centre.
Second, the Swiss DBS centers are aware of the many controversial issues of DBS and consequentially they have developed a standard for patient management that partly exceeds international benchmarks (e.g., by including social workers in the selection process or by addressing issues of conflicting interpretation of therapy outcome). However, there is still a need to assess the long-term benefit of the intervention including evaluations by third parties (e.g., close relatives) and cost-benefit comparisons. Given the cultural diversity of the country, Switzerland may provide a suitable model to assess these issues. Therefore, we propose advancing the collaboration between the DBS centres in Switzerland for allowing for long-term follow-up studies with higher case numbers involving psychosocial aspects. When introducing a DBS case registry, it should allow for comparison with nonsurgical patients to evaluate clinical results as well as cost efficacy, since systematic assessment and presentation of treatment results is a vital prerequisite to reinforce the integration of surgical and nonsurgical caregivers in a unified approach.
Third, DBS may be a promising tool for the therapy of various diseases. However, alternatives should still be valued as well. For example lesion procedures – performed either by microsurgery or by radiosurgery (e.g., Gamma Knife) – could remain an option for some special patient groups, e.g., for patients who would be non-compliant with the long-term follow-up after DBS, for patients who could neither tolerate the stress of a wake-operation nor an operation under full anesthesia, and for patients who could not accept any devices in their body (e.g., patients with obsessive compulsion disorders, especially compulsive skin-picking, see [35, 36]). This opinion is supported by a recent expert consensus regarding DBS [37].
Finally, the reduction of the number of DBS centres in Switzerland has been justified by ensuring the quality of medical care. However, one has to take into account that the reduction may also have effects that counteract this goal: First, DBS interventions establish a long-term commitment between a patient and an interdisciplinary team of experts, requiring the build-up of close relationships and regular contacts. This speaks in favour of more localised centres. Second, the specifics of the intervention (e.g., with respect to the brain target, selection procedure, or professionals involved in the interdisciplinary teams) lead to different “cultures” in centres that reflect current discussions in the global DBS community (e.g., whether STN still should be the “standard” target or not). Reducing the number of centers affects this “cultural diversity” and may lead to premature standardisations, limiting the choice of patients. Third, the current state of research does not allow for making reliable prognoses for the future development of the number of patients suitable for DBS. If these numbers increase – which is likely given the various trends described in the introduction, the annual case-load per centre could reach 50 or more patients, requiring considerable investment in resources in the remaining centres. Whether this will be realised is an open question given the cost pressure many hospitals experience. Therefore, we suggest also carefully investigating potential negative effects of concentrating DBS when the HSM decision will be re-evaluated in 2014.
The last point gains importance with respect to research for novel DBS applications as well as for technological improvements, for which Switzerland is particularly suited given its past achievements and the economic importance of the DBS industry. With respect to research, a growing case load in the remaining centres may have an ambivalent effect. On the one hand, clinical research will be facilitated as the number of potential subjects a researcher has directly access to increases. On the other hand, a growing case load for established DBS applications may hinder clinicians investigating novel applications due to restricted time resources. We clearly agree with the principal aim of the HSM decision that quality should be crucial for the decision whether a DBS centre should stay operative or not. Nevertheless, a transparent quality competition between the centres may be better suited to fulfill this aim and is also more compatible with the research process for establishing future application of DBS for patients with psychiatric and other neurological diseases.
Acknowledgement:We thank Merlin Bittlinger for his support in obtaining data on the German DBS infrastructure.
References
1 Sironi VA. Origin and evolution of deep brain stimulation. Front Integr Neurosci. 2011;5:42.
2 Hariz MI, Blomstedt P, Zrinzo L. Deep brain stimulation between 1947 and 1987: The untold story. Neurosurg Focus. 2010;29(2):E1.
3 Christen M, Bittlinger M, Ineichen C, Müller S (in preparation): Assessing the international research, practice, and infrastructure in deep brain stimulation.
4 Krames EF, Peckham PH, Rezai AR (eds.). Neuromodulation. London: Academic Press; 2009.
5 Siegfried J. Effect of stimulation of the sensory nucleus of the thalamus on dyskinesia and spasticity. Revue Neurologie (Paris). 1986;142(4):380–3.
6 Siegfried J, Shulman J. Deep brain stimulation. Pacing Clin Electrophysiol. 1987;10(1 Pt 2):271–2.
7 Müller S, Christen M. Deep brain stimulation in Parkinsonian patients – ethical evaluation of cognitive, affective, and behavioral sequelae. AJOB Neurosci. 2011;2(1):3–13.
8 Hardesty DE, Sackeim HA. Deep brain stimulation in movement and psychiatric disorders. Biol Psychiatry. 2007;61:831–5.
9 Pilitsis JG, Burrows A, Peters ML, Sargent S, Ng SC, Tseng JF. Changing practice patterns of deep brain stimulation in Parkinson’s disease and essential tremor in the USA. Stereotact Funct Neurosurg. 2012;90:25–9.
10 Lad SP, Kalanithi PS, Patil CG, Itthimathin P, Batya S, Bronte-Stewart H, Boakye M, Henderson JM. Socioeconomic trends in deep brain stimulation (DBS) surgery. Neuromodulation 2010;13:182–6.
11 Ponce FA, Lozano AM. Deep brain stimulation state of the art and novel stimulation targets. Prog Brain Res. 2010;184:311–24.
12 Schüpbach WM, Maltête D, Houeto JL, du Montcel ST, Mallet L, Welter ML, et al. Neurosurgery at an earlier stage of Parkinson disease: a randomized, controlled trial. Neurology. 2007;68(4):267–71.
13 Awan NR, Lozano A, Hamani C. Deep brain stimulation: current and future perspectives. Neurosurg Focus. 2009;27(1):E2.
14 Zacks Equity Research. Medical devices industry outlook – April 2011. March 31, 2011: http://www.zacks.com/stock/news/50398/Medical+Devices+Industry+Outlook+%96+April+2011 (accessed on September 19th 2011).
15 Infiniti Research Limited. Global Neuromodulation Market 2010-2014. June 13, 2011: http://www.technavio.com/content/global-neuromodulation-market-2010-2014 (accessed on September 19th 2011).
16 Cavuoto J. Neurotech Report. Neuromodulation. 2011;14:206–7.
17 Hemm S, Wårdell K. Stereotactic implantation of deep brain stimulation electrodes: a review of technical systems, methods and emerging tools. Med Biol Eng Comput. 2010;48(7):611–24.
18 Wider C, Pollo C, Bloch J, Burkhard PR, Vingerhoets FJ. Long-term outcome of 50 consecutive Parkinson’s disease patients treated with subthalamic deep brain stimulation. Parkinsonism Relat Disord. 2008;14(2):114–9.
19 Maes H, Vingerhoets F, Berney A. Prévalence des troubles de l’humeur dans la maladie de Parkinson traitée par stimulation cérébrale profonde. Rev Med Suisse. 2011;7(282):385–8.
20 Christen M, Bittlinger M, Walter H, Brugger P, Müller S. Dealing with side effects of deep brain stimulation: Lessons learned from stimulating the STN. AJOB Neurosci. 2012;3(1):37–43.
21 Entscheid zur Planung der hochspezialisierten Medizin (HSM) im Bereich der stereotaktischen Chirurgie der anormalen/ungewollen Bewegungen und tiefe Hirnstimulation (Deep Brain Stimulation) beim Erwachsenen. Bundesblatt vom 21. Juni 2011. Available through: http://www.gdk-cds.ch/fileadmin/docs/public/gdk/Themen/HSM/HSM_Spitalliste/BB_DC_NCH_DBS_20110520_def_d.pdf.
22 Linstone HA, Turoff M (eds.). The Delphi Method: Techniques and Applications. New Jersey: Science and Technology University; 2002.
23 Krauss JK, Broggi B, Reulen HJ, Trojanowski T, Lazorthes Y. Training chart in movement disorders surgery added competence. Acta Neurochirurgica. 2009;151:1505–9.
24 Siegfried J. Les pacemakers neurologiques. Bilan de trois décennies. Neurochirurgie. 1991;37:81–5.
25 Wirdefeldt K, Adami H-O, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol. 2011;26:S1–S58.
26 Grey sources for estimating the PD incidence in Germany. MedKolleg: http://www.med-kolleg.de/magazin/parkinson-0806081.html; Parkinson-Web: http://www.parkinson-web.de/content/was_ist_parkinson/die_parkinson_krankheit/haeufigkeit/index_ger.html; Medworld: http://www.medworld.de/therapiegebiete/morbus-parkinson/grundlagen/haeufigkeit.htm (websites accessed on November 8th 2011)
27 Thümler R. Die Parkinson-Krankheit. Mehr wissen, besser verstehen. Stuttgart: Thieme; 2006.
28 Morgante L, Morgante F, Moro E, Epifanio A, Girlanda P, Ragonese P, et al. How many Parkinsonian patients are suitable candidates for deep brain stimulation of subthalamic nucleus? Results of a questionnaire. Parkinsonism Relat Disord. 2007;13:528–31.
29 Cacciola F. Letter to the Editor. Parkinsonism Relat Disord. 2008;14:264–5.
30 Okun MS, Foote KD. Parkinson’s disease DBS: what, when, who and why? The time has come to tailor DBS targets. Expert Rev Neurother. 2010;10(12):1847–57.
31 Hariz MI, Rehncrona S, Quinn NP, Speelman JD, Wensing C, and the Multicentre Advanced Parkinson’s Disease Deep Brain Stimulation Group. Multicenter study on deep brain stimulation in Parkinson’s disease: An independent assessment of reported adverse events at 4 years. Mov Disord. 2008;23(3):416–21.
32 Agid Y, Schüpbach M, Gargiulo M, Mallet L, Houeto JL, Behar C, et al. Neurosurgery in Parkinson’s disease: the doctor is happy, the patient less so? J Neural Transm Suppl. 2006;70:409–14.
33 Blomstedt P, Hariz MI. Hardware-related complications of deep brain stimulation: a ten year experience. Acta Neurochir. 2005;147:1061–4.
34 Moro E, Poon Y-YW, Lozano AM, Saint-Cyr JA, Lang AE. Subthalamic nucleus stimulation. Improvements in outcome with reprogramming. Arch Neurol. 2006;63:E1–E7.
35 Kondziolka D, Flickinger JC, Hudak R. Results following Gamma Knife radiosurgical anterior capsulotomies for obsessive compulsive disorder. Neurosurgery. 2011;68:28–33.
36 Gabriëls L, Cosyns P, Nuttin B, Demeulemeester H, Gybels J. Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: psychopathological and neuropsychological outcome in three cases. Acta Psychiatr Scand. 2003;107:275–82.
37 Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, et al. Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol. 2011;68(2):165–71.
38 Benabid AL, Pollak P, Louveau A, Henry S, de Rougemont J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987;50:344–6.
39 Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1998;12:366–75.
40 DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–5.

