Figure 1
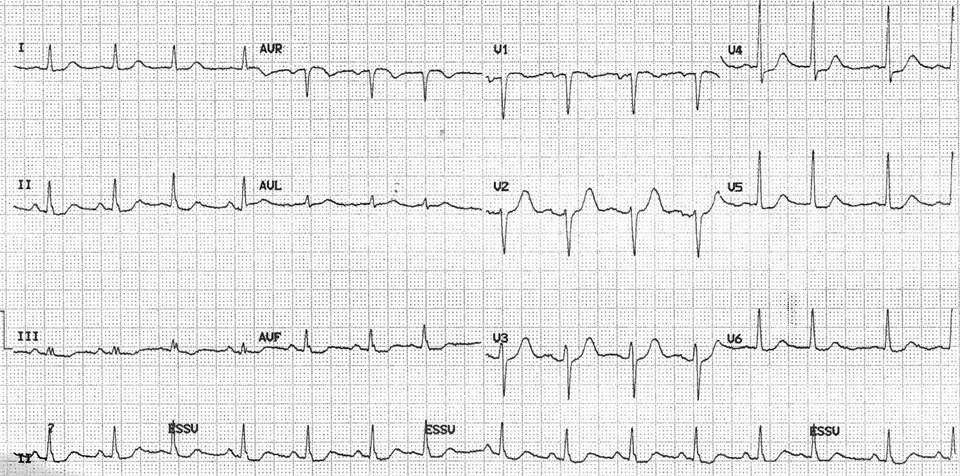
A 12 lead electrocardiogram showing sinus rythm with a heart rate of 86 beats per minute, a borderline 0.1 mV horizontal ST depression in V4-V5 and minors ST abnormalities in V6, II, III and aVF.
DOI: https://doi.org/10.4414/smw.2020.13543
Churg-Strauss syndrome (CSS) is a rare multisystemic disorder of unknown origin. The disease is defined by the American Rheumatological Society (ARA) as eosinophilic vasculitis in addition to one or more of the following: asthmatic airway obstruction, more than 10% eosinophils on the differential leucocyte blood count, migratory or transient pulmonary infiltrates, sinusitis and mononeuropathy or polyneuropathy [1]; or by Lanham criteria as the concomitant presence of asthma, peak peripheral blood eosinophilia of >1500 cell/µL and systemic vasculitis involving two or more extrapulmonary organs [2].

Figure 1
A 12 lead electrocardiogram showing sinus rythm with a heart rate of 86 beats per minute, a borderline 0.1 mV horizontal ST depression in V4-V5 and minors ST abnormalities in V6, II, III and aVF.


Figure 2
CMR and DE-CMR images.Fig. 2A: Short axis view of left ventricular myocardium without contrast showing an aspecific hypocaptation in the infero-basal portion. Fig. 2B: Long-axis DE-CMR images showing no late enhancement.
The annual incidence of CSS ranges from 0.5 to 6.8 per million with a higher prevalence in certain areas of France and Norway and a mean age at diagnosis of 48 years, affecting both sexes equally [3]. There are no laboratory tests specific for CSS, but eosinophilia is the most characteristic finding and the anti-neutrophil cytoplasmatic antibodies (ANCA) are positive in 40–60% of patients [4, 5].
Cardiac involvement is one of the most serious manifestations, accounting for approximately one-half of deaths attributable to CSS [6, 7]. Cardiac manifestation can be acute and mimic acute coronary syndrome (ACS). In this setting the differential leucocyte count may suggest an alternative diagnosis and the presence of hypereosinophilia may lead to a diagnosis of CSS. We take advantage of this rare presentation of CSS to discuss the related cardiac involvement, the differential diagnosis and current literature.
A 76-year-old woman was admitted for atypical chest pain and fatigue of 10 days’ duration. The clinical findings were irrelevant except for epigastric tenderness. The electrocardiogram (ECG) showed a borderline 0.1 mV horizontal ST depression in V4-V5 and minor ST abnormalities in V6, II, III and aVF leads (fig. 1). The chest radiography was normal. Blood analysis disclosed pathological levels of troponin-I (6.1ug/L; normal <0.30), total creatine phosphokinase (424 U/L; normal <167), creatine phosphokinase MB isoenzyme (84.1 µg/L, normal <3.4) and lactate dehydrogenase (243 U/L, normal <232). The leukocyte count was elevated (16.7 G/L, normal 4.0–10.0). Transthoracic echocardiography (TTE) showed a hyperkinetic left ventricle with a thickened interventricular septum and minimal pericardial effusion. Non-ST segment elevation myocardial infarction was diagnosed on the basis of the association of chest pain and a further increase of troponin-I (peak value 13.7 µg/L). The emergency angiogram showed normal coronary arteries. In the meantime, haematological study revealed persistent leucocytosis (peak value 19.9 G/L) with 67% eosinophilia, corresponding to an eosinophilic count of 13.2 G/L, elevation of eosinophil cationic protein (ECP; >200 µg/L, normal <16.0), IgE (685 kU/L, normal <100) as well as serum tryptase (12.2 µg/L, normal 1.0–11.4). Cardiac magnetic resonance (CMR) was performed on a Siemens Avanto 1.5T (Siemens Medical, Erlangen, Germany). The cine and delayed enhancement images (DE-CMR) revealed only aspecific hypocaptation in the infero-basal portion of left ventricular myocardium, without late enhancement (fig. 2). As further workup we performed a myocardial biopsy demonstrating an important sub-endocardial eosinophilic infiltrate with fibrin deposition and focal extension into the myocardium (fig. 3 A, B). Numerous intra- and perivascular eosinophils were also present in the interstitium. An extended aetiological workup was performed. The patient had a history of recurrent sinusitis and allergy to pollen and asthma. A solid neoplastic process was ruled out by thoraco-abdominal CT scan. Screening for parasites in the serum and stools was negative, as were serological tests for antibodies to viruses and biopsy for a myocardial infection. Haematological screening showed normal beta2-microglobulin and vitamin B12, no FIP1L1-PDGFRA mutation and normal immunoelectrophoresis. The immunological workup for vasculitis (ANCA, antimyeloperoxydase and anti-proteinase 3) was negative. On the basis of asthma, recurrent sinusitis, hypereosinophilia and the results of the heart biopsy, Churg-Strauss syndrome was diagnosed (table 1). Pulse endovenous corticosteroid treatment (500 mg i.v. prednisolone) was started just after the cardiac biopsy. At day 1 levels of blood eosinophils dropped to 0.4 G/L and the ECP level returned to normal values. The hospital course was uneventful and 3 months later the patient was still asymptomatic with a normal eosinophil count on oral prednisone.
| Table 1 | ||
| Hypereosinophilic syndromes (1) diagnostic criteria | Churg-Strauss Syndrome ARA Classification Criteria (2–3) | |
| Blood eosinophilia > 1500/mm³ on at least 2 occasions OR evidence of prominent tissue eosinophilia associated with symptoms and marked blood eosinophilia | Biopsy including artery, arteriole or venule with extravascular eosinophils | |
| Eosinophils > 10% on with blood cell differential count | ||
| Exclusion of secondary causes of eosinophilia: parasitic or viral infections, allergic diseases, drug-induced or chemical-induced eosinophilia, hypoadrenalism, and neoplasia | Asthmatic airway obstruction | |
| History of acute or chronic paranasal sinus abnormality (nasal polyps, allergic rhinitis, recurrent or chronic sinusitis) | ||
| Migratory or or transitory infiltrates on radiographs | ||
| Mononeuropathy (included multiplex) or polyneuropathy | ||
| Cardiac damage secondary to hypereosinophilia in HES or CSS | ||
| Endocardium | Endomyocarditis, mural thrombi formation along the damaged endocardium of both ventricles or atrium, endomyocardial fibrosis (obliteration at apex of both ventricles): more frequent in HES. | |
| Myocardium | Acute myocarditis, arrhythmias. | |
| Pericardium | Eosinophils effusions, acute pericarditis, costrictive pericarditis, pericardial fibrosis, tamponade: more frequent in CSS. | |
| Valves | Valve regurgitation (entrapement of chordae tendineae, dilated cardiomyopathy, thickening of the posterior leaflet of mitral valve) | |
| Cardiac function | Acute or chronic heart failure (secondary to restrictive or dilated cardiomiopathy | |
| Coronary arteries | Acute coronary syndromes and acute miocardial infarction (due to coronary vasospasm, intracoronary thrombus, coronary aneurysms or dissection): more frequent in HES. | |
| Pattern of cardiac involvement in CSS | ||
| ANCA-negative Shorter interval of CSS-specific symptoms duration until diagnosis Higher FFS (4) Higher peripheral blood eosinophils count than other CSS patients | ||
| (1): Hans-Uwe Simon et al. Refining the definition of hypereosinophilic syndrome. J. Allergy Clin Immunol. Vol.126,1:45-49. (2): Masi AT, Hunder GG, Lie JT, et al. The American College of Rheumatology 1990 criteria fort the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis) Arthritis Rheum 1990; 33:1094. (3): The presence of four or more of these criteria yields a sensitivity of 85% and a specificity of 99.7% for CSS. (4): Five factor score: 1.Proteinuria > 1g/day; 2.Renal insufficiency (serum creatinine > 141 umol/l); 3. Gastrointestinal tract involvement; Cardiomyopathy; 5. Central nervous system involvement. | ||
Cardiac involvement is one of the more serious manifestations of CSS [6, 7] and hypereosinophilic syndromes (HES) [8, 9], accounting for approximately one-half of deaths attributable to CSS (table 1). The eosinophil-mediated heart damage evolves through three stages: acute necrotic, intermediate thrombotic and fibrotic stage. The first corresponds to our case and is usually clinically silent.

Figure 3A
Intramyocardic vessels showing luminal eosinophils which were also found in the surroundig interstitium and between the myocardial fibers (H&E stain, 100x).
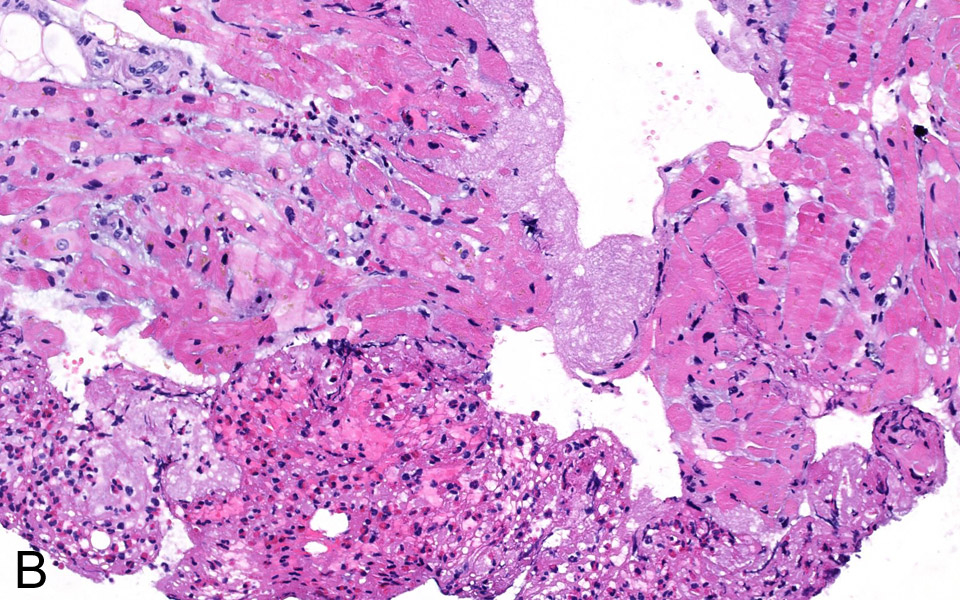
Figure 3B
Fibrinous thrombotic material mixed with numerous eosinophils was attached To the endocardium (H&E stain, 200x).
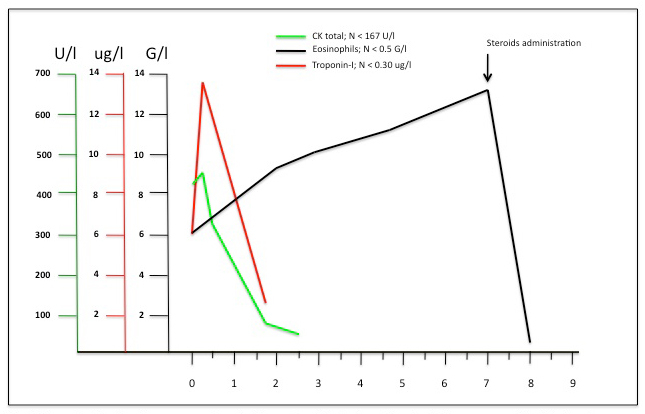
Figure 4
Representation of cardiac enzymes and eosinophils count evolution between the arrive in the emergency unit and the day after corticosteroid administration.
The cardiovascular imaging workup in the presence of hypereosinophilia and suspected heart involvement includes the ECG, which may show non-specific conductance disturbances or, very rarely, patterns typical of acute coronary syndromes due to coronary vasospasm [10, 11] or intracoronary thrombi [12]; TTE, which may be normal or show contractility impairment, ventricular wall thickness, and presence of intracardiac thrombi or pericardial effusion; CMR and DE-CMR, which may confirm and characterise myocardial necrosis [13], inflammation [14] or fibrosis [15, 16]. Nevertheless, if the myocardial involvement occurs in the early phase, as in this case, the CMR may be non-contributive and only endomyocardial biopsy is diagnostic and usually mandates the indication for immunosuppressive treatment.
Organ involvement in the presence of hypereosinophilia imposes a careful laboratory workup. This requires the exclusion of secondary causes of eosinophilia, including drug reaction and systemic symptoms (DRESS), parasite infestations and haematological malignancies. Among primary causes it is often difficult to distinguish between CSS and non-vasculitic conditions. In our patient the serum IgE were moderately elevated and are often very elevated in the HES lymphocytic variant, characterised by abnormal IL-5-producing T cells and consequent secondary polyclonal blood hypereosinophilia [17, 18]; serum tryptase was borderline and is most consistently elevated in myeloproliferative variants of HES and variable in the other subtypes; FIP1L1/PDGFRalpha genetic mutation of HES myeloproliferative forms (absent in our case) is associated with an increased incidence of cardiac involvement and potentially lethal complications in the absence of therapy [19].
We also determined the level of ECP, a toxic protein derived from eosinophil degranulation which appears to be a valuable indicator for diagnosis and an objective parameter in monitoring disease activity [20].
There are data indicating different manifestations of ANCA-positive and ANCA-negative CSS [21], the latter associated with heart disease [5]. Guillevin et al. identified five factors in CSS associated with poor prognosis (“five factor score”) with myocardial involvement being one of the factors [22]. The classical markers creatinine kinase, creatinine kinase MB subtype and troponin can be sensitive indicators of early and ongoing eosinophil-associated myocardial damage in CSS [23, 24]. Some authors evoked a possible role of troponin T as non-invasive marker of cardiac damage in hypereosinophilia, reporting its augmentation during the early stage and normalisation after steroid administration and eosinophil count reduction in three cases [25].
In our case cardiac marker kinetics is typical of acute myocardial infarct but contrasts with the progressive linear rise of eosinophils until corticosteroid treatment (fig. 4). Is this the biological expression of a vasospasm or intracoronary thrombus with spontaneous resolution? Or is the manifestation of endomyocardial cytotoxic effect of eosinophil degranulation with a non-linear expression? Our case seems to confirm the importance of troponin elevation as an early marker for acute myocardial infarction and question its role in CSS and HES evolution. Further studies to clarify cardiac marker kinetics in this setting are needed.
In summary this case shows early cardiac involvement of CSS mimicking an acute coronary syndrome, and underlines the importance of checking the leucocyte count in this setting and the possible value of myocardial biopsy in the diagnostic workup.
1 Masi AT, Hunder GG, Lie JT, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis) Arthritis Rheum. 1990;33:1094.
2 Lanham JG, Elkon KB, Pusey CD, Hughes GR. Systemic vasculitis with asthma and eosinophilia: A clinical approach to the Churg-Strauss syndrome. Medicine. 1984;63:65.
3 Noth I, Strek ME, LeV AR. Churg-Strauss syndrome. Lancet. 2003;361:587–94.
4 Della Rossa A, Baldini C, Tavoni A, et al. Churg-Strauss syndrome: clinical and serological features of 19 patients from a single Italian centre. Rheumatology. (Oxford) 2002;41:1286.
5 Sable-Fourtassou R, Cohen P, Mahr A, et al. Antineutrophil cytoplasmic antibodies and the Churg-Strauss syndrome. Ann Intern Med. 2005;143:632.
6 Neumann T, Manger B, Schmid M, et al. Cardiac involvement in Churg-Strauss syndrome: impact of endomyocarditis. Medicine. (Baltimore) 2009;88:236.
7 Corradi D, Maestri R, Facchetti F. Post-partum Churg-Strauss syndrome with severe cardiac involvement: description of a case and review of the literature. Clin Rheumatol. 2009;28:739.
8 Ogbogu PU, Rosing DR, Horne MK, 3rd. Cardiovascular manifestations of hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007;27:457.
9 Weller PF, Bubley GJ. The idiopathic hypereosinophilic syndrome. Blood. 1994;83:2759.
10 Hirakawa Y, Koyanagi S, Matsumoto T, Takeshita A, Nakamura M. A case of variant angina associated with eosinophilia. Am J Med. 1989;87:472–4.
11 Hertzman PA, Maddoux GL, Stenberg EM, Heyes MP, Mefford IN, Kephart GM, et al. Repeated coronary artery spasm in a young woman with the eosinophilia-myalgia syndrome. JAMA. 1992;267:1932–34.
12 Naohiko Takahashi, Katsuhiro Kondo, Juntaro Aoyagi. Acute myocardial infarction associated with hypereosinophilic syndrome in a young man. Jpn Circ J. 1997;61:803–6.
13 Mavrogeni S, Manoussakis MN, Karagiorgia TC, et al. Detection of coronary artery lesions and myocardial necrosis by magnetic resonance in systemic necrotizing vasculitides. Arthritis Rheum. 2009;61:1121.
14 De Cobelli F, Pieroni M, Esposito A, Chimenti C, et al. Delayed gadolinium-enhanced cardiac magnetic resonance inpatients with chronic myocarditis presenting with heart failure or recurrent arrhythmias. J Am Coll Cardiol. 2006;47(8):1649–54.
15 Jen Li Looi, Peter Ruygrok, Gordon Royle, et al. Acute eosinophilic endomyocarditis: early diagnosis and localization of the lesion by cardiac magnetic resonance imaging. Int J Cardiovasc Imaging. 2010;26:151–4.
16 Alter P, Maisch B. Endomyocardial fibrosis in Churg-Strauss syndrome assessed by cardiac magnetic resonance imaging. Int J Cardiol. 2006;108:112–3.
17 Hans-Uwe Simon, et al. Refining the definition of hypereosinophilic syndrome. J. Allergy Clin Immunol. Vol.126,1:45–9.
18 Vassina EM, Yousefi S, Simon D, Zwicky C, Conus S, Simon HU. cIAP2 and surviving contribute to cytokine-mediated delayed eosinophil apoptosis. Eur J Immunol. 2006;36:1975–84.
19 Klion AD, Noel P, Akin C, et al. Elevated serum tryptase levels identify a subset of patients with a myeloproliferative variant of idiopathic hypereosinophilic syndrome associated with tissue fibrosis, poor prognosis and imatinib responsiveness. Blood. 2003;101:4660.
20 Desreumaux P, Janin A, Dubucquoi S, et al. Synthesis of interleukin-5 by activated eosinophils in patients with eosinophilic heart diseases. Blood. 1993;82:1553–60.
21 Pagnoux C, Guillevin L. Churg-Strauss syndrome: evidence for disease subtypes? Curr Opin Rheumatol. 2010;22:21.
22 Guillevin L, Lhote F, Gayraud M, Cohen P, Jarrousse B, Lortholary O, et al. Prognostic factors in polyarteritis nodosa and Churg-Strauss syndrome. A prospective study in 342 patients. Medicine (Baltimore). 1996;75:17–28.
23 Pitini V, Arrigo C, Azzarello D, La Gattuta G, Amata C, Righi M, et al. Serum concentration of cardiac Troponin T in patients with hypereosinophilic syndrome treated with imatinib is predictive of adverse outcomes. Blood. 2003;102:3456.
24 Zaky J, Caraang C, Yu R, El-Bialy A. Elevated troponins and the Churg-Strauss syndrome: a case report. J Cardiovasc Pharmacol Ther. 2005;10:131.
25 Sato Y, Taniguchi R, Yamada T, et al. Measurements of serum concentrations of cardiac troponin T in patients with hypereosinophilic syndrome: a sensitive non-invasive marker of cardiac disorder. Intern Med. 2000;39(4):350.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.