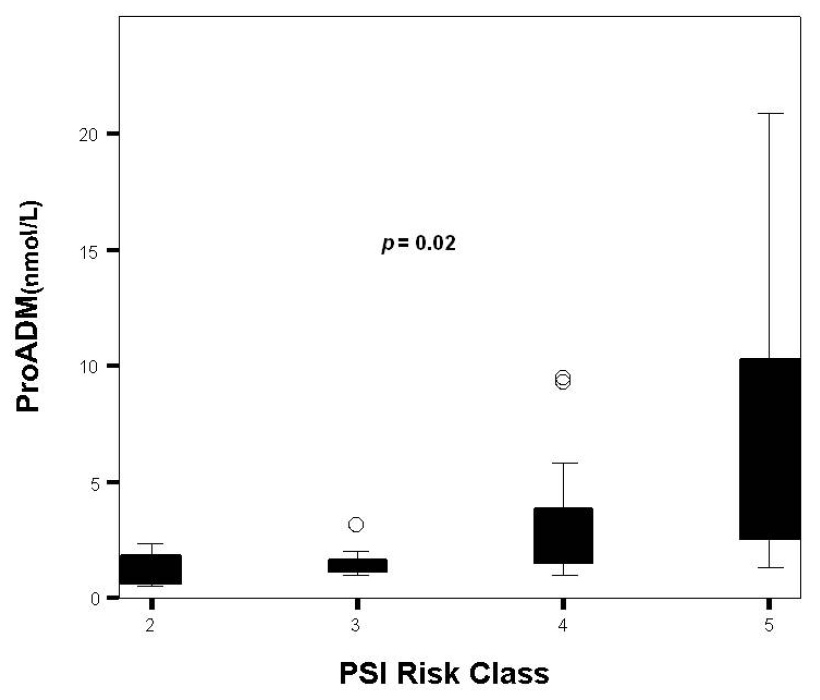
Figure 1
Distribution of proADM levels by PSI class.
DOI: https://doi.org/10.4414/smw.2012.13542
Community-acquired pneumonia (CAP) is the leading cause of death from infectious disease in western countries and involves major consumption of healthcare resources [1]. CAP mortality is reported to be between 5.7% and 14%, and is a common ground for intensive care unit admission [2]. Prompt antibiotic administration and ICU transfer if required are essential to improve survival in these patients [3, 4]. Hence early and accurate diagnosis and risk assessment are vital for optimal care of critically ill patients. Novel laboratory methods, such as real-time multiplex PCR or MALDI TOF MS, have been developed to improve the diagnosis aspect but early identification of high risk patients continues to be a challenge [5]. Likewise, several scores have been proposed to define severe CAP and identify patients at high risk. Nevertheless, these scores may underestimate severity in young people and do not perform so well when outcomes such as ICU admission or need for mechanical ventilation are taken into account [6]. Given these areas of uncertainty in clinical decision-making, a concerted effort has been undertaken to develop reliable and practical biomarkers for diagnosis, risk prediction and management of CAP.
Adrenomedullin is a peptide produced by multiple tissue types during physiological stress, and has pluripotent function including vasodilatory, antimicrobial, and antiinfammatory activity [7]. Quantification of ADM may be helpful in diagnosing and monitoring sepsis as well as establishing prognosis [8]. Unfortunately, ADM is rapidly cleared from the circulation and hence reliable measurement is almost impossible. Recently the more stable mid-regional fragment of proadrenomedullin, which directly reflects levels of the active peptide ADM, was identified in plasma of patients with septic shock [9]. ProADM may be as good as validated severity scores at detecting critically ill patients with CAP, and is probably better than other biomarkers such as procalcitonin [10, 11].
The aims of this study are to determine whether by using proADM we shall be able to detect patients with CAP and a high risk of mortality, and to compare this prognostic value with other biomarkers and scores used commonly in clinical practice.
This study was performed with the approval of the hospital ethics committee and written informed consent was obtained from patients or their relatives to allow blood sampling.
We conducted a single-centre prospective observational study between January and September 2009. The study was performed in a 30-bed adult intensive care department at Marques de Valdecilla University Hospital in Spain. This department consists of two general medical and surgical units and a neurotrauma unit. Eligible patients were all consecutive adult patients, aged 17 or over and admitted to the ICU with both a clinical and radiological diagnosis of pneumonia as defined by Fine et al. [12] and fulfilling criteria for severe sepsis or septic shock according to the 2001 International Sepsis Definitions Conference. We excluded all subjects who had been discharged from a hospital within the 10 days prior to diagnosis of CAP and those with hospital-acquired pneumonia defined as development of pneumonia 48 hours or more following hospital admission.

Figure 1
Distribution of proADM levels by PSI class.
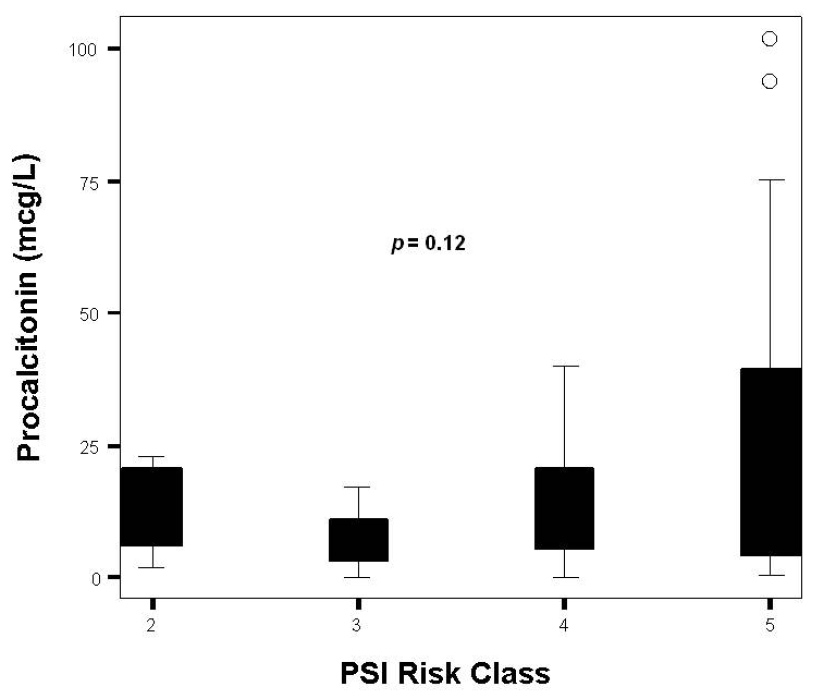
Figure 2
Distribution of PCT levels by PSI class.
Clinical and demographic characteristics of all patients, including age, gender, comorbidities, immunosuppression (AIDS, neutropenia [neutrophil count <1 × 109/L], exposure to glucocorticoids [>0.5 mg/kg for >30 d] and/or immunosuppressive or cytotoxic medications, solid organ transplantation, allogeneic or autologous stem cell transplantation, haematological malignancy, or solid tumour), Acute Physiology and Chronic Health Evaluation II score at 24 hrs [13], Sequential Organ Failure Assessment score [14] at admission, location before ICU admission, source of infection, sepsis category and organ dysfunction at ICU admission were recorded. We prospectively assessed severity of illness using the Pneumonia Severity index (PSI) [12]. We calculated CURB-65 [15] retrospectively using altered mental status or a new change in the Glasgow Coma Scale as proxy measures for confusion. Both scores were determined on ICU admission.
Venous blood samples were obtained at ICU admission and collected in tubes containing EDTA. After centrifugation, these were kept frozen at –80 ºC until assayed. MR-proADM was measured using a new sandwich immunoassay (MR-proADM; BRAHMS; Hennigsdorf, Germany). Intra-assay imprecision was under 10% over the entire measuring range, and the functional assay sensitivity (interassay coefficient of variation [CV] <20%) was 0.12 nmol/L. ProADM levels were considered normal when <4 nmol/L based in the median value of proADM observed in healthy adults [16].
For serum PCT measurement we used a time-resolved amplified cryptate emission technology assay (Kryptor PCT; Brahms, Hennigsdorf, Germany). This assay is based on a polyclonal antibody against calcitonin and on a monoclonal antibody against katacalcin. Antibodies bind to the calcitonin and katacalcin sequence of precursor molecules. This assay has an optimised functional sensitivity of 0.06 mcg/L.
Serum CRP concentrations were measured by immunoturbidimetric assay on Modular analyser (Roche Diagnostics, Meylan, France).
Discrete variables were expressed as counts (percentage) and continuous variables as means ± standard except for biomarker levels which were expressed as median and quartiles. Statistical differences between groups were assessed by chi-square test, using Yates’ correction or Fisher’s exact test when appropriate, for categorical variables, and by the Kruskal-Wallis for continuous. Those variables with P values less than 0.15 on univariate analysis were then entered into a multivariate logistic regression analysis to further identify the independent predictors of hospital mortality and their adjusted odds ratios (OR) with 95% confidence intervals (CIs) (95% CI). A P value less than 0.05 was considered significant.
To compare the predictive value of CRP, PCT, and proADM, receiver operating characteristic (ROC) curves were constructed and the area under the curve (AUC) was determined. The outcome variable was in-hospital mortality. On the basis of optimal thresholds determined according to ROC curve analysis, prognostic parameters (positive and negative predictive values and positive and negative likelihood ratios) were also calculated.
All tests were two-tailed. We used the SPSS statistical software package 15.0 (SPSS, Inc., Chicago, Ill) for all statistical analyses.
The demographic characteristics of the study population are presented in table 1. Forty-nine patients with severe sepsis and/or septic shock due to CAP were included in the study (33 men and 16 women) with a mean age at admission of 59.4 ± 13.4 years. At ICU admission mean Apache II (at 24 hours) and SOFA scores were 20.5 ± 6.8 and 8.7 ± 2.2 respectively. Immunosuppression was present in 12 (24.5%) of the patients; 8 cases due to exposure to glucocorticoids or immunosuppressive medications, 1 AIDS, 1 solid organ (lung) transplantation, 1 haematological malignancy and 1 solid tumor. Microbiological determinations were positive in 32 (65%) of the patients. With 20 (40%) isolations Streptococcus pneumoniae was the most frequently isolated bacterium followed by Legionella pneumophila (7%) and Haemophilus influenzae (5%) respectively. 12 patients (24%) presented bacteraemic pneumonia. The mortality rate was 24.5% for ICU and 34.7% for hospital mortality. Non-survivors were more frequently immunosuppressive, had higher Apache II, SOFA and PSI scores on ICU admission and needed mechanical ventilation in a higher percentage of cases (table 1).
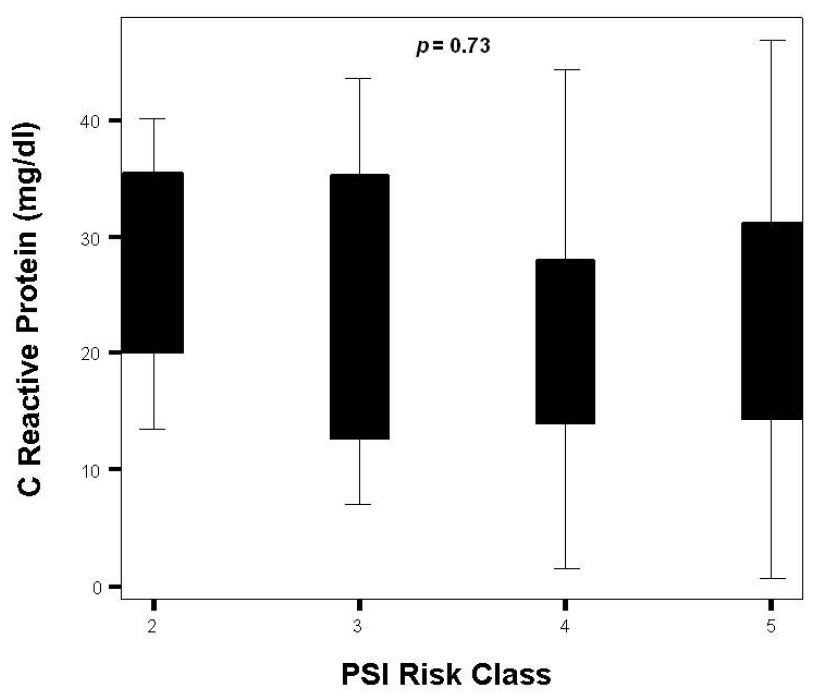
Figure 3
Distribution of CRP levels by PSI class.

Figure 4
ROC curve analysis of PSI+ProADM on predicting hospital mortality.
Overall measurements of the three biomarkers are presented in table 1. In all cases, proADM values at ICU admission were pathological. Although not statistically significant, patients in septic shock had higher levels of proADM than the group of patients in severe sepsis [2.7 (1.5–8.9) nmol/L vs. 1.3 (1.0–3.2); p= 0.08]. Neither PCT nor CRP levels could discriminate between these populations [9.3 (3.3–29.2) ng/ml vs. 13.1 (3.3-23.7) ng/ml; p = 0.51] and [25.1 (14.4–32.7) vs. 17.8 (10.1–28.8); p= 0.18] respectively. ProADM consistently rose as PSI class advanced from II to V (p = 0.02) (fig. 1 and table 2). Differences across PSI class were not significant for CRP (p = 0.73) and PCT (p = 0.12) (fig. 2 and 3 and table 2). Median proADM levels were higher in non-survivors than in survivors 5.0 (1.9–10.1) nmol/L vs. survivors 1.7 (1.3–3.1) nmol/L; p <0.01). These differences were also significant with respect to ICU mortality 6.6 (1.5–15.4) nmol/L vs 2.0 (1.4–3.4) nmol/L; p <0.01. Patients with renal failure (defined as serum creatinine higher than 2 mg/dl) presented higher levels of proADM than patients with normal function, although the difference did not reach statistical significance: 1.6 (1.2–3.6) vs 2.8 (1.5–7.7) nmol/L; p = 0.34.
The receiver-operating characteristic curve for proADM yielded an AUC of 0.72, higher than the AUC for PCT and CRP (0.40 and 0.44 respectively) and similar to PSI (0.74).The association of proADM and PSI only slightly increased the accuracy of PSI in determining the risk of hospital mortality (AUC = 0.76) (fig. 4). The optimal prognostic cut-off (maximum combined sensitivity and specificity) related to in-hospital mortality for proADM was 4.86 nmol/L, with a sensitivity of 0.53, specificity of 0.84, positive likelihood ratio of 3.39, negative likelihood ratio of 0.56, positive predictive value of 64.3 and negative predictive value of 77.1. Patients with a plasma proADM level higher than 4.86 nmol/L on ICU admission had in-hospital mortality significantly higher than those with a lower value (60% vs. 23.5%; p= 0.02).
In the multivariate logistic regression analysis, Apache II score was the only independent predictor of hospital mortality after adjustment by age, SOFA score, PSI score, immunosupression, proADM levels, ARDS and need for mechanical ventilation (OR: 1.24; CI 95%: 1.07–1.43; p= 0.003).
| Table 1: Baseline characteristics of the study population. | ||||
| Overall population n = 49 | Survivors n = 32 | Non-survivors n = 17 | P value | |
| Age (years) | 59.4 ± 13.4 | 58.5 ± 14.3 | 61.0 ± 12.0 | 0.55 |
| Male sex, n (%) | 33 (67.3) | 23 (71.8) | 10 (58.8) | 0.52 |
| Immunosuppression, n (%) | 12 (24.5) | 11 (34.3) | 14 (82.3) | <0.01 |
| Comorbidities, n (%) COPD Hypertension Chronic heart failure Diabetes Chronic renal failure Cancer | 32 (65.3) 16 (32.6) 15 (30.6) 3 (6.1) 10 (20.4) 2 (4.0) 10 (20.4) | 18 (56.2) 7 (21.8) 12 (37.5) 2 (6.2) 7 (22.5) 2 (6.2) 5 (15.6) | 14 (82.3) 9 (52.9) 3 (17.6) 1 (5.8) 3 (17.6) 0 (0) 5 (29.4) | 0.11 0.05 0.21 1.00 1.00 0.56 0.22 |
| Septic shock, n (%) | 36 (73.5) | 21 (65.6) | 15 (88.2) | 0.99 |
| Scores: Apache II SOFA PSI | 20.5 ± 6.8 8.7 ± 2.2 126.7 ± 41.9 | 18.3 ± 5.6 8.1 ± 2.0 114.3 ± 35.9 | 26.5 ± 6.2 10.3 ± 1.7 150.0 ± 43.5 | <0.01 <0.01 <0.01 |
| CURB 65, n (%): 2 3 4 5 | 10 (20.4) 26 (53.1) 10 (20.4) 3 (6.1) | 9 (28.1) 15 (46.8) 7 (21.8) 1 (3.1) | 1 (5.8) 11 (64.7) 3 (17.6) 2 (28.5) | 0.18 |
| PSI Risk Class, n (%): II III IV V | 4 (8.2) 7 (14.3) 17 (34.7) 21 (42.9) | 3 (9.3) 5 (15.6) 15 (46.8) 9 (28.1) | 1 (5.8) 2 (11.7) 2 (11.7) 12 (70.5) | 0.03 |
| Mechanical ventilation, n (%) | 25 (51.0) | 11 (34.3) | 14 (82.3) | <0.01 |
| ARDS, n (%) | 28 (57.1) | 14 (43.7) | 14 (82.3) | 0.01 |
| Renal failure, n (%) | 27 (55.1) | 21 (65.6) | 6 (35.2) | 0.07 |
| SvcO2 (%) | 67.4 ± 12.4 | 66.2 ± 11.8 | 69.7 ± 13.5 | 0.35 |
| Lactate (mg/dl) | 27.9 ± 18.2 | 25.7 ± 18.1 | 31.7 ± 18.2 | 0.28 |
| Biomarkers: PCT (ng/ml) CRP (mg/dl) Pro-ADM (nmol/L) | 10.0 (3.8–6.2) 24.0 (13.7–31.6) 2.4 (1.4–5.6) | 13.2 (5.6–29.2) 25.2 (14.2–32.7) 1.7 (1.3–3.1) | 6.0 (3.1–19.0) 19.9 (12.6–27.8) 5.0 (1.9–10.1) | 0.69 0.59 <0.01 |
| Table 2: Biomarker levels and CAP severity scores. | |||
| ProADM | Procalcitonin | C-reactive protein | |
| PSI Risk class | |||
| II | 1.0 (0.5–2.1) | 14.4 (4.1–21.8) | 28.8 (16.8–37.8) |
| III | 1.3 (1.3–2.0) | 6.0 (0.7–15.7) | 23.9 (7.5–38.6) |
| IV | 1.7 (1.5–4.3) | 10.0 (4.2–22.6) | 16.2 (13.5–29.6) |
| V | 5.0 (2.4–10.5) | 13.0 (3.8–41.2) | 25.0 (13.1–32.1) |
| Pvalue | 0.02 | 0.12 | 0.73 |
| CURB 65 group | |||
| 1 | 1.4 (1.1–1.9) | 10.4 (4.6–28.0) | 25.3 (12.7–32.4) |
| 2 | 2.6 (1.5–4.7) | 11.1 (5.6–23.5) | 25.1 (14.5–37.5) |
| 3 | 5.6 (2.0–10.1) | 9.5 (2.8–36.8) | 19.9 (12.5–28.8) |
| P value | 0.03 | 0.73 | 0.70 |
The accuracy of ProADM plasma levels at ICU admission in predicting the severity and outcome of severe sepsis and septic shock CAP is similar to that afforded by PSI and CURB-65 scores and higher than commonly measured laboratory parameters.
Despite the remarkable advances in antibiotic therapies, diagnostic tools, prevention campaigns and intensive care, community-acquired pneumonia (CAP) is still among the primary causes of death worldwide and there have been no significant changes in mortality over the last few decades. Taking into account the importance of CAP in terms of morbidity and mortality, early identification of patients at high risk is a key point in improving their outcome. However, commonly available diagnostic tools are of limited value in determining which patients will have a poor outcome. While several generic severity scoring systems have been developed to approach this issue, they may be too complicated for use in everyday practice, understimate severity in young patients [17] and perform less well when considering outcomes such as mortality [18].
Biomarkers are becoming the way to improve prognostic accuracy of clinical scores. PCT is the marker that has been most widely investigated and most frequently used in CAP. In severe sepsis and septic shock patients, PCT values on admission have better prognostic utility than other inflammatory markers such as CRP [19]. In our study neither PCT nor CRP was adequate to differentiate between different severities of CAP, as defined by PSI or CURB65, and risk of death. These results coincide with other studies which have shown the limited accuracy of PCT in predicting mortality risk in different infections including CAP, when used as a stand-alone test [20–23].
With regard to proADM there is growing knowledge of the potential role of this vasoactive pro-hormone in patients with CAP, and several studies suggest that proADM is a strong predictor for adverse outcome, probably better than scores and commonly used biomarkers. Recently Albrich et al. demonstrated in two different studies focused on emergency department patients with lower respiratory tract infections how the prognostic ability of proADM was higher than severity scores, and also how its addition to the scores improved the triage decisions, allowing better management of resources without adverse effects [24, 25]. Four other studies have evaluated its prognostic value in CAP, and in each and every one of them proADM also turned out to be better than commonly used biomarkers, PCT and CRP [10, 11, 26, 27]. All of them included only patients admitted to the emergency department, and to the best of our knowledge this is the first study focused only on ICU. In the study of Krüger et al., proADM was the one which performed best in predicting survival of CAP patients compared with the other six different cardiovascular and inflammatory biomarkers [26]. Similarly, Schuetz’s group evaluated the prognostic capacity of 5 prohormones (proADM, endothelin-1, atrial-natriuretic peptide, copeptin, and procalcitonin) in patients with lower respiratory tract infections and CAP. In their experience, while PSI and CURB65 overestimated the observed mortality, proADM alone had stronger discriminatory power than both scores to predict serious complications, and the inclusion of proADM in addition to the PSI or CURB65 scores significantly improved prediction accuracy [27]. In our study, as previously mentioned, with an AUC of 0.72 (comparable with the 0.74 of PSI), proADM was shown to be a helpful prognostic tool in individual risk assessment. Likewise, in the study of Christ-Crain et al., proADM levels on admission to the emergency department may predict outcome with a similar prognostic accuracy to the PSI score [11]. Our results are also comparable to those presented by Huang et al. in the larger published study on the use of proADM in CAP [10]. In 1,653 patients (546 in PSI class IV/V), they observed an optimal correlation of proADM levels with increasing severity of illness and death. Our study, while clearly more limited in scale, may be said to include a sicker population, as indicated by higher mortality rates similar to other studies focused on these critically ill patients [28]. Unfortunately our end points differed from theirs (30-day vs. hospital mortality) and also the lack of systemic severity scores in their study makes this comparison unfeasible. It is also important to emphasise that proADM levels in our population were significantly higher than those observed in all studies focused on CAP and other respiratory tract infections, and similar to those including only septic shock patients [16, 29]. This prompts us to question whether a specific cut-off is necessary for this group of patients.
Several limitations in our study need to be mentioned. The first is that we conducted a single centre study. Second, the generalisability of our findings is limited by the small sample size. Third, the role of renal failure in pro-ADM metabolism is not well known, and it is probable that impaired clearance of proADM in the kidney due to renal failure may have influenced our results. This hypothesis is supported by the results of the study by Christ-Crain et al. However, our data exhibit only slightly higher levels of proADM in the patients with renal dysfunction. Fourth, as previously explained, this is the first study to be focused exclusively on ICU patients with CAP, and for that reason the moment of prognostic score calculation and biomarker determination differs from the rest of the studies published so far. This particular does not minimise the importance of the results, but may render comparison with other studies unfeasible. Fifth, we have only measured single proADM and for this reason we do not know the potential value of serially measured proADM in detecting worst evolution and guiding care.
In our study, focused on patients with CAP, proADM level correlated with severity of illness and death. Our data suggest that this biomarker could potentially be clinically useful for prognosis in these patients, especially in mild/severe cases.
1 Chalmers JD, Singanayagam A, Akram AR, Mandal P, Short PM, Choudhury G, et al. Severity assessment tools for predicting mortality in hospitalised patients with community-acquired pneumonia. Systematic review and meta-analysis. Thorax. 2010;65:878–83.
2 Angus DC, Marrie TJ, Obrosky DS, Clermont G, Dremsizov TT, Coley C, et al. Severe community-acquired pneumonia: use of intensive care services and evaluation of American and British Thoracic Society Diagnostic criteria. Am J Respir Crit Care Med. 2002;166:717–23.
3 Renaud B, Santin A, Coma E, Camus N, Van Pelt D, Hayon J, et al. Association between timing of intensive care unit admission and outcomes for emergency department patients with community-acquired pneumonia. Crit Care Med. 2009;37:2867–74.
4 Restrepo MI, Mortensen EM, Rello J, Brody J, Anzueto A. Late admission to the ICU in patients with community-acquired pneumonia is associated with higher mortality. Chest. 2010;137(3):552–7.
5 Schaub N, Frei R, Müller C. Addressing unmet clinical needs in the early diagnosis of sepsis. Swiss Med Wkly. 2011;141:w13244
6 Chalmers JD. ICU admission and severity assessment in community-acquired pneumonia. Crit Care. 2009;13:156.
7 Linscheid P, Seboek D, Zulewski H, Keller U, Müller B. Autocrine/paracrine role of inflammation-mediated calcitonin gene-related peptide and adrenomedullin expression in human adipose tissue. Endocrinology. 2005;146:2699–708.
8 Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev. 2000;21:138–67.
9 Struck J, Tao C, Morgenthaler NG, Bergmann A. Identification of an adrenomedullin precursor fragment in plasma of sepsis patients. Peptides. 2004;25(8):1369–72.
10 Huang DT, Angus DC, Kellum JA, Pugh NA, Weissfeld LA, Struck J, et al. Midregional proadrenomedullin as a prognostic tool in community-acquired pneumonia. Chest. 2009;136(3):823–31.
11 Christ-Crain M, Morgenthaler NG, Stolz D, Müller C, Bingisser R, Harbarth S, et al. Pro-adrenomedullin to predict severity and outcome in community-acquired pneumonia [ISRCTN04176397]. Crit Care. 2006;10:R96.
12 Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–50.
13 Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE. APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med. 1981;9:591–7.
14 Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–800.
15 Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–82.
16 Christ-Crain M, Morgenthaler NG, Struck J, Harbarth S, Bergmann A, Müller B. Mid-regional pro-adrenomedullin as a prognostic marker in sepsis: an observational study. Crit Care. 2005;9:R816–24.
17 Chalmers JD, Singanayagam A, Hill AT. Predicting the need for mechanical ventilation and/or inotropic support for young adults admitted to the hospital with community-acquired pneumonia. Clin Infect Dis. 2008;47:1571–4.
18 Richards G, Levy H, Laterre PF, Feldman C, Woodward B, Bates BM, et al. CURB-65, PSI, and APACHE II to assess mortality risk in patients with severe sepsis and community acquired pneumonia in PROWESS. J Intensive Care Med. 2011;26:34–40.
19 Luzzani A, Polati E, Dorizzi R, Rungatscher A, Pavan R, Merlini A. Comparison of procalcitonin and C-reactive protein as markers of sepsis. Crit Care Med. 2003;3:1737–41.
20 Schuetz P, Suter-Widmer I, Chaudri A, Christ-Crain M, Zimmerli W, Mueller B. Prognostic value of procalcitonin in community-acquired pneumonia. Eur Respir J. 2011;37:384–92.
21 Huang DT, Weissfeld LA, Kellum JA, Yealy DM, Kong L, Martino M, et al. Risk prediction with procalcitonin and clinical rules in community-acquired pneumonia. Ann Emerg Med. 2008;52:48–58.
22 Polzin A, Pletz M, Erbes R, Raffenberg M, Mauch H, Wagner S, et al. Procalcitonin as a diagnostic tool in lower respiratory tract infections and tuberculosis. Eur Respir J. 2003;21:939–43.
23 Brunkhorst FM, Al-Nawas B, Krummenauer F, Forycki ZF, Shah PM. Procalcitonin, C-reactive protein and APACHE II score for risk evaluation in patients with severe pneumonia. Clin Microbiol Infect. 2002;8:93–100.
24 Albrich WC, Rüegger K, Dusemund F, Bossart R, Regez K, Schild U, et al. Optimised patient transfer using an innovative multidisciplinary assessment in Kanton Aargau (OPTIMA I): an observational survey in lower respiratory tract infections. Swiss Med Wkly. 2011;141:w13237
25 Albrich WC, Dusemund F, Rüegger K, Christ-Crain M, Zimmerli W, Bregenzer T, et al. Enhancement of CURB65 score with proadrenomedullin (CURB65-A) for outcome prediction in lower respiratory tract infections: derivation of a clinical algorithm. BMC Infect Dis. 2011;11:112.
26 Krüger S, Ewig S, Giersdorf S, Hartmann O, Suttorp N, Welte T. Cardiovascular and inflammatory biomarkers to predict short- and long-term survival in community-acquired pneumonia: Results from the German Competence Network, CAPNETZ. Am J Respir Crit Care Med. 2010;182:1426–34.
27 Schuetz P, Wolbers M, Christ-Crain M, Thomann R, Falconnier C, Widmer I, et al. Prohormones for prediction of adverse medical outcome in community-acquired pneumonia and lower respiratory tract infections. Crit Care. 2010;14(3):R106.
28 Valencia M, Badia JR, Cavalcanti M, Ferrer M, Agustí C, Angrill J, et al. Pneumonia severity index class v patients with community-acquired pneumonia: characteristics, outcomes, and value of severity scores. Chest. 2007;132:515–22.
29 Guignant C, Voirin N, Venet F, Poitevin F, Malcus C, Bohé J, et al. Assessment of pro-vasopressin and pro-adrenomedullin as predictors of 28-day mortality in septic shock patients. Intensive Care Med. 2009;35:1859–67.
Funding / potential competing interests:Borja Suberviola has received remuneration for lectures on the topic of inflammation markers by BRAHMS Iberia, Spain. BRAHMS Iberia had no influence on study design, data analysis, or final preparation of this manuscript.