Figure 1
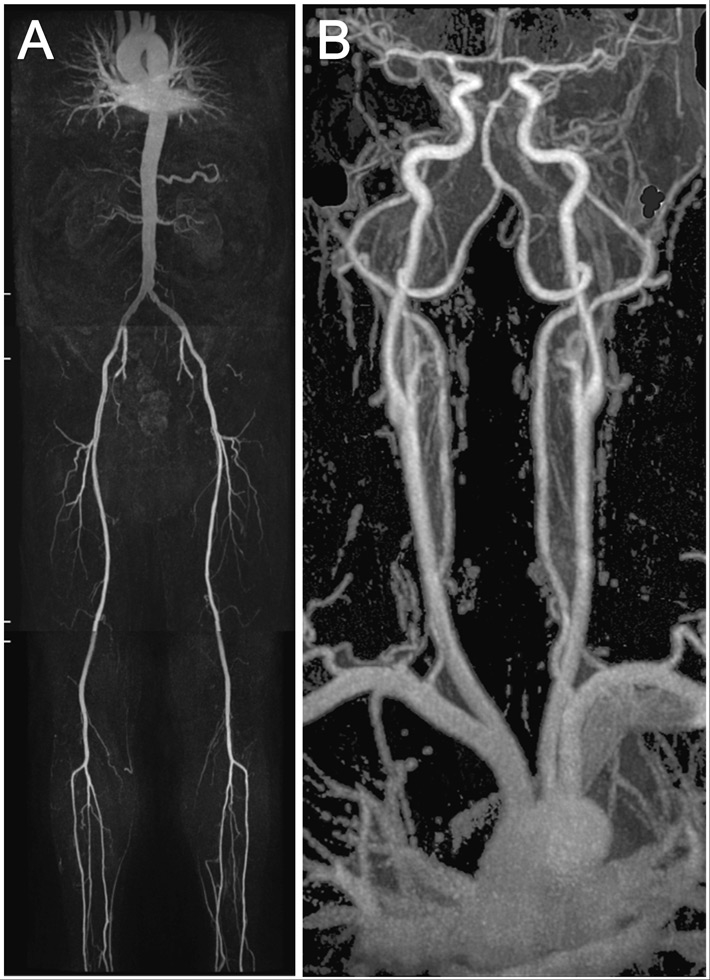
An example showing the MR whole body angiography.
The first part of the MR whole body angiography included thoracic/renal-, pelvis/thigh-, knee/calf-stations (A). The second part included the thoracic/carotid-station (B).
DOI: https://doi.org/10.4414/smw.2012.13538
Spontaneous coronary artery dissection (SCAD) occurs typically in younger women with otherwise low cardiovascular risk [1–5].
The etiology of SCAD remains unclear. Vigorous exercise, vasospasm, coronary artery ectasy, oral contraceptives/hormones, cocaine and cyclosporine have been associated with the condition. Finally, an association of SCAD and fibromuscular dysplasia (FMD) has been suggested [6].
As of now, no study has investigated whether patients with SCAD also present with abnormalities in vessels other than the coronaries. The aim of this study was to investigate whether patients presenting with spontaneous coronary artery dissections (SCAD) have lesions in other vessels than the coronaries thus reflecting a systemic disease.
Patient population and study protocol. The University Hospital Zurich is a tertiary referral clinic treating about 500 acute coronary syndromes (ACS, i.e., unstable angina pectoris and acute myocardial infarction) each year. Between January 2003 and December 2009, we identified a total of 24 patients who presented consecutively at the cardiac catheterisation laboratory with SCAD as the cause of ACS which represented 0.7% of all ACS seen at our institution. Patients with SCAD and relevant atherosclerotic coronary artery disease were excluded since dissection may be a manifestation of a plaque rupture.

Figure 1
An example showing the MR whole body angiography.
The first part of the MR whole body angiography included thoracic/renal-, pelvis/thigh-, knee/calf-stations (A). The second part included the thoracic/carotid-station (B).
Out of these 24 patients, one patient had to be excluded due to an implanted ICD which is a contraindication for MR tomography. Two patients died after hospital admission and could not be investigated. Two were living abroad, four were not reachable and five refused to participate in the study. The remaining 12 patients were included in the study. All patients were examined utilising whole-body MR angiography and duplex sonography of the carotid and renal arteries. Clinical and medical history of the index event was taken utilising a questionnaire. The study was approved by the local ethics committee and all included patients provided written informed consent.
All MR angiography (MRA) examinations were performed on a 1.5-T whole-body MR system (Philips Achieva, Release 2.6, Philips Healthcare, Best, the Netherlands) with a gradient strength of 30 mT/m and a slew rate of 150 (mT/m/msec) according to previously described protocols [7, 8]. The scanning protocol involved a 2 step approach. The first part of the examination was in feet first, supine position, for the renal arteries down to the calf arteries with automatic table movement between the stations, using a 12-element phased-array peripheral vascular coil (Philips Healthcare). A biphasic intravenous administration of gadobenate dimeglumine with 0.15 mmol per kilogram of body weight at flow rates of 2.0 ml/sec followed by 0.033 mmol per kilogram of body weight at flow rates of 0.9 ml/sec, followed by a 30 mL saline flush at a flow rate of 0.8 ml/sec (fig. 1A). In the second part of the examination a 16 -element phased-array Neurovascular coil (Philips Healthcare) was used for the carotid and thoracic arteries in head first and supine position. An intravenous administration of gadobenate dimeglumine with 0.15 mmol per kilogram of body weight at flow rates of 1.5 ml/sec followed by a 30 ml saline flush at a flow rate of 1.5 ml/sec (fig. 1B).
The angiograms were analysed by a board-certified radiologist (DB) and a skilled vascular specialist (CT) independently, in case of differing findings an additional consensus reading was performed.
In all patients, colour coded duplex ultrasound of the extracranial cerebral arteries and the renal artery was performed. A high-end ultrasound machine (iU22, Philips Healthcare) with a linear 9–3 MHz or 17–5 MHz (carotid artery) and a curved 5–1 MHz (renal artery) transducer was used by an experienced investigator (CT). All vessels were examined using B-mode ultrasound, colour Doppler imaging and Doppler spectral analysis. Examination of the extracranial cerebral arteries included the common, external and internal carotid artery, as well as the vertebral and subclavian artery. Intima media thickness of both common carotid arteries was measured using an integrated quantification software package of the ultrasound machine (QLAB, Philips Healthcare) [9]. IMT was analysed over a vessel length of 10 mm and reported as mean millimeters and technical success [%] as a quality control. Investigation of the renal perfusion included Doppler spectral analysis in different areas of the renal artery and the documentation of the intrarenal perfusion including measuring the resistive index in three areas of the renal parenchyma (upper pole, middle, lower pole). A significant (>70%) stenosis of the renal artery was excluded with a peak systolic velocity lower than 200 cm/s or a renal aortal ratio lower than 2.5 [10].
Continuous variables with a normal distribution are described as means with standard deviations. Discrete variables are presented as frequencies and percentages.
A total of 12 patients with SCAD were investigated, 9 (75%) women, mean age at the time of SCAD was 48 ± 10 years (range: 35 to 65 years). Patients presented with ST elevation myocardial infarction (STEMI, n = 8) or Non-STEMI (NSTEMI, n = 4). All patients had chest pain at rest, all but one had an elevated Troponin. Possible risk factors for SCAD were present in 5 patients: sport or exercise during the same day in 3, pregnancy within 6 month in 2, a positive family history for dissections in 2. Baseline characteristics are presented in table 1.
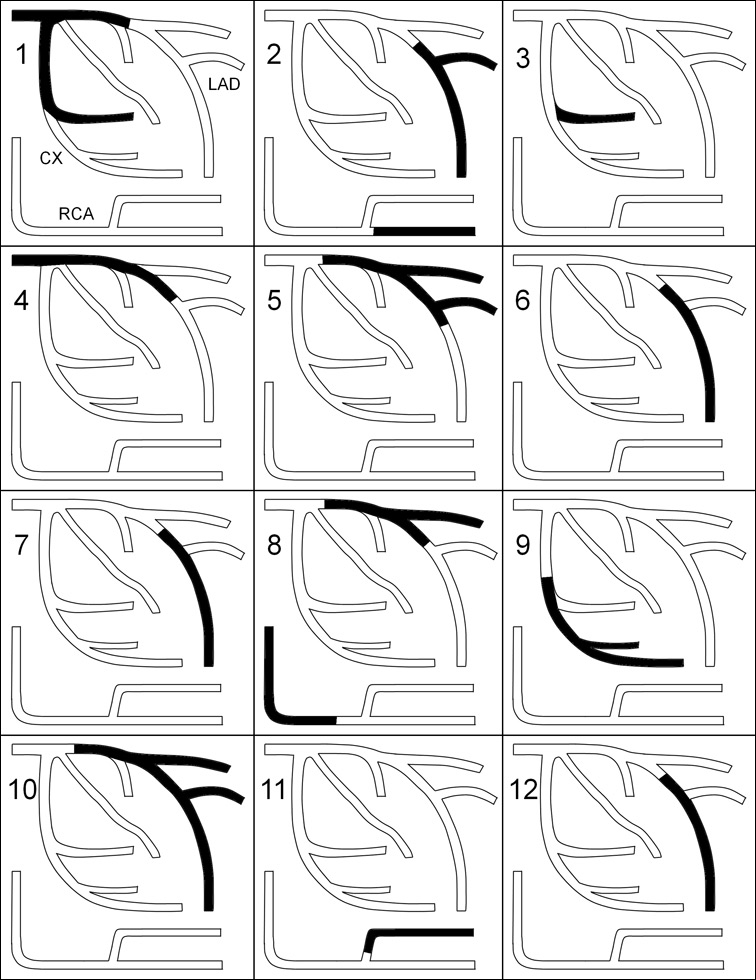
Figure 2
Segmental distribution of coronary artery dissections in patients with (1–3) and without (4–12) renal artery abnormalities.
As shown in table 2 and figure 2, multivessel involvement was present in 3 (25%). All patients presented with typical findings of SCAD such as distal, long lesions, abrupt demarcation from normal proximal segments, double lumen, spiral lucency and contrast staining. Initial treatment was conservative in 7 (58%) patients, balloon dilatation was performed in 2 (17%) and balloon dilatation with stent implantation in the remaining 3 (25%). An intra-aortal balloon pump (IABP) was inserted in 3 (25%) patients. One patient subsequently required surgical revascularisation with coronary artery bypass grafting. Median length of hospital stay was 6 days ranging from 2 to 17 days. After a follow-up of 444 and 782 days, 2 (17%) patients had a recurrent coronary artery dissection in a different vascular territory.
MRA and duplex sonography were performed after a median of 1,036 (range: 78–1938) days after the SCAD. Abnormalities of the renal arteries were found in 3/12 (25%, table 2), all were women. Two patients showed a transverse striated “string of beads” angiographic appearance of the renal artery, consistent with fibromuscular dysplasia (fig. 3, patients 1 and 2). In 1 patient an ostial dissection of the left renal artery without a significant stenosis was found (fig. 3, patient 3). MRA of the extracranial arteries was normal in all patients.
Pathologic findings of the renal artery were found in 2 patients (table 2). A <70% stenosis of the middle part (peak systolic velocity of 210 cm/s) of the right renal artery was observed in a 63 year old woman and a <50% stenosis (peak systolic velocity of 130 cm/s) in a 40 year old woman (fig. 3, patients 1 and 2). Discrete wall thickening of the carotid bulb was found in 3 patients, all other carotid arteries were normal. Intima media thickness was 0.54 ± 0.15 on the right side and 0.55 ± 0.12 on the left side. No dissections were identified by Duplex sonography.
| Table 1: Baseline characteristics in patients with (1–3) and without (4–12) renal artery abnormalities. | ||||||||||
| Nr | Age years | Sex | HTN | Risk factors | Clinical presentation | BP Sys/Dia | Leuk | CRP | Peak CK | LV-EF |
| 1 | 63 | F | Yes | None | STEMI | 92/59 | 11.6 | 21 | 3,120 | 52 |
| 2 | 40 | F | Yes | Pregnancy, Sport | NSTEMI | 140/91 | 12.0 | 156 | 128 | 65 |
| 3 | 55 | F | Yes | None | STEMI | 92/63 | 13.4 | 2 | 545 | 60 |
| 4 | 49 | M | No | positive FH | NSTEMI | 104/52 | 9.5 | 20 | 73 | 70 |
| 5 | 39 | M | No | Sport | STEMI | 100/70 | 6.6 | 3 | 1,837 | 38 |
| 6 | 51 | F | No | None | STEMI | 109/58 | 14.4 | <1 | 2,293 | 50 |
| 7 | 40 | F | No | Positive FH | STEMI | 108/74 | 10.1 | <1 | 1,041 | 57 |
| 8 | 65 | M | No | None | STEMI | 100/51 | 8.3 | 13 | 412 | 71 |
| 9 | 40 | F | Yes | None | NSTEMI | 142/88 | 7.8 | 11 | 286 | 75 |
| 10 | 35 | F | No | Pregnancy, Sport | STEMI | 100/76 | 12.0 | 7 | 620 | 50 |
| 11 | 54 | F | Yes | None | NSTEMI | 183/97 | 6.4 | <1 | 513 | 55 |
| 12 | 47 | F | No | None | STEMI | 124/69 | 7.7 | 2 | 249 | 55 |
| HTN: hypertension; BP: blood pressure in mm Hg; Leuk: Leukocyte count *109; CRP: C reactive protein in mg/l; CK: creatinine kinase; LV-EF: left-ventricular ejection fraction in %; STEMI: ST elevation myocardial infarction; NSTEMI: non-ST elevation myocardial infarction. | ||||||||||
| Table 2: Treatment, outcome, MR and duplex findings in patients with (1–3) and without (4–12) renal artery abnormalities. | ||||||
| Nr | Culprit | Angiographic findings | Initial treatment | Outcome | MR findings | Duplex findings |
| 1 | PL/CX | Spiral lesion, contrast staining | DES | Uncomplicated | Bilat RA FMD | Stenosis right RA |
| 2 | PDA | Two distal lesions with double lumen | Med | Recurrent dissection | Right RA FMD | Stenosis right RA |
| 3 | PL/CX | Distal spiral lesion, contrast staining | Med | Uncomplicated | Left RA Dissection | Normal |
| 4 | LM | Long spiral lesion with double lumen | Med | Uncomplicated | Normal | Normal |
| 5 | Prox LAD | Long lesion with contrast staining | Med | Recurrent dissection | Normal | Normal |
| 6 | Mid LAD | Long distal spiral lesion, contrast staining | DES | Uncomplicated | Normal | Normal |
| 7 | Mid LAD | Long distal spiral lesion, contrast staining | Med | Uncomplicated | Normal | Normal |
| 8 | Prox LAD | Abrubt long lesions with double lumen | DES | Uncomplicated | Normal | Normal |
| 9 | Mid CX | Distal spiral lesion with staining | POBA | Uncomplicated | Normal | Normal |
| 10 | Prox LAD | Abrubt long lesion with contrast staining | Med | Required ACBP | Normal | Normal |
| 11 | PL/RCA | Long distal spiral lesion | Med | Uncomplicated | Normal | Normal |
| 12 | Mid LAD | Long lesion with abrupt demarcation | POBA | Uncomplicated | Normal | Normal |
| PL: posterolateral branch; CX: circumflex; PDA: posterior descending artery; LM: left main; LAD: left anterior descending; RCA: right coronary artery; DES: drug eluting stent; Med: medical treatment; POBA: balloon angioplasty; FMD: fibromuscular dysplasia; RA: renal artery; bilat: bilateral. | ||||||
To our knowledge, this is the first study to investigate associated vascular lesions in patients with spontaneous coronary artery dissection using whole-body MR angiography and duplex sonography. We were able to identify abnormalities of the renal arteries in 3/12 (25%) patients, all women. MRA suggested fibro-muscular dysplasia of the renal arteries in 2 and a spontaneous dissection of the renal artery in 1 patient. Two of the 3 patients with renal artery abnormalities presented with multivessel dissections. Spontaneous dissection of the renal arteries is a common presentation of FMD [11, 12] suggesting that renal artery changes as they occur in FMD were found in all 3 patients with vascular abnormalities. No other relevant vascular abnormalities were found.
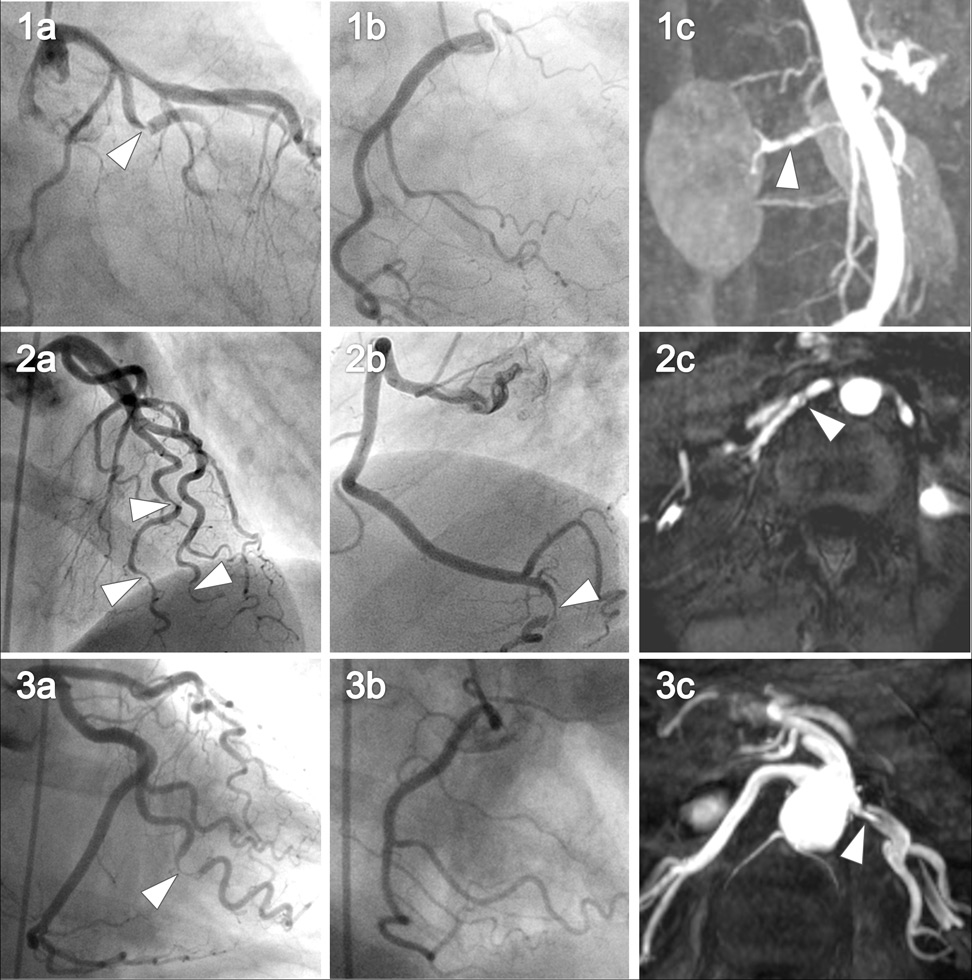
Figure 3
Coronary angiography and renal MR angiography of the 3 women with renal artery abnormalities.
The left (images 1a, 1b, 1c) and right coronary (images 2a, 2b, 2c) angiography is shown for all three patients with the corresponding renal artery abnormalities (images 3a, 3b, 3c). Arrows in images 1a–c and 2a–c point at spontaneous coronary artery dissections. Arrows in images 3a–c point at renal artery fibromuscular dysplasia (3a, 3b) and renal artery dissection (3c).
The prevalence of FMD in the general population is not precisely known. Five reports on angiographic findings in a total of 5138 potential kidney donors found 194 subjects (3.8%) with evidence of renovascular FMD [13–17]. In our study, 25% of the patients with SCAD had changes suggestive of FMD (95% Wilson-confidence interval 9–53%). Although this is a small study, these data suggest that SCAD may be caused by renal artery FMD in some patients.
Spontaneous coronary dissection is a rare form of ACS. In our hospital, 0.7% of all ACS were identified to be due to SCAD. In another report by Mortensen et al., the incidence of SCAD among patients with ACS was 0.2% [18]. Early mortality in such patients is usually high [19]. Case reports of patients with SCAD and FMD have previously been published [20, 21]. One report by Pate et al. included seven women with documented medial pattern of renal FMD and acute coronary syndromes [6]. These patients presented with coronary stenoses in which the proximal vessel appeared normal but in the middle or distal segment there was an abrupt transition to diffuse obliterative disease.
According to the findings of our study, a diagnostic study such as MR angiography, Duplex sonography, renal artery angiography or an aortogram of the renal and visceral segments following coronary angiography in patients presenting with SCAD may be considered to identify possible abnormalities of the renal arteries. A better understanding of diseases that may cause SCAD (e.g., FMD) may help in initial risk stratification at presentation, acute treatment and long-term counselling of these often very unsettled patients.
Due to the low prevalence of the disease investigated, the number of patients included in this study is small. Most of the MR- and colour-Doppler examinations were carried out late after the SCAD, and it is possible that we did not detect transient vascular abnormalities. The study did not include a matched control group of healthy individuals.
Abnormalities of the renal arteries were found in 3/12 (25%) of the patients with SCAD. No other vascular abnormalities were identified. Additional diagnostic tests of the renal arteries such as MR angiography, duplex sonography or angiography should be considered in patients presenting with SCAD.
1 Leone F, Macchiusi A, Ricci R, Cerquetani E, Reynaud M. Acute myocardial infarction from spontaneous coronary artery dissection a case report and review of the literature. Cardiol Rev. 2004;12:3–9.
2 Mahenthiran J, Revankar R, Koka V, Hoo J, Shenoy M. Spontaneous coronary artery dissection presenting as acute myocardial infarction. J Natl Med Assoc. 2000;92:87–90.
3 DeMaio SJ, Jr., Kinsella SH, Silverman ME. Clinical course and long-term prognosis of spontaneous coronary artery dissection. Am J Cardiol. 1989;64:471–4.
4 Bac DJ, Lotgering FK, Verkaaik AP, Deckers JW. Spontaneous coronary artery dissection during pregnancy and post partum. Eur Heart J. 1995;16:136–8.
5 Jorgensen MB, Aharonian V, Mansukhani P, Mahrer PR. Spontaneous coronary dissection: a cluster of cases with this rare finding. Am Heart J. 1994;127:1382–7.
6 Pate GE, Lowe R, Buller CE. Fibromuscular dysplasia of the coronary and renal arteries? Catheter Cardiovasc Interv. 2005;64:138–45.
7 Wyttenbach R, Braghetti A, Wyss M, Alerci M, Briner L, Santini P, et al. Renal artery assessment with nonenhanced steady-state free precession versus contrast-enhanced MR angiography. Radiology. 2007;245:186–95.
8 Willinek WA, Gieseke J, Conrad R, Strunk H, Hoogeveen R, von Falkenhausen M, et al. Randomly segmented central k-space ordering in high-spatial-resolution contrast-enhanced MR angiography of the supraaortic arteries: initial experience. Radiology. 2002;225:583–8.
9 Lim TK, Lim E, Dwivedi G, Kooner J, Senior R. Normal value of carotid intima-media thickness – a surrogate marker of atherosclerosis: quantitative assessment by B-mode carotid ultrasound. J Am Soc Echocardiogr. 2008;21:112–6.
10 Staub D, Canevascini R, Huegli RW, Aschwanden M, Thalhammer C, Imfeld S, et al. Best duplex-sonographic criteria for the assessment of renal artery stenosis – correlation with intra- arterial pressure gradient. Ultraschall Med. 2007;28:45–51.
11 Lacombe M. Isolated spontaneous dissection of the renal artery. J Vasc Surg. 2001;33:385–91.
12 Paris B, Bobrie G, Rossignol P, Le Coz S, Chedid A, Plouin PF. Blood pressure and renal outcomes in patients with kidney infarction and hypertension. J Hypertens. 2006;24:1649–54.
13 Spring DB, Salvatierra O, Jr., Palubinskas AJ, Amend WJ, Jr., Vincenti FG, Feduska NJ. Results and significance of angiography in potential kidney donors. Radiology. 1979;133:45–7.
14 Cragg AH, Smith TP, Thompson BH, Maroney TP, Stanson AW, Shaw GT, et al. Incidental fibromuscular dysplasia in potential renal donors: long-term clinical follow-up. Radiology. 1989;172:145–7.
15 Neymark E, LaBerge JM, Hirose R, Melzer JS, Kerlan RK Jr, Wilson MW, et al. Arteriographic detection of renovascular disease in potential renal donors: incidence and effect on donor surgery. Radiology. 2000;214:755–60.
16 Andreoni KA, Weeks SM, Gerber DA, Fair JH, Mauro MA, McCoy L, et al. Incidence of donor renal fibromuscular dysplasia: does it justify routine angiography? Transplantation. 2002;73:1112–6.
17 Lorenz EC, Vrtiska TJ, Lieske JC, Dillon JJ, Stegall MD, Li X, et al. Prevalence of renal artery and kidney abnormalities by computed tomography among healthy adults. Clin J Am Soc Nephrol. 2010;5:431–8.
18 Mortensen KH, Thuesen L, Kristensen IB, Christiansen EH. Spontaneous coronary artery dissection: a Western Denmark Heart Registry study. Catheter Cardiovasc Interv. 2009;74:710–7.
19 Koller PT, Cliffe CM, Ridley DJ. Immunosuppressive therapy for peripartum-type spontaneous coronary artery dissection: case report and review. Clin Cardiol. 1998;21:40–6.
20 Lie JT, Berg KK. Isolated fibromuscular dysplasia of the coronary arteries with spontaneous dissection and myocardial infarction. Hum Pathol. 1987;18:654–6.
21 Brodsky SV, Ramaswamy G, Chander P, Braun A. Ruptured cerebral aneurysm and acute coronary artery dissection in the setting of multivascular fibromuscular dysplasia: a case report. Angiology. 2007;58:764–7.
Funding / potential competing interests: Dr. Toggweiler is supported by a grant of the Swiss National Foundation.