Statins and stent thrombosis
DOI: https://doi.org/10.4414/smw.2012.13525
Vincent
Braunersreuther, François
Mach, Fabrizio
Montecucco
Summary
Thrombosis is a rare but serious complication of stent implantation in atherosclerotic arteries, affecting both bare-metal and drug-eluting stents. Diagnostic criteria for stent thrombosis have recently been updated with the time and probability of the event being considered as crucial parameters. To be considered as “definite”, the diagnosis of stent thrombosis has to be confirmed by angiography or histology. This statement position has clearly rendered more difficult the clinical assessment of stent thrombosis in randomised clinical trials. Considering these limitations, stent thrombosis represents a dramatic complication for both patients and cardiologists. In coronary plaques, thrombosis is often associated with death, acute coronary syndromes and arrhythmias. For these reasons, the pharmacological improvement of this outcome represents a “hot-topic” field for research. Among several medications, statins have been shown to potentially reduce the incidence of coronary stent thrombosis in humans. However, randomised clinical trials focussing on “definite” diagnosis are still needed to confirm these promising results. In addition, the use of statins in patients implanted with stents in other arteries is largely unexplored. Finally, statin-eluting stents (only tested in pigs) have to be evaluated in other animal models and human beings. Therefore, a clear recommendation on the use of statins to prevent stent thrombosis is not available and caution should be used. The “pleiotropic” anti-atherosclerotic properties of statins might represent a crucial investigation field to pathophysiologically clarify the role of statins in stent complications.
Introduction
The prompt pharmacological or mechanical restoration of the blood flow in a coronary artery has been recommended as the gold standard treatment to allow reperfusion [1] and to improve cardiac function and mortality after an ST elevation myocardial infarction (STEMI). The endovascular approach to accomplishing coronary revascularisation (using balloon angioplasty) was started in 1977 in Switzerland by Grüntzig and co-workers [2]. The authors showed for the first-time in humans that this technique efficaciously reduced the vessel stenosis and the coronary pressure gradient [2]. To improve the clinical outcomes (mainly reduction of restenosis after balloon dilatation), Palmaz and co-workers tested with success the implantation of expandable intraluminal endoprostheses in different arteries in dogs [3]. These encouraging results were confirmed two years later by the same research group in dog coronary arteries [4]. In the same year, Sigwart and co-workers performed the first-in-man endovascular implantation of a self-expandable stainless-steel endoprosthesis [5]. After more than 20 years of training and thousands of procedures performed, the placement of a “stent” in the coronary artery tree is considered as “routine” by interventional cardiologists. However, some serious complications (such as stent restenosis or thrombosis) are still reported and might be reduced by a concomitant pharmacological treatment. Anti-platelet and immunomodulatory drugs have been shown to improve stent complications. Other drugs (such as statins, which have been shown to reduce cardiovascular events) [6] might further reduce stent complications. In this review, we will update evidence on the potential anti-atherothrombotic role of statins in patients implanted with coronary stents.
Coronary stents
The evolution of stent materials was a consequence of the coronary tissue response to the device placement [7]. In order to reduce late stent restenosis, the bare-metal stents (BMS, first stents used) were replaced in the clinical management of coronary diseases by drug-eluting stents (DES) [8, 9]. These new devices releasing anti-proliferative and immunomodulatory medications have been shown to improve composite outcomes (such as major adverse cardiac events and vessel failure) when compared with BMS. A recent systemic review and meta-analysis (including 47 randomised clinical trials enrolling more than 14,500 patients) also showed some improvements in lesion and vessel revascularisation for stents releasing sirolimus, paclitaxel, dexamethasone and zotarolimus [10]. In selective populations (such as patients with chronic total coronary occlusion), DES have been demonstrated to cause less restenosis as compared to BMS [11, 12]. Also the target lesion revascularisation rate was significantly reduced by DES when compared with BMS (respectively 7% vs 13%; HR 0.49, 95% CI 0.28–0.86; p = 0.01) [13]. Conversely, despite a low incidence for both stent types [14], no relevant benefit was shown for DES in stent thrombosis in a more general population of coronary heart disease (CHD) patients [10]. More recently, a second generation of DES (characterised by more biocompatible polymers) has been investigated with promising beneficial results in a follow-up period of about 2–3 years [15, 16]. New materials (such as biodegradable or nonerodable polymers and nonpolymeric compounds) and designs have been shown to further influence cardiovascular outcomes after stent implantation in coronary atherosclerotic disease [17, 18]. Revolutionary advances have been recently achieved using nanotechnology in stent material and design. In particular, polyhedral oligomeric silsesquioxane (POSS) appears to be a promising material for coronary stents [19]. Recent microfabrication technologies might further minimise stent dimension and improve biocompatibility [7]. The use of more selective anti-proliferative agents is also under investigation, but randomised clinical trials and meta-analyses on this developmental topic are still missing.
Stent thrombosis
A standard definition of stent thrombosis has recently been proposed by academic organisations in the United States and Europe [20]. This Academic Research Consortium established the criteria for diagnosis of coronary stent thrombosis, classifying it as a “definite or confirmed” (symptoms of an acute coronary syndrome and angiographic or pathological confirmation), “probable” (unexplained death or target vessel myocardial infarction within 30 days without angiographic confirmation) and “possible” (any unexplained death after 30 days) event. The Experts also considered the time to thrombotic event after stent implantation as a pivotal parameter for the definition of the disease. They proposed early (0–30 days), late (>30 days), very late (>12 months) stent thrombosis after the implantation [20]. Since the definite diagnosis of stent thrombosis would require post-mortem examination, its real incidence is likely to be underestimated. For the same reasons, coronary stenting thrombosis has been regarded as a rare complication. The first European multicentre study (enrolling 105 patients submitted to coronary or saphenous-vein bypass graft stenting) showed a relatively high incidence (23%) of early stent occlusion, suggesting that, if angioplasty might result in an increased risk of restenosis, stent implantation might have a higher incidence of “in-stent thrombotic occlusion” [21]. Some years later, a multicentre, randomised, prospective Acute Catheterisation and Urgent Intervention Triage Strategy (ACUITY) trial (enrolling 7162 patients with moderate- and high-risk acute coronary syndrome [ACS] and treated with coronary stent implantation) the incidence of early definite coronary stent thrombosis was 0.9% [22]. On the other hand, a recent meta-analysis of 38 randomised clinical trials enrolling 18023 patients implanted with BMS or DES confirmed the low incidence of thrombotic complications (reported in the ACUITY trial) [23]. However, stent thrombosis should be considered as a serious event underlying sudden death or re-infarction in CHD patients. This complication was associated with mortality in about 25% of patients and with myocardial infarction in 80% of cases [22]. Thus, despite being rare, stent thrombosis remains a pivotal concern for cardiologists and their patients. Given these limitations, stent thrombosis remains a severe clinical entity particularly difficult to study. Therefore, further improvements in our understanding of the pathophysiology of stent thrombosis and the identification of novel therapeutic targets are desperately needed in the near future.
Pathophysiology of stent thrombosis
In the last 150 years, the pathophysiology and risk factors of thrombotic events have been widely described [24]. Differently from venous thrombosis, arterial thrombosis has been described as rather associated with vessel wall injury and a hypercoagulatory state [25]. However, considering their common pathophysiological features, thrombotic disorders have been also proposed as two potential elements underlying symptomatic atherosclerosis [26, 27]. Given these general considerations, we can easily understand that the placement of a stent in a coronary artery might favour the formation of a thrombus within the stent lumen. Blood cells (such as platelets, white blood cells and red blood cells) and coagulation factors are the major players contributing to the thrombus formation [28]. Both mononuclear and polymorphonuclear white blood cells might be attracted early within the thrombus by fibrin and its degradation products [29]. After this first phase, the local production of cytokines and chemokines might further favour the accumulation of inflammatory and vascular smooth muscle cells (VSMCs) [29]. However, the pathophysiological role of these soluble mediators has not been clarified yet. Interestingly, Cook and co-workers, performing a histopathological analysis of human coronary stent thrombus, also detected relevant infiltrates of eosinophils [30]. These cells might particularly contribute to the natural evolution and remodelling of the thrombus via the release of proteolytic products [31]. Major cellular components of stent thrombus have been summarised in figure 1 [29, 32, 33].
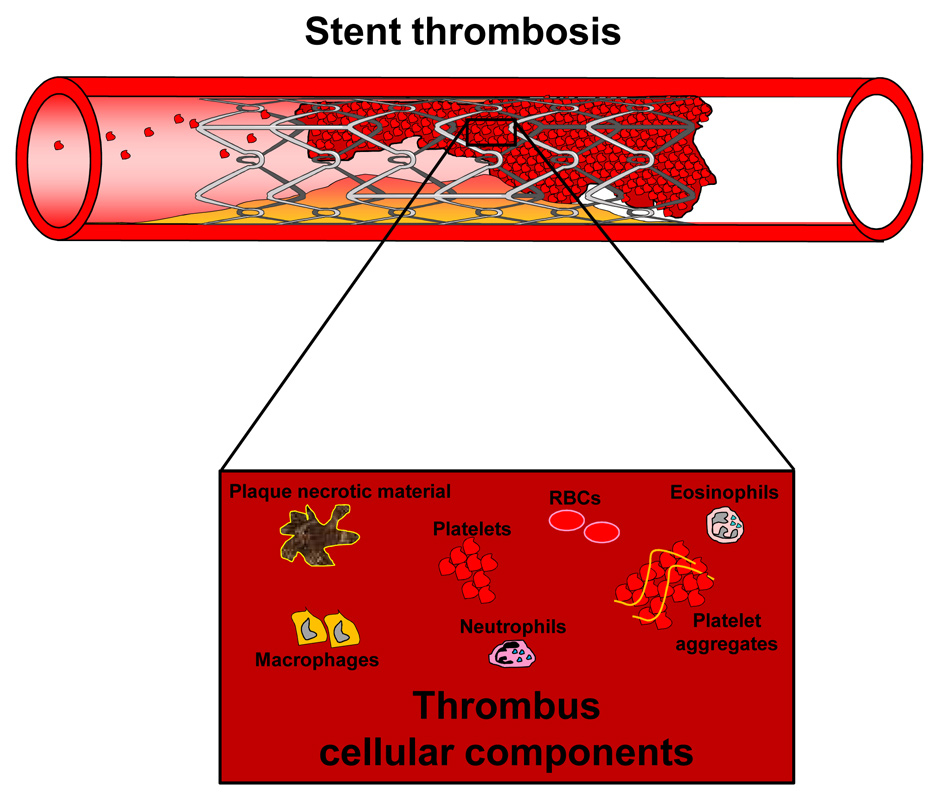
Figure 1
In-stent thrombosis: stent thrombosis is a rare but serious complication, often resulting in acute ischaemic events in downstream tissues and patient death. It is caused by atherothrombotic processes within the lumen of the stent with the subsequent acute occlusion of the vessel. The thrombus is a heterogeneous tissue, which includes platelets, platelet aggregates, inflammatory cells (eosinophils, neutrophils, macrophages), entrapped red blood cells (RBC) and necrotic parts of the ruptured atherosclerotic plaques.
Four determinants favouring stent thrombosis have been identified and classified: device-related factors, procedure-related factors, patient-related factors and lesion-related factors [34, 35].
Device-related risk is stems from the predisposition of the stent material to thrombogenicity. In fact, several determinants (such as stent geometry, coating strut thickness, polymer which houses the drug and cell-cycle inhibiting drugs) might influence thrombotic events. In a model of stents deployed in rabbit denuded iliac arteries, stent configuration and material were shown to influence the thrombosis incidence [36]. In this study, the change of stent configuration to reduce strut-strut intersections by 29% without affecting mass or surface area, reduced thrombosis by 69%. Moreover, coating the stainless steel surface with an inert polymer material also reduced thrombotic events. More recently, Gurbel and co-workers showed that stent design could be related to platelet activation in patients [37]. They observed that platelet activation was greater during the 30 days following implantation of an open-cell than a closed-cell stent, due potentially to the different stent scaffolding properties.
Stent coating is also considered as a pro-thrombotic determinant [38, 39]. Indeed, DES have been shown to cause both systemic and “intrastent” hypersensitivity reactions that are related to late stent thrombosis and death [40]. DES restenosis is associated with a larger amount of old and fibrinoid thrombus compared with BMS, probably due to the delayed tissue healing induced by the drug [41, 42]. Indeed, up to 2 years after percutaneous coronary intervention (PCI), an incomplete re-endothelialisation and thrombus were more frequently observed at angioscopy in DES than in BMS [43]. Although a recent meta-analysis did not show a significant difference in stent thrombosis between BMS and DES in the earliest phases after the implantation [23], other studies suggest higher “global” rates for DES [44–46]. The increased thrombogenicity of DES seems to be correlated with altered vascular healing manifested by incomplete and delayed stent re-endothelisation and persistent fibrin deposition [45]. Thus, the use of DES might even prolong the window of vulnerability to stent thrombosis. In a recent study, Kolandaivelu and co-workers investigated the inherent thrombogenicity of bare and polymer/drug-coated stents, using an integrated approach incorporating ex vivo and in vivo insights. They showed that early clotting is reduced by polymer/drug coatings, suggesting a potential thrombogenic effect of these devices [47].
The procedure of stent placement represents the second category of risk. A large number of studies suggest a relation between inadequate stent implantation and stent thrombosis [48–51]. The most important procedure-related factors for the development of stent thrombosis include: small dimensions of the final lumen (stents under-expanded), dissections at the stent margin, placement of multiple stents, stent malposition and persistent slow coronary blood flow.
The third category of risk for stent thrombosis comprises patient-related factors. Patients suffering from unstable angina, acute coronary syndrome, kidney disease or diabetes mellitus have an increased risk of stent thrombosis [52]. Genetic factors might also influence the risk stent thrombosis. For example, patients with a polymorphism of platelet glycoprotein IIIa gene (PlA2) are at high risk for stent thrombosis [53]. On the other hand, patient compliance with pharmacological anti-platelet treatment after stent placement is the most important areas of focus for cardiologists and family medical doctors in reducing stent thrombosis. Being foreign material in the vessel wall, stents enhance platelet adhesion and induce activation of the coagulation cascade. Thus, a potent inhibition of platelet activation is pivotal to make this procedure feasible. Premature discontinuation of anti-platelet therapy has been identified as the principal parameter mediating late stent thrombosis [54, 55]. Moreover, anti-platelet hyporesponsiveness is associated with a greater risk for stent thrombosis [56]. Since heated debate on the management of anti-platelet treatment after coronary stent implantation is is on-going, a recent positioning article from the Perioperative Haemostasis of the Society on Thrombosis and Haemostasis Research (GTH), the working group on Perioperative Coagulation of the Austrian Society for Anesthesiology, Resuscitation and Intensive Care (ÖGARI) and the Working Group Thrombosis of the European Society for Cardiology (ESC) further clarified this point [57]. In patients undergoing elective PCI with BMS, experts generally recommended dual antiplatelet therapy for a period of four to six weeks. On the other hand, 12 months of treatment was recommended for DES. We remind the reader of this article oft he long list of interesting recommendations aimed at optimising the management of anti-platelet therapy in stent-implanted patients [57].
The fourth risk group is represented by lesion-related factors. Stent thrombosis occurs more frequently in complex lesions. Vessel size, low cardiac ejection fraction, old age, plaque vulnerability and coagulation disorders are all associated with increased risk of stent thrombosis [38, 48, 58, 59]. Coronary lesion position might also play a relevant role. Ong and co-workers observed that bifurcation stenting in acute myocardial infarction was a highly significant independent predictor for angiographic stent thrombosis [60]. Armstrong and co-workers recently confirmed that the implantation of a stent at coronary bifurcations was associated with a higher in-hospital and long-term mortality as compared with non-bifurcation lesions [61]. More recently, other studies suggested that local and systemic inflammation might be involved in the pathogenesis of stent thrombosis [30, 62, 63]. Indeed, inflammation and clotting cascades share common signalling pathways promoting platelet activation [64]. Finn and co-workers showed that inflammation was increased at sites of overlapping DES when multiple stents are placed [65].
Statins in stent thrombosis
Inhibitors of the 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase (also called “statins”), are potent lipid-lowering drugs, which have been shown to reduce cardiovascular events and mortality in patients with or without coronary artery disease [6]. Recent evidence suggests that statins possess several potent “pleiotropic effects” besides their lipid-lowering effects. Statins have been shown to induce anti-inflammatory effects, modulate endothelium and inhibit the thrombotic signalling cascade. All these properties potentially provide a clinical benefit in the setting of PCI by preventing post-procedural myocardial damage and cardiovascular events [66]. Indeed, several studies showed that statin therapy before and after stent implantation reduces the incidence of coronary events [67–69]. In the last decades, only few basic research studies have been conducted to elucidate the involvement of statins in stent complication pathophysiology. Zhu and co-workers showed that fluvastatin induced anti-thrombotic properties in human vascular smooth muscle cells. In particular, they showed that this drug reduced the sirolimus-induced expression of tissue factor (TF, a primary initiator of the coagulation cascade and essential for the homeostasis of SMCs in human saphenous veins and aorta) [70]. This study suggested a potential anti-thrombotic role of statins in complications after DES implantation [71]. In a model of abdominal stent implantation in rats, the administration of another statin (rosuvastatin) was associated with the reduction of neo-intima formation and, in parallel, with the improvement of endothelial-mediated vasodilation [72]. These data from basic research suggested that statin-coated stents might be a potential option alternative to paclitaxel and sirolimus reducing side effects of late stent thrombosis and endothelial dysfunction. More recently, fluvastatin has been shown to accelerate the re-endothelisation impaired after the placement of sirolimus-eluting stents [73]. In this study, Fukuda and co-workers submitted mouse femoral arteries to wire mediated vascular injury and locally treated the animals with sirolimus in the presence or absence of concomitant fluvastatin systemic treatment. Sirolimus significantly delayed the re-endothelisation. Fluvastatin showed protective effects through ameliorated proliferation and migration of mature endothelial cells impaired by sirolimus administration.
Statin treatments have been investigated with some promising preliminary results to improve cardiovascular outcomes after stent implantation in human beings (secondary prevention). Chan and co-workers investigated the potential effects in short-term survival (up to 6 months) of an on-going statin treatment in patients submitted to PCI. 5052 patients completed the clinical follow up and were included in the final analysis [74]. Treatment with a statin was shown as an independent predictor of 6-month survival when compared with untreated subjects [74]. Since the population enrolled was submitted to a stent implantation in a quite small percentage (statin-treated vs untreated patients: 49 vs 39%, p = 0.001), this study was not clearly designed to appropriately assess the incidence of stent thrombosis. However, despite some other limitations (non-randomised design and unknown compliance with statin treatment), this study showed for the first time that oral statin treatment might be beneficial in PCI patients. A few years later, Pasceri and co-workers focused on the potential benefits of statins in reducing risk of post-PCI risk of myocardial injury [69]. They investigated pre-treatment with atorvastatin (40 mg/day for 7 days before PCI) on post-procedural myocardial infarction in a randomised placebo-controlled trial enrolling 153 patients affected by stable angina and undergoing elective PCI. Since a large number of patients in both groups were implanted with coronary stents (placebo vs. atorvastatin: 95 vs 92%), the short-term outcome of this study fits very well with timing of early stent thrombosis. Pre-treatment with atorvastatin significantly reduced procedural myocardial injury (assess by serum levels of cardiac necrosis enzymes) in elective coronary intervention independently of other protective medications, such as beta-blockers, anti-platelets or angiotensin-converting enzyme (ACE)-inhibitors [69]. In 2007, a meta-analysis of randomised trials investigating statin treatment (initiated at the time of PCI) to reduce myocardial injury, was published by Mood and co-workers [74]. This systematic review analysed 6 studies enrolling 3941 patients randomised to statin (n = 1967) and placebo (n = 1974). During the clinical follow-up (ranging from 1 day to 45 months), statin treatment significantly reduced the incidence of myocardial infarction compared to the placebo (3.0 vs 5.2%, odds ratio [OR] 0.57, 95% confidence interval [CI] 0.42 to 0.78, p <0.0001). Conversely, no effect was observed on all-cause or cardiovascular mortality between statin and placebo-treated groups (OR 0.74, 95% CI 0.50 to 1.1, p = 0.14; OR 0.58, 95% CI 0.30 to 1.11, p = 0.10, respectively) [75]. Considering that this meta-analysis focused on acute myocardial infarction as a general clinical outcome, we cannot extrapolate any clear results on the potential relationship between statin treatment and coronary stent thrombosis. The protective effect of long-term statin treatment in preventing acute ischaemic events after PCI was confirmed in a more recent retrospective cohort study (enrolling 386 patients). Statin (alone or in combination with an ACE-inhibitor or beta-blocker) was associated with the reduction in the risk of clinical ischaemic events in heart and brain (HR 0.52 [95% CI 0.28–0.99], p = 0.045) during the registry follow up (median of 832 days). Importantly, the protective effect of statins was more relevant than clopidogrel alone (HR 1.19 [95% CI 0.36–3.98], p = 0.78) in the same patients [76]. However, also in this study, authors only investigated some general clinical ischaemic outcomes without assessing a “definite” diagnosis of coronary stent thrombosis. Chua and co-workers recently focused on this aspect and designed a clinical study in 455 patients undergoing primary coronary stenting for ST-elevation myocardial infarction (STEMI) and followed up for at least one month after PCI [77]. Authors showed a quite high incidence of definite stent thrombosis (assessed by coronary angiography) of 3.7% in their cohort. The use of a statin was found significantly more frequent in the group without stent thrombosis as compared to patients affected by this complication (39.5 vs 5.9%, p <0.01). Accordingly, statin use was shown to be independently associated with the reduction of the risk of stent thrombosis (HR 0.09 [95% CI 0.01–0.75], p = 0.03). Nishino and co-workers showed an independent association between the administration of a statin before stent placement and the reduction of thrombosis [78]. Since the diagnosis of stent thrombosis was performed by histological analysis of the “in-stent” tissue arterectomy, these data indicate that also a pre-procedural strategy of statin administration might induce an important reduction in this complication [78]. These results strongly support a potential benefit of statin therapy in prevention of coronary stent thrombosis [77].
In partial contrast with these studies, some recent data also suggested an unexpected positive association between statin treatment and stent thrombosis [79–81]. This paradoxical effect might be related to the potential drug-drug interaction on the cytochrome P450 3A4 that metabolises both statins and clopidogrel [82]. In particular, concomitant statin treatment might inhibit the pharmacological activation of clopidogrel by cytochrome P450 3A4, thus resulting in a final impaired inhibition of platelet activities [83]. This hypothesis was suggested by Brophy and co-workers that showed that prescription for drugs inhibiting cytochrome P450 3A4 enzyme was associated with adverse cardiovascular events after percutaneous coronary intervention [80]. Conversely, Wenaweser and co-workers did not confirm the risky impact of a concomitant treatment with statins (atorvastatin or pravastatin) in stent thrombosis [82]. Therefore, these controversial results did not clarify this important issue. Differently from proton-pump inhibitors (PPI) [81], no relevant interference between statins and clopidogrel has been described [82, 84]. However, the use of medications metabolised by this common pathway might suggest caution during clopidogrel treatment.
In order to indentify some pathophysiological mechanisms underlying statin-mediated protection, “lipid-lowering” and “pleiotropic” anti-atherosclerotic activities have been investigated in human cohort studies. The role of LDL-cholesterol levels in stent thrombosis has not been clarified yet [85, 86]. Miyamoto and co-workers showed in 2001 that LDL-cholesterol apheresis (performed immediately before and after percutaneous transluminal coronary artery angioplasty [PTCA]) prevented stent restenosis [87]. However, no data are available on stent thrombosis.
Even less is known on the potential protective role of HDL-cholesterol levels of stent thrombosis. Sakakura and co-workers recently showed an unexpected relationship between the increase of HDL-cholesterol (5 mg/dl) and early recurrence (within 1 year) of myocardial infarction [88]. However, since in their cohort of patients only 24.7% was previously implanted with a coronary stent [88], the potential association between HDL-cholesterol and stent thrombosis remain unexplored. Also the potential different activities of hydrophilic and lipophilic statins or the concomitant use of other lipid improving drugs remain unknown in stent thrombosis [89].
Statin “pleiotropic” (anti-atherosclerotic and anti-thrombotic) activities were also investigated with particular focus on the soluble and cellular mediators mediating thrombus formation.
In a cohort of patients with peripheral arterial occlusive diseases, high-dose treatment with atorvastatin has been shown to reduce thrombin generation and the expression of tissue factor, P-selectin and GPIIIa on platelet-derived microparticles [90]. Since patients with previous myocardial infarction and PTCA were excluded from this study, we cannot know more on the potential direct activity of statins in the coagulation cascade. On the other hand, statin treatment was associated with ex vivoreduced platelet reactivity in patients with acute myocardial infarction [91]. This property might clearly favour a beneficial role of statin on stent thrombosis, also independently of their lipid-lowering activity.
Although some controversies still exist [92], serum C-reactive protein (CRP) levels have been recently shown to predict stent thrombosis and major cardiovascular events in patients implanted with coronary DES [62, 93]. Statins have been shown to reduce C-reactive protein (CRP) production in vitro [94] and in vivo [95, 96]. Therefore, these preliminary studies suggest that statin treatment might reduce stent thrombosis via reduction in CRP levels. Randomised placebo-controlled clinical trials are still missing to address this research question. Also a statin-eluting stent might be proposed as a useful approach to reduce stent complications in a porcine coronary model [97, 98]. Given these preliminary results from basic research and the lack of data from randomised clinical trials, we cannot make a useful recommendation on the potential application of the “local” administration of statins. This strategy might also avoid the potential adverse effects due to common metabolic pathways in stent-implanted patients.
Conclusions
The potential benefits of statins in patients implanted with stents have been only marginally studied in coronary artery disease. Considering a “definite” diagnosis of stent thrombosis, only preliminary results from clinical trials indicate that pre-procedural statin use is associated with a reduction of this complication early (first month) after coronary stent placement. On the other hand, these promising studies specifically focused on coronary stent thrombosis. Thus, scientific evidence is still lacking for other arteries. Considering the low incidence of stent thrombosis, the realisation of randomised placebo-controlled clinical trials requires the enrolment of great numbers of patients. Since this aspect markedly increases the research costs, we doubt that this interesting research question will be clarified in the near future for statins. Despite these limitations, we believe that treatment with statins can be considered as a useful systemic drug strategy to prevent stent thrombosis. On the other hand, the local activity of such drugs in eluting stents most likely will be explored in humans. Although some promising data exist, the proof of concept on statin-mediated reduction of stent thrombosis is still missing. Therefore, the use of a statin, which has been indicated as a safe treatment in cardiovascular patients [99], cannot reach a clear recommendation in stent-implanted patients to reduce thrombotic complications.
References
1 Cuculi F, De Caterina AR, Kharbanda RK, Banning AP. Optimal reperfusion in ST-elevation myocardial infarction – the role of the coronary microcirculation. Swiss Med Wkly. 2011; 141:w13313. doi: 10.4414/smw.2011.13313.
2 Gruntzig AR, Senning A, Siegenthaler WE. Nonoperative dilatation of coronary-artery stenosis: percutaneous transluminal coronary angioplasty. N Engl J Med. 1979;301:61–8.
3 Palmaz JC, Sibbitt RR, Reuter SR, Tio FO, Rice WJ. Expandable intraluminal graft: a preliminary study. Work in progress. Radiology. 1985;156:73–7.
4 Schatz RA, Palmaz JC, Tio FO, Garcia F, Garcia O, Reuter SR. Balloon-expandable intracoronary stents in the adult dog. Circulation. 1987;76:450–7.
5 Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med. 1987;316:701–6.
6 Veillard NR, Mach F. Statins: the new aspirin? Cell Mol Life Sci. 2002;59:1771–86.
7 Martinez AW, Chaikof EL. Microfabrication and nanotechnology in stent design. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3:256–68.
8 Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–23.
9 Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–31.
10 Greenhalgh J, Hockenhull J, Rao N, Dundar Y, Dickson RC, Bagust A. Drug-eluting stents versus bare metal stents for angina or acute coronary syndromes. Cochrane Database Syst Rev. 2010: CD004587.
11 Saeed B, Kandzari DE, Agostoni P, Lombardi WL, Rangan BV, Banerjee S, et al. Use of drug-eluting stents for chronic total occlusions: a systematic review and meta-analysis. Catheter Cardiovasc Interv. 2011;77:315–32.
12 Colmenarez HJ, Escaned J, Fernandez C, Lobo L, Cano S, del Angel JG, et al. Efficacy and safety of drug-eluting stents in chronic total coronary occlusion recanalization: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1854–66.
13 Mehilli J, Pache J, Abdel-Wahab M, Schulz S, Byrne RA, Tiroch K, et al. Drug-eluting versus bare-metal stents in saphenous vein graft lesions (ISAR-CABG): a randomised controlled superiority trial. Lancet. 2011;378:1071–8.
14 Urban P, Abizaid A, Banning A, Bartorelli AL, Baux AC, Dzavik V, et al. Stent thrombosis and bleeding complications after implantation of sirolimus-eluting coronary stents in an unselected worldwide population: a report from the e-SELECT (Multi-Center Post-Market Surveillance) registry. J Am Coll Cardiol. 2011;57:1445–54.
15 Garg S, Serruys P, Onuma Y, Dorange C, Veldhof S, Miquel-Hebert K, et al. 3-year clinical follow-up of the XIENCE V everolimus-eluting coronary stent system in the treatment of patients with de novo coronary artery lesions: the SPIRIT II trial (Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients with de novo Native Coronary Artery Lesions). JACC Cardiovasc Interv. 2009;2:1190–8.
16 Stone GW, Midei M, Newman W, Sanz M, Hermiller JB, Williams J, et al. Randomized comparison of everolimus-eluting and paclitaxel-eluting stents: two-year clinical follow-up from the Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients with de novo Native Coronary Artery Lesions (SPIRIT) III trial. Circulation. 2009;119:680–6.
17 Diehm N, Katzen BT, Dick F, Kovacs M, Zemel G, Powell A, et al. Influence of stent type on hemodynamic depression after carotid artery stent placement. J Vasc Interv Radiol. 2008;19:23–30.
18 Tsetis D, Uberoi R. Quality improvement guidelines for endovascular treatment of iliac artery occlusive disease. Cardiovasc Intervent Radiol. 2008;31:238–45.
19 Ghanbari H, de Mel A, Seifalian AM. Cardiovascular application of polyhedral oligomeric silsesquioxane nanomaterials: a glimpse into prospective horizons. Int J Nanomedicine. 2011;6:775–86.
20 Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–51.
21 Serruys PW, Strauss BH, Beatt KJ, Bertrand ME, Puel J, Rickards AF, et al. Angiographic follow-up after placement of a self-expanding coronary-artery stent. N Engl J Med. 1991;324:13–7.
22 Aoki J, Lansky AJ, Mehran R, Moses J, Bertrand ME, McLaurin BT, et al. Early stent thrombosis in patients with acute coronary syndromes treated with drug-eluting and bare metal stents: the Acute Catheterization and Urgent Intervention Triage Strategy trial. Circulation. 2009;119:687–98.
23 Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schomig A, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370:937–48.
24 Virchow RLK. Gesammelte Abhandlungen zur Wissenschaftlichen Medicin. Frankfurt, Meidinger Sohn & Co., 1856. In, Virchow RLK. Thrombosis and Emboli (1846–1856). Matzdorff AC, Bell WR (transl). Canton, Science History Publications, 1998;110:5–11.
25 Martinelli I, Bucciarelli P, Mannucci PM. Thrombotic risk factors: basic pathophysiology. Crit Care Med. 2010;38(2 Suppl):S3–9.
26 Piazza G, Goldhaber SZ, Lessard DM, Goldberg RJ, Emery C, Spencer FA. Venous thromboembolism in patients with symptomatic atherosclerosis. Thromb Haemost. 2011;106:1095–102.
27 Montecucco F, Mach F. Should we focus on “venous vulnerability” instead of “plaque vulnerability” in symptomatic atherosclerotic patients? Thromb Haemost. 2011;106:995–6.
28 Wyss CA, Neidhart M, Altwegg L, Spanaus KS, Yonekawa K, Wischnewsky MB, et al. Cellular actors, Toll-like receptors, and local cytokine profile in acute coronary syndromes. Eur Heart J. 2010;31:1457–69.
29 Miller DD, Karim MA, Edwards WD, Schwartz RS. Relationship of vascular thrombosis and inflammatory leukocyte infiltration to neointimal growth following porcine coronary artery stent placement. Atherosclerosis. 1996;124:145–55.
30 Cook S, Ladich E, Nakazawa G, Eshtehardi P, Neidhart M, Vogel R, et al. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation. 2009;120:391–9.
31 Moir E, Booth NA, Bennett B, Robbie LA. Polymorphonuclear leucocytes mediate endogenous thrombus lysis via a u-PA-dependent mechanism. Br J Haematol. 2001;113:72–80.
32 Agostoni P, Vermeersch P, Knaapen M, Verheye S. Stent thrombosis is not always stent thrombosis: de novo atherosclerosis in a stented coronary segment. Int J Cardiol. 2010;144:e19–21.
33 Zavalloni D, Bossi P, Rossi ML, Gasparini GL, Lisignoli V, Presbitero P. Inflammatory substrate with eosinophils may be present in bare-metal stent thrombosis. J Cardiovasc Med (Hagerstown). 2009;10:942–3.
34 Honda Y, Fitzgerald PJ. Stent thrombosis: an issue revisited in a changing world. Circulation. 2003;108:2–5.
35 Luscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. 2007;115:1051–8.
36 Rogers C, Edelman ER. Endovascular stent design dictates experimental restenosis and thrombosis. Circulation. 1995;91:2995–3001.
37 Gurbel PA, Callahan KP, Malinin AI, Serebruany VL, Gillis J. Could stent design affect platelet activation? Results of the Platelet Activation in STenting (PAST) Study. J Invasive Cardiol. 2002;14:584–9.
38 Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–30.
39 Stone GW, Rizvi A, Newman W, Mastali K, Wang JC, Caputo R, et al. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med. 2010;362:1663–74.
40 Nebeker JR, Virmani R, Bennett CL, Hoffman JM, Samore MH, Alvarez J, et al. Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) project. J Am Coll Cardiol. 2006;47:175–81.
41 van Beusekom HM, Saia F, Zindler JD, Lemos PA, Swager-Ten Hoor SL, van Leeuwen MA, et al. Drug-eluting stents show delayed healing: paclitaxel more pronounced than sirolimus. Eur Heart J. 2007;28:974–9.
42 Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202.
43 Awata M, Kotani J, Uematsu M, Morozumi T, Watanabe T, Onishi T, et al. Serial angioscopic evidence of incomplete neointimal coverage after sirolimus-eluting stent implantation: comparison with bare-metal stents. Circulation. 2007;116:910–6.
44 Jensen LO, Maeng M, Kaltoft A, Thayssen P, Hansen HH, Bottcher M, et al. Stent thrombosis, myocardial infarction, and death after drug-eluting and bare-metal stent coronary interventions. J Am Coll Cardiol. 2007;50:463–70.
45 Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation. 2007;115:1440–55; discussion 55.
46 Wenaweser P, Daemen J, Zwahlen M, van Domburg R, Juni P, Vaina S, et al. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4-year results from a large 2-institutional cohort study. J Am Coll Cardiol. 2008;52:1134–40.
47 Kolandaivelu K, Swaminathan R, Gibson WJ, Kolachalama VB, Nguyen-Ehrenreich KL, Giddings VL, et al. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation. 2011;123:1400–9.
48 Schuhlen H, Kastrati A, Dirschinger J, Hausleiter J, Elezi S, Wehinger A, et al. Intracoronary stenting and risk for major adverse cardiac events during the first month. Circulation. 1998;98:104–11.
49 Cutlip DE, Baim DS, Ho KK, Popma JJ, Lansky AJ, Cohen DJ, et al. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation. 2001;103:1967–71.
50 Uren NG, Schwarzacher SP, Metz JA, Lee DP, Honda Y, Yeung AC, et al. Predictors and outcomes of stent thrombosis: an intravascular ultrasound registry. Eur Heart J. 2002;23:124–32.
51 Chieffo A, Bonizzoni E, Orlic D, Stankovic G, Rogacka R, Airoldi F, et al. Intraprocedural stent thrombosis during implantation of sirolimus-eluting stents. Circulation. 2004;109:2732–6.
52 Park DW, Park SW, Park KH, Lee BK, Kim YH, Lee CW, et al. Frequency of and risk factors for stent thrombosis after drug-eluting stent implantation during long-term follow-up. Am J Cardiol. 2006;98:352–6.
53 Walter DH, Schachinger V, Elsner M, Dimmeler S, Zeiher AM. Platelet glycoprotein IIIa polymorphisms and risk of coronary stent thrombosis. Lancet. 1997;350:1217–9.
54 McFadden EP, Stabile E, Regar E, Cheneau E, Ong AT, Kinnaird T, et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet. 2004;364:1519–21.
55 Eisenstein EL, Anstrom KJ, Kong DF, Shaw LK, Tuttle RH, Mark DB, et al. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA. 2007;297:159–68.
56 Wenaweser P, Dorffler-Melly J, Imboden K, Windecker S, Togni M, Meier B, et al. Stent thrombosis is associated with an impaired response to antiplatelet therapy. J Am Coll Cardiol. 2005;45:1748–52.
57 Korte W, Cattaneo M, Chassot PG, Eichinger S, von Heymann C, Hofmann N, et al. Peri-operative management of antiplatelet therapy in patients with coronary artery disease: joint position paper by members of the working group on Perioperative Haemostasis of the Society on Thrombosis and Haemostasis Research (GTH), the working group on Perioperative Coagulation of the Austrian Society for Anesthesiology, Resuscitation and Intensive Care (ÖGARI) and the Working Group Thrombosis of the European Society for Cardiology (ESC). Thromb Haemost. 2011;105:743–9.
58 Moussa I, Di Mario C, Reimers B, Akiyama T, Tobis J, Colombo A. Subacute stent thrombosis in the era of intravascular ultrasound-guided coronary stenting without anticoagulation: frequency, predictors and clinical outcome. J Am Coll Cardiol. 1997;29:6–12.
59 Holmes DR, Jr., Kereiakes DJ, Garg S, Serruys PW, Dehmer GJ, Ellis SG, et al. Stent thrombosis. J Am Coll Cardiol. 2010;56:1357–65.
60 Ong AT, Hoye A, Aoki J, van Mieghem CA, Rodriguez Granillo GA, Sonnenschein K, et al. Thirty-day incidence and six-month clinical outcome of thrombotic stent occlusion after bare-metal, sirolimus, or paclitaxel stent implantation. J Am Coll Cardiol. 2005;45:947–53.
61 Armstrong EJ, Yeo KK, Javed U, Mahmud E, Patel M, Shunk KA, et al. Angiographic stent thrombosis at coronary bifurcations short- and long-term prognosis. JACC Cardiovasc Interv. 2012;5:57–63.
62 Park DW, Yun SC, Lee JY, Kim WJ, Kang SJ, Lee SW, et al. C-reactive protein and the risk of stent thrombosis and cardiovascular events after drug-eluting stent implantation. Circulation. 2009;120:1987–95.
63 Virmani R, Guagliumi G, Farb A, Musumeci G, Grieco N, Motta T, et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation. 2004;109:701–5.
64 Croce K, Libby P. Intertwining of thrombosis and inflammation in atherosclerosis. Curr Opin Hematol. 2007;14:55–61.
65 Finn AV, Kolodgie FD, Harnek J, Guerrero LJ, Acampado E, Tefera K, et al. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation. 2005;112:270–8.
66 Mihos CG, Salas MJ, Santana O. The pleiotropic effects of the hydroxy-methyl-glutaryl-CoA reductase inhibitors in cardiovascular disease: a comprehensive review. Cardiol Rev. 2010;18:298–304.
67 Serruys PW, de Feyter P, Macaya C, Kokott N, Puel J, Vrolix M, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;287:3215–22.
68 Briguori C, Colombo A, Airoldi F, Violante A, Focaccio A, Balestrieri P, et al. Statin administration before percutaneous coronary intervention: impact on periprocedural myocardial infarction. Eur Heart J. 2004;25:1822–8.
69 Pasceri V, Patti G, Nusca A, Pristipino C, Richichi G, Di Sciascio G. Randomized trial of atorvastatin for reduction of myocardial damage during coronary intervention: results from the ARMYDA (Atorvastatin for Reduction of MYocardial Damage during Angioplasty) study. Circulation. 2004;110:674–8.
70 Walter DH, Fichtlscherer S, Britten MB, Rosin P, Auch-Schwelk W, Schachinger V, et al. Statin therapy, inflammation and recurrent coronary events in patients following coronary stent implantation. J Am Coll Cardiol. 2001;38:2006–12.
71 Zhu S, Viswambharan H, Gajanayake T, Ming XF, Yang Z. Sirolimus increases tissue factor expression but not activity in cultured human vascular smooth muscle cells. BMC Cardiovasc Disord. 2005;5:22.
72 van der Harst P, Groenewegen HC, Roks AJ, Buikema H, Zijlstra F, van Gilst WH, et al. Rosuvastatin attenuates angiotensin II-induced neointimal formation after stent implantation in the rat. Coron Artery Dis. 2008;19:47–53.
73 Fukuda D, Enomoto S, Shirakawa I, Nagai R, Sata M. Fluvastatin accelerates re-endothelialization impaired by local sirolimus treatment. Eur J Pharmacol. 2009;612:87–92.
74 Chan AW, Bhatt DL, Chew DP, Quinn MJ, Moliterno DJ, Topol EJ, et al. Early and sustained survival benefit associated with statin therapy at the time of percutaneous coronary intervention. Circulation. 2002;105:691–6.
75 Mood GR, Bavry AA, Roukoz H, Bhatt DL. Meta-analysis of the role of statin therapy in reducing myocardial infarction following elective percutaneous coronary intervention. Am J Cardiol. 2007;100:919–23.
76 Aronow HD, Strawderman RL, Moscucci M, Cowen ME. Duration of evidence-based medical therapy and the hazard for atherothrombotic events following percutaneous coronary intervention. Int J Cardiol. 2011;153:262–6.
77 Chua SK, Hung HF, Cheng JJ, Wang JH, Lo HM, Kuan P, et al. Incidence, predictors and outcomes of subacute stent thrombosis following primary stenting for ST-elevation myocardial infarction. J Formos Med Assoc. 2010;109:430–7.
78 Nishino M, Hoshida S, Kato H, Egami Y, Shutta R, Yamaguchi H, et al. Preprocedural statin administration can reduce thrombotic reaction after stent implantation. Circ J. 2008;72:232–7.
79 Lauer MS. Cardiovascular medicine update 2007: perioperative risk, carotid angioplasty, drug-eluting stents, stronger statins. Cleve Clin J Med. 2007;74:505–11.
80 Brophy JM, Babapulle MN, Costa V, Rinfret S. A pharmacoepidemiology study of the interaction between atorvastatin and clopidogrel after percutaneous coronary intervention. Am Heart J. 2006;152:263–9.
81 Evanchan J, Donnally MR, Binkley P, Mazzaferri E. Recurrence of acute myocardial infarction in patients discharged on clopidogrel and a proton pump inhibitor after stent placement for acute myocardial infarction. Clin Cardiol. 2010;33:168–71.
82 Wenaweser P, Windecker S, Billinger M, Cook S, Togni M, Meier B, et al. Effect of atorvastatin and pravastatin on platelet inhibition by aspirin and clopidogrel treatment in patients with coronary stent thrombosis. Am J Cardiol. 2007;99:353–6.
83 Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821–30.
84 Blagojevic A, Delaney JA, Levesque LE, Dendukuri N, Boivin JF, Brophy JM. Investigation of an interaction between statins and clopidogrel after percutaneous coronary intervention: a cohort study. Pharmacoepidemiol Drug Saf. 2009;18:362–9.
85 Rodés-Cabau J, Bertrand OF, Larose E, Déry JP, Rinfret S, Bagur R, et al. Comparison of plaque sealing with paclitaxel-eluting stents versus medical therapy for the treatment of moderate nonsignificant saphenous vein graft lesions: the moderate vein graft lesion stenting with the taxus stent and intravascular ultrasound (VELETI) pilot trial. Circulation. 2009;120:1978–86.
86 Almalla M, Schröder J, Deserno V, Vogt F, Koos R, Koch KC, et al. Long-term clinical outcome of sirolimus-eluting stent implantation in metabolic syndrome and diabetes. J Invasive Cardiol. 2010;22:317–21.
87 Miyamoto T, Niwa A, Sinoda T. State of percutaneous transluminal coronary artery angioplasty and effectiveness of low-density lipoprotein apheresis. Ther Apher. 2001;5:226–31.
88 Sakakura K, Kubo N, Ako J, Ikeda N, Funayama H, Hirahara T, et al. Clinical features of early recurrent myocardial infarction. Heart Vessels. 2009;24:347–51.
89 Montecucco F, Mach F. Update on statin-mediated anti-inflammatory activities in atherosclerosis. Semin Immunopathol. 2009;31:127–42.
90 Mobarrez F, He S, Bröijersen A, Wiklund B, Antovic A, Antovic J, et al. Atorvastatin reduces thrombin generation and expression of tissue factor, P-selectin and GPIIIa on platelet-derived microparticles in patients with peripheral arterial occlusive disease. Thromb Haemost. 2011;106:344–52.
91 Matetzky S, Fefer P, Shenkman B, Shechter M, Novikov I, Savion N, et al. Statins have an early antiplatelet effect in patients with acute myocardial infarction. Platelets. 2011;22:103–10.
92 Delhaye C, Sudre A, Lemesle G, Marechaux S, Broucqsault D, Hennache B, et al. Preprocedural high-sensitivity C-reactive protein predicts death or myocardial infarction but not target vessel revascularization or stent thrombosis after percutaneous coronary intervention. Cardiovasc Revasc Med. 2009;10:144–50.
93 Park DW, Lee SW, Yun SC, Song HG, Ahn JM, Lee JY, et al. A Point-of-Care Platelet Function Assay and C-Reactive Protein for Prediction of Major Cardiovascular Events After Drug-Eluting Stent Implantation. J Am Coll Cardiol. 2011;58:2630–9.
94 Arnaud C, Burger F, Steffens S, Veillard NR, Nguyen TH, Trono D, et al. Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes: new evidence for direct antiinflammatory effects of statins. Arterioscler Thromb Vasc Biol. 2005;25:1231–6.
95 Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr., Kastelein JJ, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373:1175–82.
96 Andrie RP, Bauriedel G, Braun P, Hopp HW, Nickenig G, Skowasch D. Increased expression of C-reactive protein and tissue factor in acute coronary syndrome lesions: Correlation with serum C-reactive protein, angioscopic findings, and modification by statins. Atherosclerosis. 2009;202:135–43.
97 Kaesemeyer W. Statin drug eluting stent (DES) for early stent thrombosis. Atherosclerosis. 2009;207:343.
98 Miyauchi K, Kasai T, Yokayama T, Aihara K, Kurata T, Kajimoto K, et al. Effectiveness of statin-eluting stent on early inflammatory response and neointimal thickness in a porcine coronary model. Circ J. 2008;72:832–8.
99 Mills EJ, Wu P, Chong G, Ghement I, Singh S, Akl EA, et al. Efficacy and safety of statin treatment for cardiovascular disease: a network meta-analysis of 170,255 patients from 76 randomized trials. QJM. 2011;104:109–24.
