Colonisation with Pseudomonas aeruginosa and antibiotic resistance patterns in COPD patients
DOI: https://doi.org/10.4414/smw.2012.13509
Kathrin
Engler, Kathrin
Mühlemann, Christian
Garzoni, Henrik
Pfahler, Thomas
Geiser, Christophe
von Garnier
Summary
QUESTIONS: P. aeruginosa infections are assumed to play a major role in the frequency of exacerbations and severity of chronic obstructive pulmonary disease (COPD). Colonisation with P. aeruginosa accelerates lung function decline, most probably due to more frequent exacerbations. In this retrospective study we aimed to determine the prevalence of colonisation with P. aeruginosa in COPD patients treated in a tertiary hospital centre.
METHODS: 112 patients diagnosed with COPD testing positive for P. aeruginosa in at least one respiratory sample during the study period (2004–2008) were retrospectively analysed to estimate GOLD stage-specific prevalences, colonisation patterns, morphology and antibiotic resistance profiles of P. aeruginosa strains.
RESULTS: Colonisation with P. aeruginosa was present in all COPD stages, but prevalence significantly increased with disease severity (GOLD 1: 0.7%, GOLD 2: 1.5%; GOLD 3: 1.5%; GOLD 4: 2.6%; p= 0.0003). 41% of COPD patients with P. aeruginosa-positive respiratory samples were chronic carriers, of whom 8% had mucoid strains. Carriage of a mucoid strain was associated with advanced COPD stage GOLD 4 (p= 0.01). Resistance to cephalosporins was most frequently encountered and resistance to ciprofloxacin was found in more advanced stages of COPD.
CONCLUSIONS: Colonisation with P. aeruginosa was present in all COPD severity stages and colonisation with mucoid strains was more frequent in advanced COPD. Resistance to the only oral anti-pseudomonas antibiotic ciprofloxacin was more frequently encountered in severe COPD stages.
Introduction
Bacterial infections play a central role in the pathogenesis and clinical course of chronic obstructive pulmonary disease (COPD) [1]. COPD is characterised by non-reversible obstruction of lower airways and its severity is graded according to the Global Initiative for Obstructive Lung Disease (GOLD) classification into four categories, ranging from stage 1 (mild) to 4 (severe) ( http://www.goldcopd.com ). In patients with a more advanced disease state, colonisation with bacteria during a clinically stable episode is common, subsequently increasing the risk of exacerbations, and a higher frequency of exacerbations is, in turn, known to accelerate lung function decline [2].
P. aeruginosa is a non-fermentative, gram-negative bacterium known for its capacity to generate resistances against broad-spectrum antibiotics [3, 4]. It is one of the most important pathogens in patients with chronic lung infections and is commonly found in severe COPD during exacerbations as well as during clinically stable periods [1].
Colonisation with P. aeruginosa has been reported to require a structurally altered mucosa, was associated with more advanced COPD, and has been identified as the most frequent bacterial pathogen in severe COPD stages [1, 5–7].
In COPD patients the majority of P. aeruginosa infections are probably transient in nature with a colonisation time of less than one month [1, 8, 9]. Once chronic infection is established, mucoid P. aeruginosa strains emerge, displaying higher mutation rates, less motility, and increased propensity to generate a bio-film [8, 10, 11]. The overall frequency of chronic infection with P. aeruginosa in all COPD stages, defined by Murphy et al. as a colonisation longer than 6 months, is low (22.8%) [12]. Mucoid strains emerge with time, rendering eradication increasingly difficult, if not impossible [1, 4, 10]. Moreover, hypermutation, a spontaneous mutation rate above normal levels, leads to multi-resistance [4, 11]. This correlates with higher antibiotic resistance rates in chronic infection [11]. Furthermore, patients with severe COPD have more frequent exacerbations and therefore undergo more antibiotic treatments that may select for resistant pathogens [1, 5, 8]. The presence of multi-resistant P. aeruginosa strains, defined as the absence of susceptibility to three or more antibiotic families, significantly increases 2-year mortality to 60%, as compared to 28% in patients with less resistant organisms [3, 13]. This is probably related to inappropriate choice of antibiotic treatment rather than increased clone virulence.
The aims of this single centre retrospective study were: first, to estimate the prevalence of P. aeruginosa in respiratory samples of COPD patients and, second, to analyse antibiotic resistance patterns in P. aeruginosa isolates from COPD patients.
Methods
Study design
The local ethics committee (Kantonale Ethikkommission, Bern) approved the study. All COPD patients treated at Bern University Hospital during the period from 1 January 2004 to 31December 2008, with isolation of P. aeruginosa from at least one respiratory tract sample (sputum, induced sputum, tracheo-bronchial aspirate, broncho-alveolar lavage) were eligible for our retrospective study.
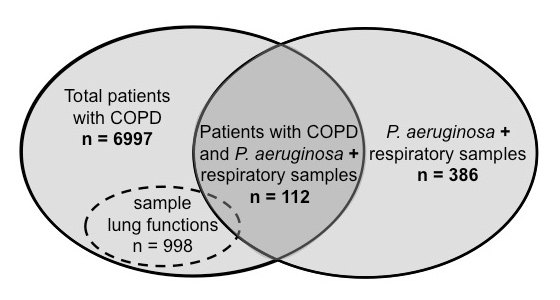
Figure 1
Overview of study population, sample lung function sub-population, and respiratory samples included in the analyses during the study period from 1 January 2004 to 31 December 2008. Total patients with COPD, patients either hospitalised or seen as outpatients between 1 January 2004 and 31 December 2008 at Bern University Hospital who had COPD as primary diagnosis or co-morbidity; lung functions, representative sample population of COPD patients who underwent lung function testing in the respiratory medicine department during the study period; P. aeruginosa + respiratory samples, isolates (sputum, induced sputum, tracheo-bronchial aspirate, broncho-alveolar lavage) from which P. aeruginosa was cultured.
In a first analysis, based on the ICD-10 database of the institutional coding department ProCod, we determined that n = 6,997 patients, either hospitalised or seen as outpatients between 1 January 2004 and 31 December 2008 at the Bern University Hospital, had COPD as the primary diagnosis or as a co-morbidity. The diagnosis of COPD was based on the ICD-10 codes for emphysema (ICD-10 code J43) and other chronic obstructive pulmonary diseases (ICD-10 code J44).
In a second analysis we identified n = 386 patients colonised with P. aeruginosa through the laboratory database of the Institute of Infectious Diseases, University of Bern.
In a third analysis, patients identified by laboratory database were matched with hospital ICD-10 statistics from ProCod. From n = 386 patients colonised with P. aeruginosa, COPD was also diagnosed in n = 112. An overview of the patient populations included for analyses is given in figure 1. Records from COPD patients in whom P. aeruginosa was isolated in at least one respiratory sample were analysed for socio-demographic and clinical characteristics, including severity of COPD according to GOLD classification ( http://www.goldcopd.com ), and P. aeruginosa colonisation patterns. In patients colonised by P. aeruginosa but not suffering from COPD the underlying diagnoses were also obtained. Microbiological data on bacterial colony characteristics and antibiotic resistances were retrieved from the laboratory database. Chronic P. aeruginosa colonisation was defined as at least two positive respiratory samples isolated more than one month apart. Resistance to anti-pseudomonas antibiotics was categorised in five groups, i.e. (1) extended spectrum penicillins, (2) monobactams and cephalosporins, (3) carbapenems, (4) aminoglycosides and (5) quinolones, as summarised in table 1.
To determine GOLD stage specific P. aeruginosa colonisation prevalences, we stratified GOLD stages in a representative sample population of n = 998 COPD patients who underwent lung function testing in the respiratory medicine department during the study period (fig. 1). The reason for adopting this approach was that complete lung function data was not sufficiently available for the 6,997 COPD patients. The GOLD stage distribution derived from this sample population made it possible to calculate the expected numbers of individuals within each GOLD stage for the total of n = 6,997 COPD patients seen during the study period at our institution. In a final step, specific prevalences for P. aeruginosa colonisation for each GOLD stage were estimated on the basis of the number of respiratory samples positive for P. aeruginosa (for which GOLD stages were known). Details of the algorithms for the analyses performed are shown in figure 2.
Statistics
Statistical analyses were performed using the two-tailed Chi-square test and a Chi-square test for trend with Prism (Graph Pad, San Diego). A p-value <0.05 was considered statistically significant.
|
Table 1: Classes of anti-pseudomonas antibiotics. |
|
Antibiotic
|
Class
|
| Ticarcillin
Ticarcillin / Clavulanic acid
Piperacillin
Piperacillin / Tazobactam |
Extended spectrum penicillins |
| Ceftazidim
Cefepime
Aztreonam |
Monobactams and Cephalosporins |
| Imipenem
Meropenem |
Carbapenems |
| Gentamicin
Amikacin
Netilmicin
Tobramycin |
Aminoglycosides |
| Ciprofloxacin |
Fluoroquinolones |
Results
Study population
A total of n = 386 patients in whom P. aeruginosa was detected in respiratory samples were analysed, of whom n = 112 (29.0%) were previously diagnosed with COPD (fig. 1). At the time when culture was performed, 80% of the COPD patients were hospitalised and approx. one third were admitted to intensive care (table 2). Of n = 274 patients colonised with P. aeruginosa but with no diagnosis of COPD, n = 165 (42.7%) were treated in the intensive care unit (ICU). One third of this group (n = 118, 30.6%) had a respiratory disease (other than COPD), most frequently pneumonia in n = 68 (17.6%) cases (data not shown). 72 patients (18.6%) not hospitalised in the ICU and without the diagnosis of COPD had another respiratory diagnosis, most frequently pneumonia (n = 36, 9.3%), bronchiectasis (n = 13, 3.4%), pleural effusion/empyema (n = 10, 2.6%), asthma/bronchitis (n = 7, 1.8%), or interstitial lung disease (n = 6, 1.6%) (data not shown).
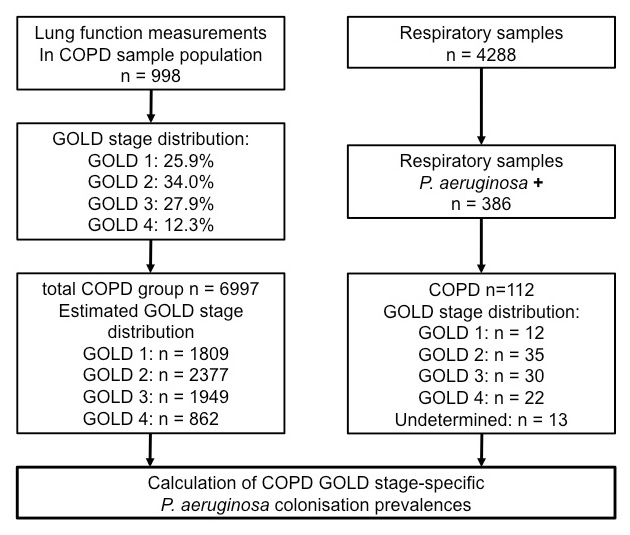
Figure 2
Algorithm used to perform analyses and estimations of prevalences. Patient populations, lung functions and respiratory samples were defined in the methods section. COPD severity was graded according to the GOLD classification as detailed in the methods section.
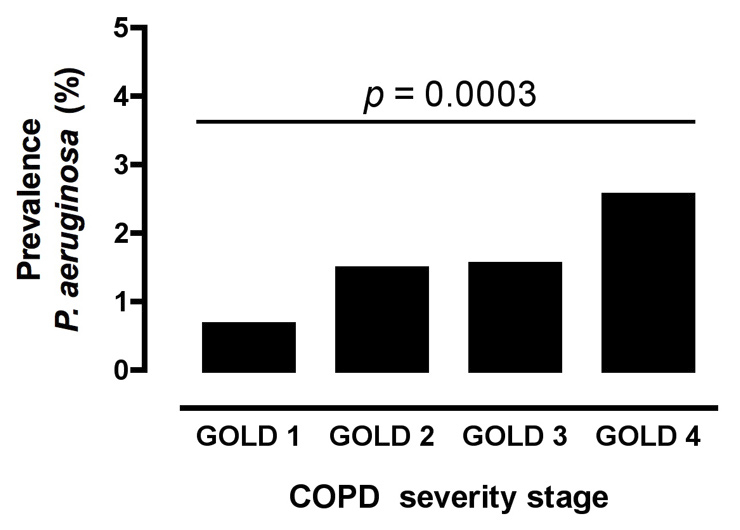
Figure 3
Estimated prevalences of P. aeruginosa colonisation in COPD GOLD stages 1–4. Prevalences were estimated as outlined in the Methods section and in figures 1–2. The p-value was obtained with the Chi-square test for trend comparing all GOLD stages for colonisation with P. aeruginosa in the COPD cohort of n = 6,997 patients.
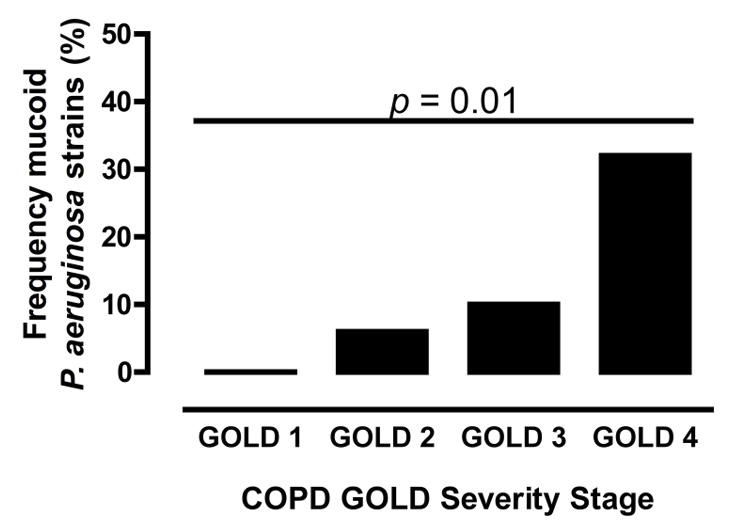
Figure 4
Frequency of mucoid phenotype in P. aeruginosa isolates from COPD patients stratified according to severity of disease (GOLD1-4). p-values were obtainedutilising the Chi-square test.
In n = 37 patients (9.6%) without a respiratory diagnosis and not hospitalised on the ICU, the most frequently reported disorders were cancer (n = 14, 3.6%), gastrointestinal or renal diseases (n = 10, 2.6%), heart disease or transplantation (kidney, heart) (n = 4 each, 1.0%), neurological disease (n = 3, 0.8%), and others (n = 2, 0.5%) (data not shown).
Prevalence of P. aeruginosa colonisation in COPD patients
According to the database of the institutional coding department, during the study period a total of n = 6,997 patients with COPD as primary diagnosis or co-morbidity had been treated at Bern University Hospital. Respiratory samples were obtained from approximately two thirds (61.3%), i.e. from n = 4,288 COPD patients (conventional sputum (n = 399), induced sputum (n = 14), tracheo-bronchial aspirates (n = 380), broncho-alveolar lavage (n = 134), not further specified (n = 3,361)) (data not shown).
In n = 112 COPD patients in whom P. aeruginosa isolated in respiratory samples (54 conventional sputum, 5 induced sputum, 41 tracheo-bronchial aspirates, 12 broncho-alveolar lavage) (table 2), the distribution of COPD GOLD severity stages was: n = 12 (10.7%) GOLD 1, n = 35 (31.2%) GOLD 2, n = 30 (26.8%) GOLD 3, and n = 22 (19.6%) GOLD 4 (fig. 2). Despite an extensive search in lung function data, as well as contact with the treating physician, and/or general practitioner responsible for the patient, the degree of COPD severity could not be determined in n = 13 cases (12%).
To calculate the prevalences of P. aeruginosa colonisation within different COPD severity stages, the relative distribution of GOLD stages was estimated on the basis of a sample of n = 998 well-characterised COPD patients who underwent lung function testing at our institution during the study period (fig. 2). Estimated stage-specific prevalence rates for colonisation with P. aeruginosa in the total COPD cohort were: GOLD 1: 0.7%, GOLD 2: 1.5%; GOLD 3: 1.5%; GOLD 4: 2.6%. There was a significant association between severity of COPD and prevalence of colonisation (Chi square test for trend p= 0.0003) (fig. 3).
In our analysis we defined chronic P. aeruginosa colonisation as at least two positive respiratory samples more than one month apart. Based on this definition, n = 46 (41.1%) COPD patients with P. aeruginosa isolated from respiratory samples were chronically colonised. In this COPD population, the prevalence of chronic carriage in different GOLD COPD stages was as follows: n = 2 (0.1%) GOLD 1; n = 18 (0.8%) GOLD 2; n = 11 (0.6%) GOLD 3; n = 12 (1.4%) GOLD 4. The frequency of mucoid strain carriage in different GOLD stages was significantly associated with severity of COPD (fig. 4).
Antibiotic resistance patterns in P. aeruginosa-colonised COPD patients
P. aeruginosa isolates were routinely analysed for resistance patterns to different anti-pseudomonas antibiotic classes (table 1). In one case no information on antibiotic resistances was available.
The 112 COPD patients analysed were all colonised with non-mucoid P. aeruginosa strains, with evidence for colonisation with an additional mucoid strain in 12 patients. Among non-mucoid P. aeruginosa strains (n = 112), 58% (n = 65) of the isolates obtained from COPD patients were sensitive to all antibiotics tested (pan-sensitive) and resistances were distributed as follows: n = 27 (24.1%) resistant to one antibiotic group; n = 7 (6.3%) resistant to two antibiotic groups; n = 10 (8.9%) resistant to three groups; and n = 2 (1.8%) resistant to four groups (fig. 5). Analysis of the mucoid P. aeruginosa strains (n = 12) showed that n = 5 (41.6%) of the pathogens were sensitive to all antibiotic groups and relatively more strains were resistant to three or more antibiotic groups than in non-mucoid strains (fig. 5).
In n = 46 patients with chronic P. aeruginosa colonisation, n = 19 bacterial isolates (41.3%) were sensitive to all antibiotics, n = 12 (26.1%) had strain resistances to one antibiotic group, n = 5 (10.9%) to two antibiotic groups, n = 8 (17.4%) to three antibiotic groups, and n = 2 (4.3%) to four antibiotic groups (data not shown). In resistant P. aeruginosa isolates (n = 74), resistance to monobactams and cephalosporins (ceftazidim, cefepime, and aztreonam) occurred most frequently, i.e. in n = 28 isolates (37.4%), whereas resistance to aminoglycosides (gentamicin, amikacin, netilmicin, and tobramycin) was observed in n = 16 isolates (21.6%) (fig. 6). Resistances to the other anti-pseudomonas antibiotic classes occurred at similar frequencies: extended spectrum penicillins (n = 9; 12.2%), carbapenems (n = 11; 14.9%), and fluoroquinolones (n = 10; 13.5%). In COPD GOLD 4, resistance to ciprofloxacin (fluoroquinolones) was the most frequent resistance (60.0%) encountered (fig. 6). Due to low numbers (i.e. GOLD 1: n = 0; GOLD 2: n = 3; GOLD 3: n = 1; GOLD 4: n = 6) there is a fluctuation for ciprofloxacin resistance between GOLD 2 and GOLD 4 in figure 6.
In summary, this data showed that chronic colonisation, mucoid strains, and more extensive antibiotic resistances occurred in patients with advanced COPD GOLD stages.
|
Table 2: Characteristics of COPD study population with P. aeruginosa identified in a respiratory sample. |
|
|
COPD No. (%)
|
| Patients |
112 |
| Sex (male) |
89 (79) |
| Mean age (yrs), SD |
67, (11) |
| Hospitalised |
90 (80) |
| ICU |
36 (32) |
| Respiratory sample:
Sputum
Induced sputum
Tracheo-bronchial aspirate
Broncho-alveolar lavage |
54 (48)
5 (4)
41 (37)
12 (11) |
Discussion
This retrospective analysis performed during the study period January 2004 – December 2008 showed that 1.4% of COPD patients in our institution were colonised with P. aeruginosa. Though colonisation prevalences were highest in advanced COPD GOLD 4 (2.6%), P. aeruginosa was also isolated in less severe COPD stages, i.e. in 0.7% GOLD 1 and 1.5% GOLD 2. The overall colonisation prevalence of 1.4% P. aeruginosa in patients with COPD found in our retrospective study is lower than the reported prevalences published by other authors (3–15%) [1, 4, 5, 8, 11, 12, 14–16]. There are several possible explanations for our lower prevalence: first, the retrospective nature of our study might have introduced selection biases. Inclusion of patients on the basis of their positive sputum isolates implies that only those suffering from a disease or worsening of their clinical condition severe enough to warrant hospital admission, in our study mainly inpatients (80%), were analysed. A significant number of COPD outpatients with less severe disease but colonisation withP. aeruginosa may not have been considered in this hospital-based study and may account for higher prevalences observed in other studies in which sputum samples were routinely analysed in all COPD patients. Second, prevalence of P. aeruginosa may vary due to epidemiological factors, national antibiotic prescription policies and/or different attitudes towards management of P. aeruginosa colonisation in COPD patients, accounting for regional differences observed.
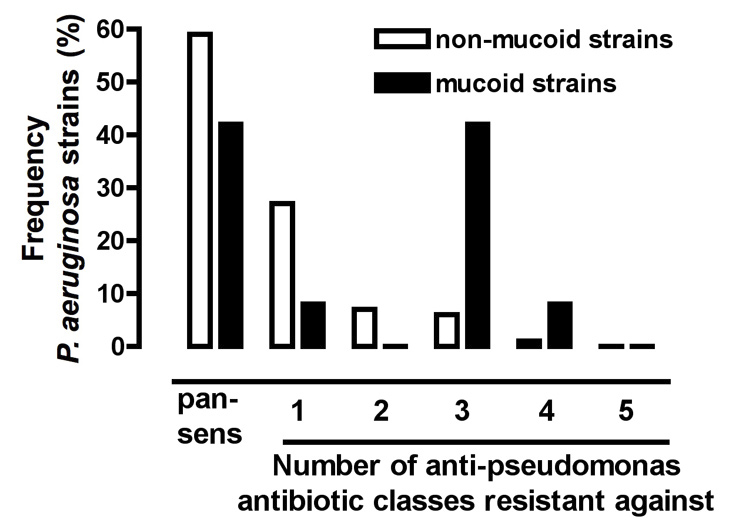
Figure 5
Frequency of resistances against 5 anti-pseudomonas antibiotic classes in 112 COPD patients, all colonised with non-mucoid (n = 112) and some additionally with mucoid (n = 12) P. aeruginosa strains.The anti-pseudomonas antibiotic classes tested are detailed in the Methods section and in table 1. Pan-sens, pan-sensitive P. aeruginosa isolates.
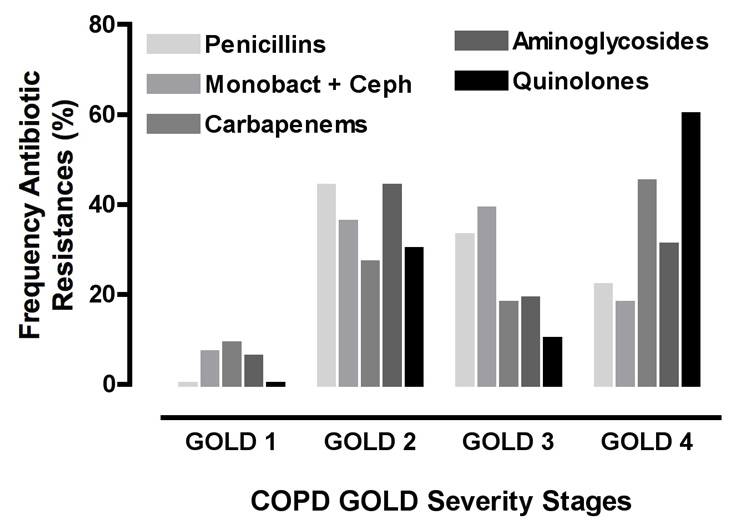
Figure 6
Frequency of resistances against anti-pseudomonas antibiotic classes in different COPD stages (GOLD 1-4) for patients colonised with P. aeruginosa (n = 112). Anti-pseudomonas antibiotic groups: Penicillins, extended spectrum penicillins (Ticarcillin, Ticarcillin/Clavulanic acid, Piperacillin, Piperacillin /Tazobactam), Monobact + Ceph, monobactams and cephalosporins, (Ceftazidim, Cefepime, Aztreonam), carbapenems (Imipenem, Meropenem), aminoglycosides (Gentamicin, Amikacin, Netilmicin, Tobramycin), and quinolones, fluoroquinolones (Ciprofloxacin).
The exact prevalence of COPD in Switzerland is currently unclear. Russi et al. estimated that more than 350,000 patients suffer from this disease, corresponding to a prevalence of about 4.8% in the year 2002 [17]. The baseline characteristics of participants in SAPALDIA study 1 and 2 (1991, 2002) showed a prevalence of 7.6%, including both symptomatic and asymptomatic patients [18]. On the basis of a prevalence of 4.8–7.6% for COPD in the Swiss population (7.7 million in 2008), it can be expected that as many as 5,174–8,192 COPD patients may currently be colonised with P. aeruginosa, when considering a conservative overall colonisation rate of 1.4%. Jochmann et al. estimated the distribution of different COPD severity stages in Switzerland in n = 346 COPD outpatients, showing a stage distribution from GOLD 1 to 4 of 8%, 45%, 38%, and 9%, respectively [19]. In comparison, our study population of n = 998 inpatients showed a GOLD 1 to 4 stratification of 26%, 34%, 28%, and 12% respectively. Though several factors may account for the differences observed, the most obvious one is that unequal patient populations were analysed, i.e. outpatients versus a majority of inpatients (80%) in our COPD population. In the latter population, patients had been admitted for a variety of reasons, explaining the higher rates of GOLD 1 stages in our study, whereas only outpatients in more advanced GOLD stages would have consulted in the study by Jochmann et al., introducing a bias towards GOLD stages 2 and 3.
Two salient findings emerge from our study: first, stage-specific distribution of prevalence rates for colonisation with P. aeruginosa showed that about 0.7% of GOLD 1, 1.5% GOLD 2 / 3, and 2.6% of GOLD 4 were affected. Second, as much as 40% of P. aeruginosa colonisation occurred in COPD patients with mild and moderately severe stages (GOLD 1+2). These data contrast with figures reported by other authors, i.e. that P. aeruginosa colonisation predominantly occurred in more advanced disease (GOLD 3 and 4) [1, 5–8, 12, 16, 20]. Only a few studies described infection with P. aeruginosa in less severe stages [2, 7, 14, 21]. In patients with mild COPD stages (GOLD 1), P. aeruginosa was predominantly limited to those who were mechanically ventilated [22]. Consistent with this fact, our study shows that out of n = 47 patients with COPD GOLD 1 or GOLD 2 infected with P. aeruginosa, n = 17 (36%) had received prior ICU treatment. Furthermore, as already discussed above, our data were obtained from patients with a clinical condition severe enough to warrant hospital admission.
Despite the shortcomings of our retrospective analysis, we found that 41% of COPD patients with P. aeruginosa isolated from a respiratory sample were chronically colonised – a figure situated within the wide range of 20-66% previously reported in the literature [1, 4, 8, 11, 14]. In agreement with Murphy et al., we also found that chronic colonisation occurred more frequently in severe COPD (GOLD 4) disease [1].
Consistent with findings previously reported by Garcia-Vidal et al., sensitivity testing to antibiotics showed that 58% of the P. aeruginosa isolates were pan-sensitive, with no evidence of pan-resistant strains [6]. Sensitivity to antibiotics was affected by the chronicity of colonisation, as illustrated in our study in which 42% of the isolates in chronically colonised patients were resistant to at least one anti-pseudomonas antibiotic. One explanation for this finding may be hypermutation such as frequently occurs in mucoid strains [4]. A limitation of our retrospective study is that we could not obtain data on the number, timing and duration of previous antibiotic treatments to understand the association with increased GOLD stage and antibiotic resistance.
The resistance (38%) against monobactams and cephalosporins most frequently detected in our study was previously also described by Miravitlles et al. [5]. Contrary to our findings, bacterial strains in this study were mostly sensitive to ciprofloxacin [5]. Miravitlles et al. also reported that sensitivity to aminoglycosides was maintained in multi-resistant strains and therefore aminoglycosides may represent the treatment of choice in these cases [5]. Our results, however, showed that resistances to aminoglycosides occurred most frequently after those against monobactams / cephalosporins and may therefore influence the choice of antibiotics used in this situation. It is most likely that these discrepancies are due to varying antibiotic prescription policies in different countries that may select for specific antibiotic resistance patterns.
Our retrospective study has several noteworthy limitations, i.e. its retrospective nature, as well as lack of serial lung function, systematic serial evaluation of colonisation for P. aeruginosa-positive patients, and information on previous antibiotic treatments. Nevertheless, despite these shortcomings, the underlying data strongly suggest that the colonisation with P. aeruginosa occurs in all severity stages of COPD, but prevalence increases with disease severity. Though colonisation with P. aeruginosa occurred most frequently in severe COPD stages, it may also manifest early in the course of the disease. Lung function decline in COPD is accelerated by exacerbations that are more frequent with P. aeruginosa infection and/or colonisation. An important clinical corollary is therefore to diagnose, treat, and attempt eradication of P. aeruginosa infection as early as possible during the course of COPD.
In clinical practice, based on the assumption and empirical observation that in vitro resistances do not always correlate with the observed clinical response to an anti-pseudomonas antibiotic, some clinicians may attempt treatment with an antibiotic to which isolates are resistant. Although this clinical attitude may be justified in the light of previously observed responses to an anti-pseudomonas antibiotic in a given patient, resistances may significantly limit the effectiveness of these agents. Therefore, if patients do not respond to anti-pseudomonas monobactams / cephalosporins, or ciprofloxacin, alternative antibiotic regimens should be administered in these situations, such as the use of i.v. antibiotics in combination with inhaled colistin or tobramycin.
In conclusion, the underlying retrospective study allows several important conclusions for physicians treating COPD patients: First, based on patient characteristics (i.e. COPD severity), P. aeruginosa colonisation increases in frequency in severe COPD, although colonisation may occur in the early stages. Second, this should prompt physicians to consider, search, and treat P. aeruginosa if clinical response to therapy is inadequate in all COPD stages in the presence of acute exacerbations. Third, as P. aeruginosa is related to increased exacerbations and accelerated lung function decline in COPD patients, it is important to search for P. aeruginosa when a patient has frequent exacerbations despite appropriate treatment. Fourth, resistance to ciprofloxacin, most frequently found in severe COPD, may influence empiric antibiotic treatment offered to patients in this context.
References
1 Murphy TF. Pseudomonas aeruginosa in adults with chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2009;15(2):138–42.
2 Wilkinson TM, Patel IS, Wilks M, Donaldson GC, Wedzicha JA. Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167(8):1090–5.
3 Montero M, Domínguez M, Orozco-Levi M, Salvadó M, Knobel H. Mortality of COPD patients infected with multi-resistant Pseudomonas aeruginosa: a case and control study. Infection. 2009;37(1):16–9.
4 Macia MD, Blanquer D, Togores B, Sauleda J, Pérez JL, Oliver A. Hypermutation is a key factor in development of multiple-antimicrobial resistance in Pseudomonas aeruginosa strains causing chronic lung infections. Antimicrob Agents Chemother. 2005;49(8):3382–6.
5 Miravitlles M, Espinosa C, Fernández-Laso E, Martos JA, Maldonado JA, Gallego M, and the Study Group of Bacterial Infection in COPD. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Study Group of Bacterial Infection in COPD. Chest. 1999;116(1):40–6.
6 Garcia-Vidal C, Almagro P, Romaní V, Rodríguez-Carballeira M, Cuchi E, Canales L, et al. Pseudomonas aeruginosa in patients hospitalized for COPD exacerbation: a prospective study. Eur Respir J. 2009;34:1072–8.
7 Eller J, Ede A, Schaberg T, Niederman MS, Mauch H, Lode H. Infective exacerbations of chronic bronchitis: relation between bacteriologic etiology and lung function. Chest. 1998;113(6):1542–8.
8 Murphy TF, Brauer AL, Eschberger K, et al. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177(8):853–60.
9 Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359(22):2355–65.
10 Juan C, Gutiérrez O, Renom F, Garau M, Gallegos C, Albertí S, et al. Chronic respiratory infections by mucoid carbapenemase-producing Pseudomonas aeruginosa strains, a new potential public health problem. Antimicrob Agents Chemother. 2008;52(6):2285–6.
11 Martinez-Solano L, Macia MD, Fajardo A, Oliver A, Martinez JL. Chronic Pseudomonas aeruginosa infection in chronic obstructive pulmonary disease. Clin Infect Dis. 2008;47(12):1526–33.
12 Murphy TF. The many faces of Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Clin Infect Dis. 2008;47(12):1534–6.
13 Montero M, Horcajada JP, Sorlí L, Alvarez-Lerma F, et al. Effectiveness and safety of colistin for the treatment of multidrug-resistant Pseudomonas aeruginosa infections. Infection. 2009;37(5):461–5.
14 Soler N, Ewig S, Torres A, Filella X, Gonzalez J, Zaubet A. Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur Respir J. 1999;14(5):1015–22.
15 Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173(10):1114–21.
16 Alamoudi OS. Bacterial infection and risk factors in outpatients with acute exacerbation of chronic obstructive pulmonary disease: a 2-year prospective study. Respirology. 2007;12(2):283–7.
17 Russi EW, Leuenberger P, Brändli O, Frey JG, Grebski E, Gugger M, et al. Management of chronic obstructive pulmonary disease: the Swiss guidelines. Official guidelines of the Swiss Respiratory Society. Swiss Med Wkly. 2002;132(5-6):67–78.
18 Brutsche MH, Downs SH, Schindler C, Gerbase MW, Schwartz J, Frey M, et al. Bronchial hyperresponsiveness and the development of asthma and COPD in asymptomatic individuals: SAPALDIA cohort study. Thorax. 2006;61(8):671–7.
19 Jochmann A, Neubauer F, Miedinger D, Schafroth S, Tamm M, Leuppi JD. General practitioner’s adherence to the COPD GOLD guidelines: baseline data of the Swiss COPD Cohort Study. Swiss Med Wkly. 2010;140:w13053.
20 Donaldson GC, Seemungal TA, Patel IS, Bhowmik A, Wilkinson TM, Hurst JR, et al. Airway and systemic inflammation and decline in lung function in patients with COPD. Chest. 2005;128(4):1995–2004.
21 Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–52.
22 Soler N, Torres A, Ewig S, Gonzalez J, Celis R, El-Ebiary M, et al. Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilation. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1498–505.





