
DOI: https://doi.org/10.4414/smw.2012.13513
Not even two decades ago, two case series – the first originated from the USA, the second from Switzerland and was published in this Journal – independently described adult patientssuffering clinically from dysphagia associated histologically with an eosinophil-predominant infiltration. This pattern was immediately recognised as being different from GERD and was therefore labeled primary or idiopathic eosinophilic esophagitis [1, 2]. The presentation of dysphagia and food impaction in atopic individuals with endoscopic findings of esophageal rings and longitudinal furrows was distinct from the heartburn, regurgitation, and erosive esophagitis that typified GERD. Not more than one year later, Kelly and colleagues reported on a series of allergic childrensuffering from severeGERD-like symptoms refractoryto medical or surgery therapy. These pediatric patients again had a relevant infiltration of the esophagus with eosinophils and responded to treatment with a hypo-allergic diet [3]. Further studies demonstrated that these two different clinical presentations were probably two sides of the same coin, a clinicopathological disease later termed eosinophilic esophagitis (EoE) [4, 5].
EoE affects individuals at any age [6] but has a striking male predominance [1, 2, 4, 5]. Originally, EoE was considered more of a rare curiosity than an epidemiologically relevant disease [4, 5]. However, in recent years, adult and pediatric gastroenterologists observed a dramatic increase in the number of newly-diagnosed EoE cases [6, 7]. This observation raised the question of whether the occurrence of EoE was truly increasing and the disease affecting more individuals, or whether the diagnosis was simply more frequently made as a consequence of heightened awareness by health care providers. Results from a recently published population-based long-term study have clarified this uncertainty and demonstrate that the accelerated EoE incidence represents, in fact, a true increase and not merely the consequence of greater disease awareness by gastroenterologists [8]. Currently, this chronic disease affects in westernised areas between 40 and 55 individuals per 100,000 inhabitants [7, 8]; this prevalence is comparable with those of Crohn’s disease. However, whether this current increase is an ongoing or only a temporary phenomenon is not yet known.
The clinical manifestation of EoE is strongly age-related [9] and there are therefore substantial differences in EoE symptom presentation between children and adults. In neonates and infants, food refusal is a common symptom of EoE and may denote dysphagia [9], which cannot be easily expressed in this age group. Children often complain of GERD-like symptoms, such as heartburn and reflux (range of frequency 5–82%), vomiting (range 5–68%) and abdominal pain (range 8–100%). Dysphagia and food impaction are reported increasingly with age. Less frequently, children present with failure to thrive, chest pain and diarrhea.
EoE in adolescents and adults presents with a narrow spectrum of symptoms, mainly with dysphagia for solids (range of frequency 29–100%) and food impaction (range 25–100%) [10]. A minority of adult patients reports GERD-like symptoms, non-swallowing-related chest pain and upper abdominal pain (see table 1). In summary, EoE should be considered in young children with GERD-like symptoms and feeding problems, whereas in older children and adults a history of, dysphagia for solids, food impaction or refractory retrosternal pain should raise suspicion.
Physical examination and standard laboratory analyses are typically normal [4, 5] except a mild peripheral eosinophilia and elevated total IgE values, which are found in up to 50% and 70% of EoE patients, respectively [4, 5].
Upper endoscopy is the first diagnostic step in evaluating individuals with dysphagia [11]. Although there is neither a pathognomonic endoscopic sign nor a typical pattern of abnormalities associated with EoE, a considerable number of different endoscopic features may be observed. These signs usually appear in random combination and represent evidence of active inflammation with mucosal edema (furrows, exudates, etc.) or chronic inflammation with tissue remodelling (crêpe-paper mucosa, corrugated rings, stricture) (fig. 1) [4, 5]. These abnormalities occur in children and adults, but experience suggests that evidence of active inflammation occurs more often in children whereas manifestations of chronic inflammation are observed more frequently in adults. Of note, if present these signs are suggestive of the diagnosis of EoE, but endoscopy is often confusing or even misleading and a history of dysphagia is a clear indication for biopsy sampling, even in an normal appearing esophagus [4, 5].
The healthy esophagus is devoid of eosinophils [12]. In contrast, a relevant infiltration of the esophageal epithelium with eosinophils is the leading sign of EoE and the quantity of these late-inflammatory cells is a crucial component in the diagnosis of this disorder. In addition, several other inflammatory cells such as T helper cells, mast cells [13] and regulatory T cells are found in EoE biopsies [14, 15]. The pattern of inflammatory cells and mediators involved in EoE corresponds to a Th2 type inflammation [13]. Furthermore, a number of structural abnormalities of the esophageal mucosa, for instance superficial layering, basal zone hyperplasia, papillary elongation and lamina propria fibrosis are seen regularly in EoE biopsies and have to be taken into account in the histopathologic evaluation [4, 5]. Of note, pathologic features of EoE are characteristic, but not specific because the etiology of the epithelial eosinophilic inflammation cannot be determined solely from microscopy (fig. 2).



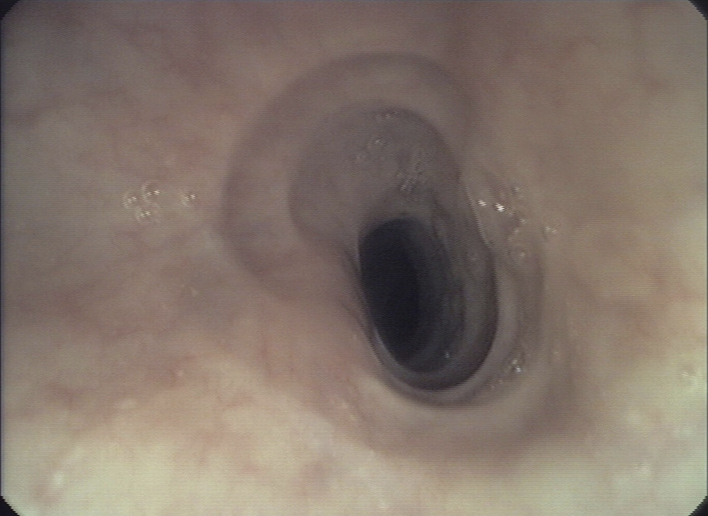
Figure 1
Endoscopic pictures illustrating the major endoscopic signs of EoE:
Panel A illustrates the endoscopic appearance of a healthy esophagus. Panel B represents a moderate inflamed esophagus with edema, longitudinal furrowing and mild non-stenosing trachealisation. Panel C shows a severe inflamed esophagus with edema, white exudates and deep furrowing. Panel D illustrate signs associated with remodeling such as stricture due to fixed esophageal rings.
Recently an international panel of EoE experts has published the following conceptual definition: “EoE represents a chronic, immune-mediated esophageal disease, characterised clinically by symptoms related to esophageal dysfunction and histologically by an eosinophil-predominant inflammation” [5]. This definition implies that for the confirmation of the diagnosis EoE the clinician as well as the pathologist are required. Neither symptoms of an esophageal dysfunction nor an esophageal eosinophilia are exclusive to EoE; both can also be associated with other diseases, such as gastro-oesophageal reflux disease (GERD), Crohn’s disease, vascular diseases, infectious esophagitis, drug-induced esophagitis, eosinophilic gastroenteritis and esophageal malignancies. The diagnosis of EoE is therefore a composite one and, exclusion of other diseases is mandatory before the EoE diagnosis can be established. Of note, endoscopic abnormalities are not included in this disease definition due to their huge variability.

Figure 2
Biopsy specimen illustrating the leading histopathologic signs of EoE:
The histograph, taken from an adult patient with active EoE, shows a dense infiltration of the squamous esophageal epithelium with eosinophils and dilated intercellular spaces. H&E staining, original magnification x200.
The clinical part of the diagnosis relies almost exclusively on the presence of esophageal symptoms, whereas physical examination, laboratory analyses and radiologic examinations have only a subsidiary value in the diagnostic work-up of EoE patients. Of note, the assessment of symptoms of an esophageal dysfunction requires a careful history in order to distinguish between dysphagia and globus sensation and between EoE-associated and reflux-induced retrosternal pain.
The histological part of the diagnosis is hampered by the patchy nature of the inflammation [16]. This characteristic has the consequence that the endoscopist has to perform a structured biopsy sampling and the pathologist a careful search of the biopsy specimens in order to prevent a misdiagnosis. In addition, the absolute number of eosinophils is a rigid parameter, and supplemental markers of eosinophil activation, for instance eosinophil degranulation, have been proposed for evaluation of an accurate EoE diagnosis [4, 5]. The diagnostic criteria of EoE are based on three pillars and are depicted in table 2.
| Table 1: Symptom presentation in Eosinophilic Esophagitis in relation to the age. | ||
| Symptoms | Children | Adults/adolescents |
| Food refusal | +++ | – |
| Vomiting/regurgitation | ++ | + |
| “GERD refractory to therapy” | +++ | + |
| Food impaction/foreign body impaction | + | ++ |
| Epigastric pain | ++ | + |
| Dysphagia | + | +++ |
| Failure to thrive | +++ | – |
| Table 2: Diagnostic criteria of Eosinophilic Esophagitis. | |
| Clinical manifestations | Symptoms of esophageal dysfunction |
| Histologic manifestations | Number of eosinophils >15 in at least one hpf |
| Exclusion criteria | Exclusion of GERD, clinically, endoscopically, histologically and if necessary by functional studies (e.g., pH-monitoring/impedance) Exclusion of other conditions that cause oesophageal eosinophilia |
| Adapted from Liacouras CA, et al. J Allergy Clin Immunol. 2011;128:3–20. | |
In the diagnostic work-up of esophageal eosinophilia EoE and GERD are the leading conditions which have to be considered. The diagnostic challenge is that these two inflammatory conditions can be related to each other in a complex way. Today at least the following four possible associations between EoE and GERD exist [17]:
GERD induces eosinophilic infiltration: The pathogenic hallmark of GERD lies in the exposure of the esophageal epithelium to gastric acid, which induces a chemokine release (e.g., platelet-activating factor, interleukin-4 or interleukin-13) that attracts eosinophils. Therefore, a mild tissue eosinophilia, mainly in the distal esophagus, is observed in GERD patients. In this situation, clinical symptoms, endoscopic findings and pH-monitoring are fully consistent with the diagnosis of GERD. Thus, a therapy with PPI can be started, leading to a decrease in the eosinophil infiltration.
EoE contributes to or causes GERD: The eosinophil inflammation may lead to an impaired function of the lower esophageal sphincter, induced either by acute inflammation or by fibrosis. This leads to secondary gastro-oesophageal reflux. In this situation, the manifestation of the disease is dominated by symptoms and signs of EoE and, on endoscopy, typical signs of GERD are absent. However, patients present with some signs of GERD and pH-monitoring documents a pathologic reflux. Patients falling into this category should be treated with a combination of anti-eosinophil and acid-suppressive medication.
Non-causative co-existence of GERD and EoE: GERD represents one of the most common gastrointestinal diseases in the population of the Western hemisphere with a prevalence of up to 20% [18]. Since EoE has a cumulative prevalence of 40 patients per 100,000 inhabitants [8], it can be expected that at least 10 to 20% of patients with EoE may also have co-existing GERD.
PPI-responsive EoE: One published case series indicates the existence of an EoE subgroup, presenting with typical symptoms and signs of EoE, but with a response of symptoms and eosinophil infiltration under PPI [19]. Of note, this patient group has no GERD manifestations with respect to symptoms, endoscopic findings, or functional studies. Therefore, the new term PPI-responsive EoE might be appropriate to describe this patient subgroup. Physiological reflux might lead to barrier disruption that would predispose patients to EoE development (increased permeability for allergens). Another potential mechanism is that PPI’s are able to suppress epithelial secretion of eotaxin-3 independently of their effects on acid-secretion.
As a consequence of this complex interplay some patients might need additional examinations such as pH-Monitoring and impedance measurement in order to dissect EoE and GERD and to perform an appropriate treatment.
Our understanding of EoE pathogenesis is still limited and an optimal treatment algorithm has not yet been defined. It is currently under debate whether physicians should focus on treatment of EoE symptoms only or whether they should also strive to achieve a histologic remission. Recent advances in understanding EoE’s natural history should help us answering these questions.
Solid food dysphagia and food impaction represent the two leading symptoms in adult EoE patients.A retrospective database analysis has demonstrated that more than one third of adult EoE patients (87 of 251) experienced long lasting food impactions requiring endoscopic intervention with bolus removal, and none of these patients was at the time point of the impaction adequately treated [10]. Furthermore, unbridled eosinophilic inflammation leads to development of fibrosis and angiogenesis. This tissue remodeling leads to a progressive loss of elasticity of the esophageal wall and luminal narrowing in patients and in animal models [20–22]. Upon treatment with the topical corticosteroid budesonide, the expression of the fibrosis-related markers, such as TGF-beta and Tenascin-C as well as the degree of fibrosis was markedly decreased in the esophageal tissue [21, 23]. It has recently been shown that anti-eosinophil treatment with topical corticosteroids even can reverse tissue remodelling [21, 23].
In summary, there are at least three good reasons to treat patients with clinically and histologically active EoE: 1) An improvement in the quality of life; 2) A reduction in the risk for severe esophageal injury by preventing long-lasting food impactions; and 3) A prevention of organ damage caused by the ongoing tissue remodeling.
Over the last 20 years, one of the major goals of EoE research was to develop an effective strategy for treatment of EoE. In this chapter, we present an overview of different treatment modalities. Currently, the treatment modalities include the 3 Ds, drugs, diets, and dilation [4, 5].
As to the role of acid suppression with PPI, it has been previously demonstrated that the efficacy of this treatment in subverting EoE symptoms was comparable to that observed following the treatment with topical corticosteroids [24]. In addition, acid suppression with PPI may also be used in patients with established EoE, who suffer from symptoms of concomitant GERD, and in those EoE patients that suffer from “PPI-responsive EoE”. Nevertheless, PPI treatment should not be considered as a primary treatment option and should rather be used as co-therapy that partially alleviates some symptoms of EoE and/or GERD.
Several clinical trials and many case series have demonstrated that systemic and topically administered corticosteroids are highly effective in resolving symptoms and signs of acute flares of EoE, in both children and adults [24–30]. Upon comparison of topical and systemic corticosteroid application, it has been shown that there is no significant difference in regards to efficacy; however, the topical steroid therapy has far fewer side effects [28]. Unfortunately, when topical or systemic corticosteroids are discontinued, the disease generally recurs within a few weeks [23, 28]. Based on these considerations, topical corticosteroids are recommended as first line medication for EoE patients. They are well tolerated and, with the exception of oro-pharyngeal Candidiasis, are almost free of side effects. The use of systemic corticosteroids may be limited to emergent cases, such as dysphagia requiring hospitalisation, or dehydration due to swallowing difficulties. Recommended treatment duration for flares ranges between 2–12 weeks. Patients are often treated with a maintenance regimen with a lower dose of topical corticosteroids for up to one year. There is an urgent need for studies that evaluate topical corticosteroid treatment schedules and pharmacodynamics in EoE patients.
Leukotriene inhibitorshave shown to induce symptomatic relief at high dosages. However, its use has not been shown to have any effect on esophageal eosinophilia [31]. Unfortunately, this symptomatic relief could recently not be confirmed in a prospective study [32]. The use of leukotriene inhibitors is therefore not recommended in the treatment of either adults or pediatric EoE [4, 5].
Respecting EoE’s immuno-pathogenesis, several biologic agentsandimmunosuppressants were investigated. Mepolizumab, a humanised anti-IL-5 antibody, led to a significant reduction of esophageal eosinophils in adult and pediatric EoE patients [33, 34]. The agent was well tolerated, but unfortunately the clinical improvement was minimal. Treatment with the anti-TNF antibody infliximab has not been proven to be effective in reducing eosinophilic tissue infiltration or improving symptoms [35], despite the fact that in active EoE the squamous epithelium expresses high amounts of TNF-α [13]. One pilot study has demonstrated that azathioprine or 6-mercaptopurinetreatment has been effective in inducing and maintaining a remission in three corticosteroid-refractory EoE patients [36]. However, before these treatment regimens are to be implemented in clinical practice, the results of these studies have to be confirmed in a larger number of patients.
Several trials have been conducted to assess the potential of different diets in alleviating EoE symptoms, mainly in pediatric populations. Protein-free elemental diet [3, 37], individually adjusted elimination diet [26] and 6-food elimination diet with removal of the 6 most common allergenic foods (dairy, wheat, eggs, soy, peanuts/nuts, fish/shellfish) [38, 39] have shown efficacy in the treatment of children and adults diagnosed with EoE. Unfortunately, as soon as the dietary restrictions are released symptoms and inflammation recur [4, 5]. When deciding on the use of a specific dietary therapy, the patient’s lifestyle and family resources also need to be considered. So far, dietary treatment has been more effective in children than in adults. Overall, the use of dietary therapy in adults requires further evaluation.
Esophageal dilation, either by the bouginage or by balloontechnique, can lead to long-lasting symptom improvement in patients, who do not adequately respond to medical therapy and mainly present with a functional narrowing of the esophagus. Earlier studies have reported on a substantial risk of esophageal perforation, especially if disimpaction of food is performed by rigid endoscopy [10].However, two recently published large series have demonstrated that esophageal dilation can be regarded as safe if performed by flexible endoscopy [40, 41]. However, dilation does not resolve the underlying eosinophilic inflammation [40] and is therefore considered as second-line treatment in cases refractory to correct medical treatment.
EoE is a young disease with the consequence that long-term perspectives regarding the risks of the disease itself as well as the risks of the performed treatments are lacking. Regular visits are therefore highly recommended in EoE patients focusing on symptoms, adherence to therapy and adverse effects to ensure quality of life and to improve the recognition of EoE-associated complications.
A few case reports illustrate an infrequent association of EoE with Barrett’s esophagus. Taking the GERD prevalence into consideration, this association is not really astonishing and further long-term data is awaited. Nevertheless, whether this chronic esophageal inflammation is a risk for the development of pre-malignant or even malignant conditions remains to be determined.
So far proper assessment of disease activity requires endoscopy with tissue sampling because reliable non-invasive markers are still lacking [4, 5]. This approach is invasive and, particularly in children, time and cost-consuming. Several groups have therefore evaluated non-invasive markers such as peripheral eosinophil counts [30] and eosinophil granule proteins regarding their correlation with disease activity. The interpretation of the studies looking at peripheral eosinophilia is hampered by the facts that various definitions for “peripheral eosinophilia” were used and that a high percentage of concurrent allergic sensitisations also contribute to the elevated eosinophil counts. In a cross-sectional analysis in 47 pediatric EoE patients the potential of blood eosinophils, eosinophil-derived neurotoxin (EDN), and eotaxin-3 as biomarkers of EoE was evaluated [42]. Blood eosinophil levels [30, 42], plasma EDN levels, as well as eotaxin-3 levels significantly correlated with esophageal eosinophil density and were increased in patients with active EoE versus controls. Eotaxin-3, a chemoattractant for eosinophils, may also be an interesting tool for discriminating EoE from GERD. Esophageal eotaxin-3 mRNA and protein levels correlated with tissue eosinophilia. Despite promising initial results, assessment of eotaxin-3 currently remains a research tool and further studies are necessary to assess its correlation with disease severity. The evaluation of peripheral blood eosinophils may provide supportive evidence for the presence of EoE and the degree of tissue involvement but they are not diagnostic and the correlation with disease activity is hampered by the high percentage of concurrent allergic diseases.
Summarised, as long as reliable non-invasive biomarkers with good test accuracy are lacking, endoscopy including biopsies remains the only tool to assess for biologic disease activity. Regarding the substantial disease-inherent and treatment-inherent uncertainties, we therefore recommend performing currently clinical, endoscopic and histologic controls, most likely, on a one-year schedule.
Eosinophilic esophagitis is a new chronic inflammatory disease of the esophagus. As recent advances in the diagnosis of EoE have been made within the past years and indications for treatment have become more specific, it is evident that the majority of EoE patients can be treated adequately with topical corticosteroids. However, the long-term medical management of this condition and the drug therapies for patients with EoE refractory to standard treatment are not yet defined. Many of the issues outlined in this review remain at the forefront of EoE research today and require the concerted effort on behalf of clinicians and scientists to bring about new and improved therapies for management of this condition for the benefit of all patients suffering from this disease.
1 Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia, a distinct clinicopathologic syndrome. Dig Dis Sci. 1993;38:109–16.
2 Straumann A, Spichtin HP, Bernoulli R, Loosli J, Vögtlin J. Idiopathic eosinophilic esophagitis: a frequently overlooked disease with typical clinical aspects and discrete endoscopic findings [in German with English abstract]. Schweiz Med Wochenschr. 1994;124:1419–29.
3 Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology. 1995;109:1503–12.
4 Furuta GT, Liacouras C, Collins MH, Gupta S, Justinich C, Putnam P, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342–63.
5 Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20.
6 Kapel RC, Miller JK, Torres C, Aksoy S, Lash R, Katzka DA. Eosinophilic esophagitis: A prevalent disease in the United States that affects all age groups. Gastroenterology. 2008;134:1316–21.
7 Prasad GA, Alexander JA, Schleck CD, Zinsmeister AR, Smyrk TC, Elias RM, et al. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2009;7:1055–61.
8 Hruz P, Straumann A, Bussmann C, Heer P, Simon HU, Zwahlen M, et al. Escalating incidence of eosinophilic esophagitis: A 20 year prospective, population-based study in Olten County Switzerland. J Allergy Clin Immunol. 2011;128:1349–50.
9 Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med. 2004;351:940–1.
10 Straumann A, Bussmann C, Zuber M, Vannini S, Simon HU, Schoepfer AM. Eosinophilic Esophagitis: Analysis of food impaction and perforation in 251 adolescent and adult patients. Clin Gastroenterol Hepatol. 2008;6:598–600.
11 Varadarajulu S, Eloubeidi MA, Patel RS, Mulcahy HE, Barkun A, Jowell P, et al. The yield and the predictors of esophageal pathology when upper endoscopy is used for the initial evaluation of dysphagia. Gastrointest Endosc. 2005;61;804–8.
12 Kato M, Kephart GM, Talley NJ, Wagner JM, Sarr MG, Bonno M, et al. Eosinophil infiltration and degranulation in normal human tissue. Anat Rec. 1998;252:418–25.
13 Straumann A, Bauer M, Fischer B, Blaser K, Simon HU. Idiopathic eosinophilic esophagitis is associated with a T-helper 2-type allergic inflammatory response. J Allergy Clin Immunol. 2001;108:954–61.
14 Fuentebella J, Patel A, Nguyen T, Sanjanwala B, Berquist W, Kerner JA, et al. Increased number of regulatory T cells in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2010;51:283–9.
15 Stuck MC, Straumann A, Simon HU. Relative lack of T regulatory cells in adult eosinophilic esophagitis – no normalization after corticosteroid therapy. Allergy. 2011;66:705–7.
16 Gonsalves N, Policarpio-Nicolas M, Zhang Q, Rao S, Hirano I. Histopathologic variability and endoscopic correlates in adults with eosinophilic esophagitis. Gastrointest Endoscopy. 2006;64:313–9.
17 Spechler SJ, Genta RM, Souza RF. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007;102:1301–6.
18 Locke GR III, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ III. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted county, Minnesota. Gastroenterology. 1997;112:1448–56.
19 Molina-Infante J, Ferrando-Lamana L, Ripoll C, Hernandez-Alonso M, Mateos JM, Fernandez-Bermejo M, et al. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol. 2011;9:110–7.
20 Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU, et al. Natural history of primary eosinophilic esophagitis: a follow-up of thirty patients for up to 11.5 years. Gastroenterology. 2003;125:1660–9.
21 Aceves SS, Newbury RO, Chen D, Mueller J, Dohil R, Hoffman H, et al. Resolution of remodeling in eosinophilic esophagitis correlates with epithelial response to topical corticosteroids. Allergy. 2010;65:109–16.
22 Mishra A, Wang M, Pemmaraju VR, Collins MH, Fulkerson PC, Abonia JP, et al. Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia. Gastroenterology. 2008;134:204–14.
23 Straumann A, Conus S, Degen L, Frei C, Bussmann C, Beglinger C, et al. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2011;9:400–9.
24 Peterson KA, Thomas KL, Hilden K, Emerson LL, Wills JC, Fang JC. Comparison of esomeprazole to aerosolized swallowed fluticasone for eosinophilic oesophagitis. Dig Dis Sci. 2010;55:1313–9.
25 Faubion WA, Perrault J, Burgart LJ, Zein NN, Clawson ML, Freese DK. Treatment of eosinophilic esophagitis with inhaled corticosteroids. J Pediatr Gastroenterol Nutr. 1998;27:90–3.
26 Liacouras CA, Spergel JM, Ruchelli E, Verma R, Mascarenhas M, Semeao E, et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol. 2005;3:1198–206.
27 Konikoff MR, Noel RJ. Blanchard C, Kirby C, Jameson SC, Buckmeier BK, et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology. 2006;131:1381–91.
28 Schaefer ET, Fitzgerald JF, Molleston JP, Croffie JM, Pfefferkorn MD, Corkins MR, et al. Comparison of oral prednisone and topical fluticasone in the treatment of eosinophilic esophagitis: A randomized trial in children. Clin Gastroenterol Hepatol. 2008;6:165–73.
29 Dohil R, Newbury R, Fox L, Bastian J, Aceves SS. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology. 2010;139:418–29.
30 Straumann A, Conus S, Degen L, Felder S, Kummer M, Engel H, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology. 2010;139:1526–37.
31 Attwood SE, Lewis CJ, Bronder CS, Morris CD, Armstrong GR, Whittam J. Eosinophilic oesophagitis: a novel treatment using Montelukast. Gut. 2003;52:181–5.
32 Lucendo AJ, De Rezende LC, Jimenez-Contreras S, Yagüe-Compadre JL, González-Cervera J, Mota-Huertas T, et al. Montelukast was inefficient in maintaining steroid-induced remission in adult eosinophilic esophagitis. Dig Dis Sci. 2011;56:3551–8.
33 Straumann A, Conus S, Grzonka P, Kita H, Kephart G, Bussmann C, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomized, placebo-controlled, double-blind trial. Gut. 2010;59:21–30.
34 Assa’ad AH, Gupta SK, Collins MH, Thomson M, Heath AT, Smith DA, Perschy TL, Jurgensen CH, Ortega HG, Aceves SS. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141:1593–604.
35 Straumann A, Bussmann C, Conus S, Beglinger C, Simon HU. Anti-TNF-alpha (infliximab) therapy for severe adult eosinophilic esophagitis. J Allergy Clin Immunol. 2008;122:425–7.
36 Netzer P, Gschossmann JM, Straumann A, Sendensky A, Weimann R, Schoepfer AM. Corticosteroid-dependent eosinophilic oesophagitis: azathioprine and 6-mercaptopurine can induce and maintain long-term remission. Eur J Gastroenterol Hepatol. 2007;19:865–9.
37 Markowitz JE, Spergel JM, Ruchelli E, Liacouras CA. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol. 2003;98:777–82.
38 Kagalwalla AF, Sentongo TA, Ritz S, Hess T, Nelson SP, Emerick KM, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4:1097–102.
39 Gonsalves N, Ritz S, Yang G, Ditto A, Hirano I. A prospective clinical trial of allergy testing and food elimination diet in adults with eosinophilic esophagitis. Gastroenterology. 2007;132:A-6 (Abstract)
40 Schoepfer AM, Gonsalves N, Bussmann C, Conus S, Simon HU, Straumann A, et al. Esophageal dilation in eosinophilic esophagitis: effectiveness, safety, and impact on the underlying inflammation. Am J Gastroenterol. 2010;105:1062–70.
41 Dellon ES, Gibbs WB, Rubinas TC, Fritchie KJ, Madanick RD, Woosley JT, et al. Esophageal dilation in eosinophilic esophagitis: safety and predictors of clinical response and complications. Gastrointest Endosc. 2010;71:706–12.
42 Konikoff MR, Blanchard C, Kirby C, Buckmeier BK, Cohen MB, Heubi JE, et al. Potential of blood eosinophils, eosinophil-derived neurotoxin, and eotaxin-3 as biomarkers of eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4:1328–36.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.