
Figure 1
Flow chart of CRB-65 data set obtained from patients with community acquired pneumonia (CAP).
DOI: https://doi.org/10.4414/smw.2012.13510
A retrospective observational study
Abbreviations
BP: Blood pressure
CAP: Community acquired pneumonia
CRB-65: Confusion, respiratory rate, blood pressure and age over 65
CRP: C reactive protein
CURB-65: Confusion, urea, respiratory rate, blood pressure and age over 65
DNT: Door-to-needle time
ED: Emergency departement
PCT: Procalcitonin
PSI: Pneumonia severity index
RR: Respiratory rate
WBC: white blood cell counts
Community acquired pneumonia (CAP) and sepsis are leading causes of hospitalisation after admission to a medical emergency department (ED) and are potential life-threatening situations [1, 2]. An early and correct diagnosis is crucial for an optimal treatment of patients with severe infections. However, that is often difficult due to symptoms, which are unspecific or similar to other noninfectious conditions. The blood culture results are not immediately available and common laboratory parameters are not specific enough to separate critical infections from other conditions [3]. The addition of procalcitonin, a laboratory value, has been proposed to guide clinicians, but has not resolved by this issue [4]. Furthermore, unnecessarily given antibiotics are known to contribute to antibiotic resistance and cause preventable health costs [5]. Therefore, guidelines for the diagnosis of sepsis as well as CAP have emerged to assist in diagnosis and empirical therapy [6–8]. However, there are several reasons that lead to non-adherence of these guidelines, e.g. comorbidities or the recommendation from a consulting physician [9]. In infectious diseases early administration of antibiotics according to evidence-based quality guidelines have been reported to be associated with decreased morbidity and mortality [10–12]. The so-called door-to-needle time (DNT) is defined as the time between arrival at the ED and the administration of intravenous antibiotics and it is used as a quality marker in treatment of CAP and sepsis. Therefore, a short DNT is pivotal for good clinical practice [12–14]. Most studies suggest a DNT of four to eight hours for better outcome and sufficient management quality [15, 16]. These recommendations are based on several studies that found an association between reduced mortality, decreased length of hospital stay and improved outcome with timely antimicrobial therapy [14, 17, 18]. To achieve the quality of care standards the resident staff and nurses must be trained and be aware of existent guidelines, definitions, and clinically helpful tools.
One such tool is the confusion, respiratory rate, blood pressure and age over 65 (CRB-65) score. It represents a clinical prediction rule that grades the severity of community-acquired pneumonia in terms of 30-day mortality [19–21]. A score from 0-1 means a predicted mortality rate of 2–5%. A score of 2 indicates a mortality rate of 12% and scores of 3 and 4 a mortality rate of 31% [22]. It is used to define the group of patients, which are most likely to present a severe pulmonary infection, and therefore needs to be treated in hospital. The CRB-65 score is a modification of the CURB-65 score, which additionally includes serum urea measurement. Both scores are claimed to perform equally and as the CRB-65 score does not require a blood test, it is the faster and easier bedside tool in primary care [21]. Furthermore, urea determination is not widely used in Europe to assess kidney function. A further pneumonia severity assessment system is the pneumonia severity index (PSI), which uses 20 clinical and investigational variables to divide patients into 5 severity classes [23]. Compared to the CURB-65 score, the PSI seems to be more specific and sensitive in predicting patients who will require admission to the intensive care unit [24]. However, the high number of variables used for the PSI is not practical in an ED, hence the CRB-65 score is often preferred. All these scores were developed to facilitate the decision-making process, if a patient needs to be hospitalised or if he can be treated ambulatorily. Therefore, these scores might help to reduce health care expenditure.
The purpose of this study was to determine DNT in our hospital setting in the treatment of patients with severe infections such as CAP and sepsis. In patients admitted with the diagnosis of CAP, the CRB-65 score was retrospectively calculated. We examined if the CRB-65 score had an influence on the timing of antibiotics administration. The correlation between DNT and the CRB-65 score was our main focus and furthermore we investigated if other parameters might lead to a shorter DNT in CAP patients and patients with sepsis.
A retrospective data analysis of adult patients (age >18 years) with demission diagnosis of CAP or sepsis was performed. We applied the following definitions. CAP refers to pneumonia acquired outside of hospitals. The diagnosis of pneumonia was ascertained by clinical signs (productive cough, fever, shortness of breath) an auscultation which was typical and an infiltrate on a chest X-ray, which was confirmed by 2 observers (radiologist and clinician). Sepsis was defined by SIRS criteria and bacterial growth in culture or by a confirmed infection in body tissue [25]. Pulmonary comorbidity includes chronic obstructive pulmonary disease, asthma or lung fibrosis. Cerebrovascular comorbidity includes ischemic or hemorrhagic stroke. Hepatic comorbidity includes liver cirrhosis, known hepatitis A-C or unknown elevated liver transaminases (4 times upper the limit of normal value). Renal comorbidity denotes acute renal failure or known a creatinin clearance <30 ml/h.

Figure 1
Flow chart of CRB-65 data set obtained from patients with community acquired pneumonia (CAP).
We included all patients admitted by the ED of the Clinic of Internal Medicine of a Swiss hospital with a reach of 160,000 habitants from June 2009 to June 2010. Only data sets with either main or second diagnosis of pneumonia or sepsis were included and one of these had to be the reason of the hospital stay. Therefore, the dismissal diagnosis had to be the same as the entrance diagnosis after quitting the ED. Data from 309 patients admitted to our hospital with the diagnosis of pneumonia were obtained and analysed for DNT. A data set was considered complete, if (1) time of admission and time of antibiotic administration were distinctively noted, (2) the first diagnosis was approved by radiology or clinical examination and (3) the first diagnosis was also the main cause of hospital stay. Excluded were patients presenting to our ED who had already received antibiotics or who had been transferred from another hospital. Also excluded were patients who did not receive antibiotic treatment in a palliative situation or because of patient preference. Patients who received antibiotics on the medical ward (e.g. due to a crowded ED) were excluded, due to difficulties in securing the precise timing of the antibiotic treatment. After application of inclusion and exclusion criteria, 113 complete data sets from a total of 309 (36%) were investigated for CAP. In figure 1 the final identification and numbers of missing respiratory rate (RR) and confusion state are illustrated.
The same selection process was applied to patients with the demission diagnosis of sepsis. Out of 120 patients, 65 (54%) data sets were complete. Forty-two patients fulfilled both sepsis and pneumonia criteria and were used in both data sets. DNT was defined as the time interval between the first contact of the nursing staff at our ED until the intravenous antibiotic therapy was given.
Due to the retrospective nature of the study, the CRB-65 score had to be calculated out of clinical examination notes. If this was not possible the data was denoted incomplete and therefore excluded. In 68 data sets (60% from the completed data sets of CAP), the CRB-65 score could be calculated. All other collected parameters, e.g. temperature, pulse and blood pressure, were only used if they had been taken before any intervention took place and they had to be clearly declared as first measurements. CRP and WBC were collected from the first blood examination, which had to be taken prior to the antibiotic treatment.
Results are displayed as mean ± standard deviation. To calculate correlation coefficients between the different parameters we applied Spearman correlation analyses (ρ). For all statistics PASW statistics (Version 18.0, SPSS Incorporated, Chicago, USA) was used. p-values <0.05 were considered statistically significant.
Patients’ characteristics are shown in table 1.
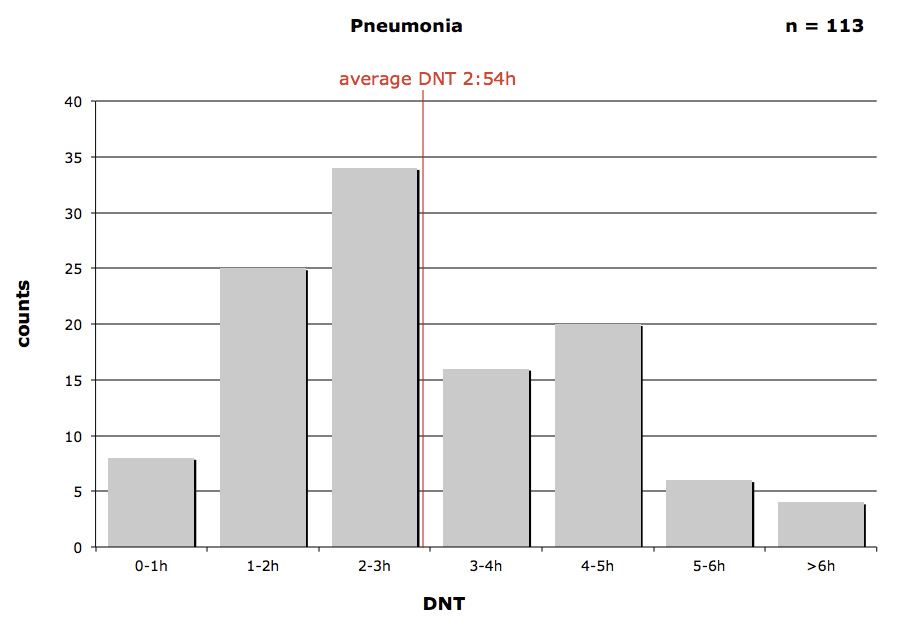
Figure 2
Door-to-needle-time (DNT) in community acquired pneumonia patients.
In figure 2 the DNT for CAP patients is presented. More than half of the patients (59.3% (67/113)) were diagnosed and treated within 3 hours of admission to our ED. 73% (83/113) of all patients received antibiotics within the recommended 4 hours. In 4 cases (3.5%; 4/113) the DNT was longer than 6 hours. The fastest application took place after 15 minutes, the longest time interval for DNT was over 10 hours.
Figure 3 shows the relation between the CRB-65 score and DNT.
In table 2 all sub-categories of the CRB-65 score and the score itself are listed. A considerable number (n = 50) of patients were hospitalised in spite of having a low risk profile (CRB-65 scores of 0–1). In those cases hospitalisation took place due to significant comorbidities (e.g. chronic obstructive pneumopathy or asthma (n = 11), immunodeficiency (n = 6; HIV, diabetes, cancer on treatment) or sepsis (n = 9). Furthermore, social situations of elderly, alone-living patients with otherwise impaired clinical performance status had led to hospital admission.
Figure 4 presents the DNT for patients with the diagnosis of sepsis. The fastest DNT was 15 minutes, the longest DNT was 9 hours after ED admission.
In table 3 the different parameters in relation to DNT are shown (n = 64, one data sheet did not contain initial blood pressure and temperature). Significant correlations were not found (highest correlation was between DNT and WBC, ρ = 0.15, p = 0.24).
| Table 1: Characteristics of the patients with community acquired pneumonia and sepsis. | ||
| CAP (n = 113) | Sepsis (n = 65) | |
| Age (years) | 67.0 ± 17.3 | 65.8 ± 16.9 |
| Number of men, n (%) | 69 (61) | 33 (50) |
| Chronic heart failure, n (%) | 15 (13) | 11 (17) |
| Diabetes mellitus, n (%) | 14 (12) | 16 (25) |
| Neoplastic disease, n (%) | 10 (8) | 8 (12) |
| Pulmonary comorbidity, n (%) | 31 (27) | 11 (17) |
| Cerebrovascular comorbidity, n (%) | 12 (10) | 1 (1) |
| Hepatic comorbidity, n (%) | 4 (3) | |
| Renal comborbidity, n (%) | 24 (21) | 13 (20) |
| CAP: community acquired pneumonia | ||
| Table 2: CRB-65 score in patients with community acquired pneumonia. | |
| n (%) | |
| Number of CRB-65 data sets | 68 |
| Confused patients | 9 (13) |
| Respiratory rate >30/min | 13 (19) |
| Systolic <90 mm Hg | 3 (4) |
| Diastolic <60 mm Hg | 13 (19) |
| Age >65 years | 42 (61) |
| CRB-65 score 0 | 18 (26) |
| CRB-65 score 1 | 32 (47) |
| CRB-65 score 2 | 11 (16) |
| CRB-65 score 3 | 5 (7) |
| CRB-65 score 4 | 2 (3) |
| BP: blood pressure | |
| Table 3: Average door-to-needle-time in sepsis patients with clinical and laboratory variables in the different time slots. | |||||||
| DNT (mean ± SD) | n (%) | Systolic BP (mm Hg) | Diastolic BP (mm Hg) | Pulse (min–1) | Temperature (°C) | CRP (mg/L) | WBC (*103/uL) |
| <1 hour | 4 (6) | 101 ± 22 | 53 ± 5 | 106 ± 47 | 37.8 ± 1 | 218 ± 192 | 10.2 ± 4 |
| <2 hours | 17 (26) | 117 ± 30 | 67 ± 14 | 105 ± 19 | 37.4 ± 2 | 199 ± 158 | 11.6 ± 6 |
| <3 hours | 19 (30) | 140 ± 38 | 74 ± 16 | 108 ± 13 | 38.2 ± 1 | 124 ± 111 | 12.2 ± 6 |
| <4 hours | 8 (12) | 123 ± 42 | 64 ± 19 | 100 ± 20 | 37.2 ± 1 | 153 ± 125 | 12.3 ± 5 |
| <5 hours | 8 (12) | 124 ± 34 | 65 ± 18 | 106 ± 22 | 37.3 ± 1 | 161 ± 196 | 17.6 ± 21 |
| <6 hours | 4 (6) | 120 ± 27 | 70 ± 20 | 107 ± 23 | 37.9 ± 1 | 50 ± 36 | 13.3 ± 5 |
| ≥6 hours | 4 (6) | 109 ± 20 | 63 ± 12 | 101 ± 9 | 37.7 ± 2 | 284 ± 71 | 16.9 ± 8 |
| DNT: door-to-needle-time; BP: blood pressure; WBC: white blood cell count; CRP: C-reactive protein; Temperature: measured axillary | |||||||
A short time to first antibiotic dose has been reported to improve patient outcome in CAP and sepsis [26–28]. In our hospital, CAP and sepsis patients received their first antibiotic dose in most of the cases within the anticipated time of 4 hours after admission to the ED. To grade quality of care, the DNT has been suggested to be an indicator. Delays in antibiotic administration may be due to different reasons. Since our hospital is engaged in education of postgraduate physicans and pregraduated nurses, persons in training may lack knowledge or awareness of the benefits of short DNT. This might be one of the factors that lead to a longer DNT. Correlation of DNT with the availability of a more experienced physician in the ED was not assessed, but might be of influence. The presence of an educated nurse might as well be of importance. Also, a crowded ED with a high patient load and restricted resources at specific hours of the day could delay the time until a physician or a nurse has contact with the patient and this might increase DNT. This however could be excluded in our hospital since an external quality assessment (performed by the Verein Outcome [29]) revealed that the time interval to first patient contact was below 15 minutes. In our sample, the main reason however seemed to be the difficulty to identify those patients suffering from CAP and/or sepsis. In elderly patients with additional comorbidities, symptoms of pneumonia or sepsis maybe less distinct or difficult to assign to a specific organ system. However, in most cases the DNT was within an acceptable time range in our ED.
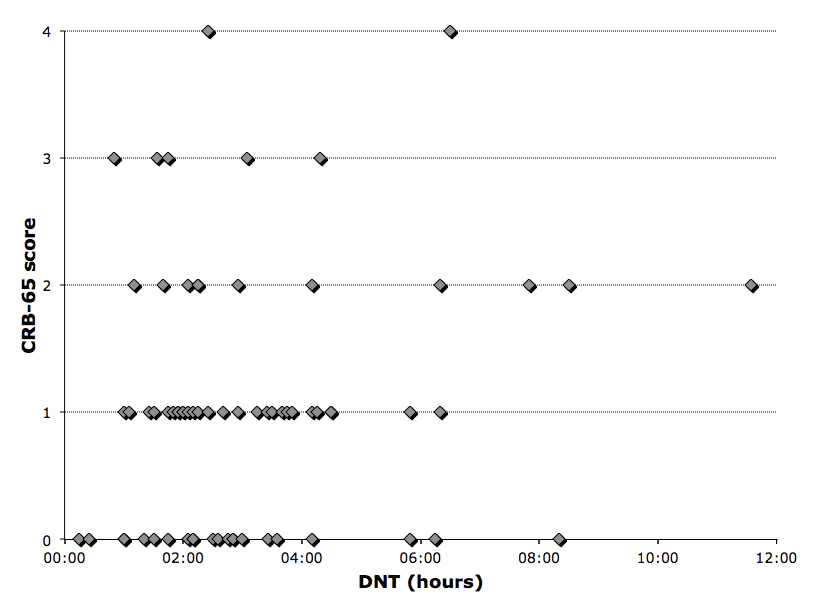
Figure 3
Relation between door-to-needle-time (DNT) und CRB-65 in community acquired pneumonia patients.
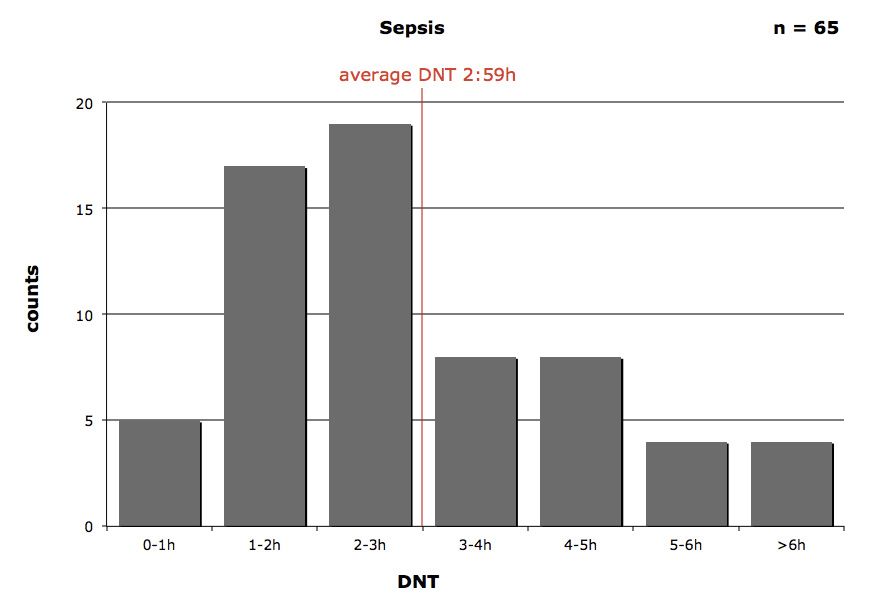
Figure 4
Average door-to-needle-time (DNT) in sepsis patients.
With respect to the CRB-65 score, our study has some limitations. The retrospective design led to the fact that some variables such as confusion and RR were not found in the medical charts. In nearly 50% of the cases the CRB-65 score could not be calculated, due to lack of this data. To determine confusion of a patient with some degree of dementia can be difficult and in some cases it was not possible to distinguish between baseline dementia and actual confusion, but mostly the physician did not comment on the confusion state of their patients at all. Unexpectedly, the biggest loss of data was attributed to missing RR documentation. Some of the missing RR was possibly due to the fact that vital signs such as temperature, blood pressure and heart rate were measured by a nurse and documented in a sheet where the RR could not be easily included. Furthermore, some of the vital signs, for instance blood pressure and heart rate were measured by an automated monitoring system, but not the RR. Most of the nurses and the physicians do not carry a watch which allows them to measure the RR reliably over 30s. Because of hygienic concerns watches on the forearm were banned in our hospital. Furthermore, this implicates that the assessment of the CRB-65 score was not routinely done. This is also reflected in the fact that there was no significant correlation between DNT and the CRB-65 score. To facilitate the application of the CRB-65 score and the 4-hours rule, we raised the awareness regarding this particular rule and score in our ER staff. Furthermore, all nurses at the ER received a watch attachable to their clothes for easy assessment of the RR and the documentation sheet has been changed accordingly. As for pneumonia the CRB-65 score was originally designed to be a tool to predict severity of the disease and to decide where to treat a patient with CAP. In our sample the most represented CRB-65 scores were 0 and 1. According to earlier published guidelines [30] the major part of our study population should actually have been dismissed from the hospital setting with an ambulant antibiotic therapy. The fact that a score from 0–1 was the most represented cannot be explained by the number of patients younger than 65 years old, conversely, the CRB-65 score might overestimate patients older than 65. These findings supported the notion that maybe not only the severity of the pneumonia had led to hospitalisation but that there were also other influences. Important parameters are not taken into account in the CRB-65 score: functional impairment, living alone at home and comorbidities, which require an inpatient treatment. However, it has already been found, that the CURB-65 score, and therefore also the CRB-65 score, does not perform equally in all cohorts of patients, because it does not assess the impact of comorbidities [24]. The CRB-65 score cannot be used as a single decision making tool and the clinical presentation does and should influence the process of decision-making. Nevertheless, as a consequence of the results of this study, clinicians were instructed to use the CRB-65 score to assess the mortality risk of the patients with pneumonia correctly. Patients with low mortality are to be managed ambulantly or if comorbidities or social circumstances do not allow this, it should be noted on admission.
As an alternative score the PSI would provide a higher sensitivity and pretest probability in patients with CAP [31, 32]. In our study we did not test the PSI due to its more complex calculation with several variables. In general, scores can be a decision-supporting tool, but only a few among them also allow an impact on treatment management [33–35]. They do not include rare medical conditions or immune-status and it lies in their nature that they oversimplify some conditions. Also do they not account for certain important states, e.g. hypothermia, functional impairment, living alone or comorbidities.
We collected further parameters, of which we thought they could support the physician in shortening DNT. The fact of present fever and high WBC did not result in significant correlations to DNT. We interpret the spread of DNT time also with the difficulty of obtaining CAP or sepsis specific parameters. To identify the patients without typical symptoms of CAP and sepsis, rapid and accurate laboratory test are necessary. The isolation of microorganisms from body fluid specimens as in blood cultures takes definitely more than 4 hours. And the quickly available parameters CRP and WBC only have a limited specificity. Having an infection-specific parameter would be of high interest.
We conclude that clinical prediction rules such as the CRB-65 were not routinely assessed by the staff in our ED, due to practical limitations such as documentation and assessment of the RR and confusion state. We are aware of the limitation of clinical prediction rules and can conclude that the DNT in CAP and sepsis patients was according to good clinical practice.
1 Greene G, Hood K, Little P, Verheij T, Goossens H, Coenen S, et al. Towards clinical definitions of lower respiratory tract infection (LRTI) for research and primary care practice in Europe: an international consensus study. In: Prim Care Respir J. 2011/04/22 ed; 2011.
2 Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–10.
3 Bauer M, Reinhart K. Molecular diagnostics of sepsis – where are we today? Int J Med Microbiol. 2010;300(6):411–3.
4 Wolff M, Bouadma L. What procalcitonin brings to management of sepsis in the ICU. Crit Care. 2010;14(6):1007.
5 Kanwar M, Brar N, Khatib R, Fakih MG. Misdiagnosis of community-acquired pneumonia and inappropriate utilization of antibiotics: side effects of the 4-h antibiotic administration rule. Chest. 2007;131(6):1865–9.
6 Levinson AT, Casserly BP, Levy MM. Reducing mortality in severe sepsis and septic shock. Semin Respir Crit Care Med. 2011;32(2):195–205.
7 ERS Task Force Report. Guidelines for management of adult community-acquired lower respiratory tract infections. European Respiratory Society. Eur Respir J. 1998;11(4):986–91.
8 Niederman MS, Mandell LA, Anzueto A, Bass JB, Broughton WA, Campbell GD, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163(7):1730–54.
9 Aujesky D, McCausland JB, Whittle J, Obrosky DS, Yealy DM, Fine MJ. Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumonia. Clin Infect Dis. 2009;49(10):e100-8.
10 Metersky ML, Sweeney TA, Getzow MB, Siddiqui F, Nsa W, Bratzler DW. Antibiotic timing and diagnostic uncertainty in Medicare patients with pneumonia: is it reasonable to expect all patients to receive antibiotics within 4 hours? 2006. Chest. 2009;136(5 Suppl):e30.
11 Battleman DS, Callahan M, Thaler HT. Rapid antibiotic delivery and appropriate antibiotic selection reduce length of hospital stay of patients with community-acquired pneumonia: link between quality of care and resource utilization. Arch Intern Med. 2002;162(6):682–8.
12 Proulx N, Frechette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. Qjm 2005;98(4):291–8.
13 Ziss DR, Stowers A, Feild C. Community-acquired pneumonia: compliance with centers for Medicare and Medicaid services, national guidelines, and factors associated with outcome. South Med J. 2003;96(10):949–59.
14 Meehan TP, Fine MJ, Krumholz HM, Scinto JD, Galusha DH, Mockalis JT, et al. Quality of care, process, and outcomes in elderly patients with pneumonia. JAMA. 1997;278(23):2080–4.
15 Mandell LA, Bartlett JG, Dowell SF, File TM, Jr., Musher DM, Whitney C. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin Infect Dis. 2003;37(11):1405–33.
16 Jaeschke RZ, Brozek JL, Dellinger RP. 2008 update of international guidelines for the management of severe sepsis and septic shock: should we change our current clinical practice? Pol Arch Med Wewn. 2008;118(3):92–5.
17 Houck PM, Bratzler DW, Nsa W, Ma A, Bartlett JG. Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community-acquired pneumonia. Arch Intern Med. 2004;164(6):637–44.
18 Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–77.
19 Guidelines for the management of community acquired pneumonia in adults, revised edition. Respirology. 2006;11(Suppl 3):S79–133.
20 Levy ML, Le Jeune I, Woodhead MA, Macfarlaned JT, Lim WS. Primary care summary of the British Thoracic Society Guidelines for the management of community acquired pneumonia in adults: 2009 update. Endorsed by the Royal College of General Practitioners and the Primary Care Respiratory Society UK. Prim Care Respir J. 2010;19(1):21–7.
21 Bauer TT, Ewig S, Marre R, Suttorp N, Welte T. CRB-65 predicts death from community-acquired pneumonia. J Intern Med. 2006;260(1):93–101.
22 Aujesky D, Auble TE, Yealy DM, Stone RA, Obrosky DS, Meehan TP, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med. 2005;118(4):384–92.
23 Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243–50.
24 Ananda-Rajah MR, Charles PG, Melvani S, Burrell LL, Johnson PD, Grayson ML. Comparing the pneumonia severity index with CURB-65 in patients admitted with community acquired pneumonia. Scand J Infect Dis. 2008;40(4):293–300.
25 Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344(10):699–709.
26 Kumar A, Safdar N, Kethireddy S, Chateau D. A survival benefit of combination antibiotic therapy for serious infections associated with sepsis and septic shock is contingent only on the risk of death: a meta-analytic/meta-regression study. Crit Care Med. 2010;38(8):1651–64.
27 Kumar A, Haery C, Paladugu B, Symeoneides S, Taiberg L, Osman J, et al. The duration of hypotension before the initiation of antibiotic treatment is a critical determinant of survival in a murine model of Escherichia coli septic shock: association with serum lactate and inflammatory cytokine levels. J Infect Dis. 2006;193(2):251–8.
28 Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–96.
29 http://www.vereinoutcome.ch
30 Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72.
31 Ewig S, de Roux A, Bauer T, Garcia E, Mensa J, Niederman M, et al. Validation of predictive rules and indices of severity for community acquired pneumonia. Thorax. 2004;59(5):421–7.
32 Brown SM, Jones BE, Jephson AR, Dean NC. Validation of the Infectious Disease Society of America/American Thoracic Society 2007 guidelines for severe community-acquired pneumonia. Crit Care Med. 2009;37(12):3010–6.
33 Buising KL, Thursky KA, Black JF, MacGregor L, Street AC, Kennedy MP, et al. A prospective comparison of severity scores for identifying patients with severe community acquired pneumonia: reconsidering what is meant by severe pneumonia. Thorax. 2006;61(5):419–24.
34 Yandiola PP, Capelastegui A, Quintana J, Diez R, Gorordo I, Bilbao A, et al. Prospective comparison of severity scores for predicting clinically relevant outcomes for patients hospitalized with community-acquired pneumonia. Chest. 2009;135(6):1572–9.
35 Chalmers JD, Mandal P, Singanayagam A, Akram AR, Choudhury G, Short PM, et al. Severity assessment tools to guide ICU admission in community-acquired pneumonia: systematic review and meta-analysis. Intensive Care Med. 2011.
Funding / potential competing interests:No financial support and no other potential conflict of interest relevant to this article was reported.