Medium from mesangial cells incubated with aggregated IgA1 from IgA nephropathy patients reduces podocyte adhesion through activation of the renin angiotensin system
DOI: https://doi.org/10.4414/smw.2011.13304
C
Wang, X
Liu, Y
Tang, H
Peng, ZC
Ye, J
Zhang, H
Tang, T
Lou
Summary
BACKGROUND: Podocyte injury plays an important role in glomerulosclerosis in IgA nephropathy (IgAN), and detachment from the glomerular basement membrane is the main cause of podocyte damage. In a previous study we found that medium from mesangial cells incubated with aggregated IgA1 (aIgA1) isolated from IgAN patients decreased podocyte adhesive capacity. However, the underlying mechanism remains unclear.
MATERIALS AND METHODS:Podocytes were incubated in medium from mesangial cells incubated with aIgA1 isolated from IgAN patients, enalaprilat (10–5 M) and chymostatin (20 μM), or with enalaprilat and chymostatin separately. Podocyte adhesive capacity was evaluated by cell counting and hexosaminidase assay. Expression of the renin angiotensin system was measured by real-time PCR, Western blot analysis and ELISA.
RESULTS: Angiotensinogen, renin and angiotensin II type 1 and 2 receptors mRNA and protein expression, angiotensin-converting enzyme activity, and angiotensin II levels increased in podocyte lysates and conditioned culture media on exposure to mesangial medium containing aIgA1 from IgAN patients (P<0.05). Enalaprilat or chymostatin partly improved the reduced adhesive capacity of podocytes compared to cells exposed to mesangial medium (P<0.05), but it was still lower than for podocytes exposed to mesangial medium containing aIgA1 from healthy controls (P<0.05).
CONCLUSION: Our findings indicate that activation of the renin angiotensin system in podocytes is partly involved in downregulation of adhesive capacity in podocytes by mesangial medium in IgA nephropathy.
Introduction
IgA nephropathy (IgAN) is the most common type of primary glomerular nephritis in China and is the main cause of end-stage renal disease [1]. The pathogenesis of IgAN is still not fully understood and its treatment is not well defined. An increasing number of studies indicate that deposition of undergalactosylated IgA1 in mesangial areas might contribute to pathogenic mechanisms in IgAN owing to an ability to bind specifically to mesangial cells and induce production of a number of cytokines [2, 3]. Recent studies have revealed that the amount of aberrantly glycosylated IgA1 correlates significantly with the severity of glomerular histological lesions and with the prognosis of IgAN [4].
Podocytes are highly differentiated, pericyte-like cells that are essential for normal kidney function. Loss of podocytes is a hallmark of progressive kidney diseases, including IgAN. It is now widely accepted that there is a strong correlation between podocyte injury and IgAN severity: IgAN patients with urinary podocyte excretion show higher proteinuria and poorer renal function compared to patients who lack podocyte excretion. Urinary podocyte excretion is also related to glomerular sclerosis and interstitial fibrosis, which indicate that podocyturia represents clinical evidence of renal damage [5]. There are two mechanisms that could be suggested for podocyte loss: detachment from the glomerular basement membrane and apoptosis [6]. Interesting experiments have explored whether urinary podocytes are fully viable, can be cultivated and continue to synthesise podocyte-specific proteins in vitro in both experimental and human glomerular disease; the ratio of apoptotic podocytes was <50% [7–9]. In addition to apoptosis, detachment of viable podocytes represents another cause of podocytopenia. We previously reported that medium from mesangial cells incubated with IgA1 from IgAN patients can inhibit podocyte adhesive capacity and can activate the renin angiotensin system in podocytes [10, 11]. Clinical studies have demonstrated that renin angiotensin system inhibitors markedly reduce podocyturia, proteinuria and progression of glomerular disease. In this regard, we hypothesise that renin angiotensin system activation in podocytes by medium from mesangial cells incubated with IgA1 from IgAN patients might play a role in inhibiting podocyte capacity. We carried out an in vitro study to gain insight into the underlying mechanism.
Materials and methods
Patients and controls
Twenty-two IgAN patients were enrolled in the study. IgAN was diagnosed by granular IgA deposits, mainly in the glomerular mesangium and occasionally along the peripheral capillary basement membrane using immunofluorescence, and by the presence of electron-dense deposits in the mesangium using ultrastructural tests. All patients were symptomatic for more than 12 months prior to the tests, and no significant renal impairment was documented. Fifteen healthy volunteers, comparable in age and race and without microscopic haematuria or proteinuria, were recruited as control subjects. Informed written consent for blood sampling was obtained from every subject. The study was carried out in accordance with the principles of the Declaration of Helsinki and was approved by the university and hospital ethics committees.
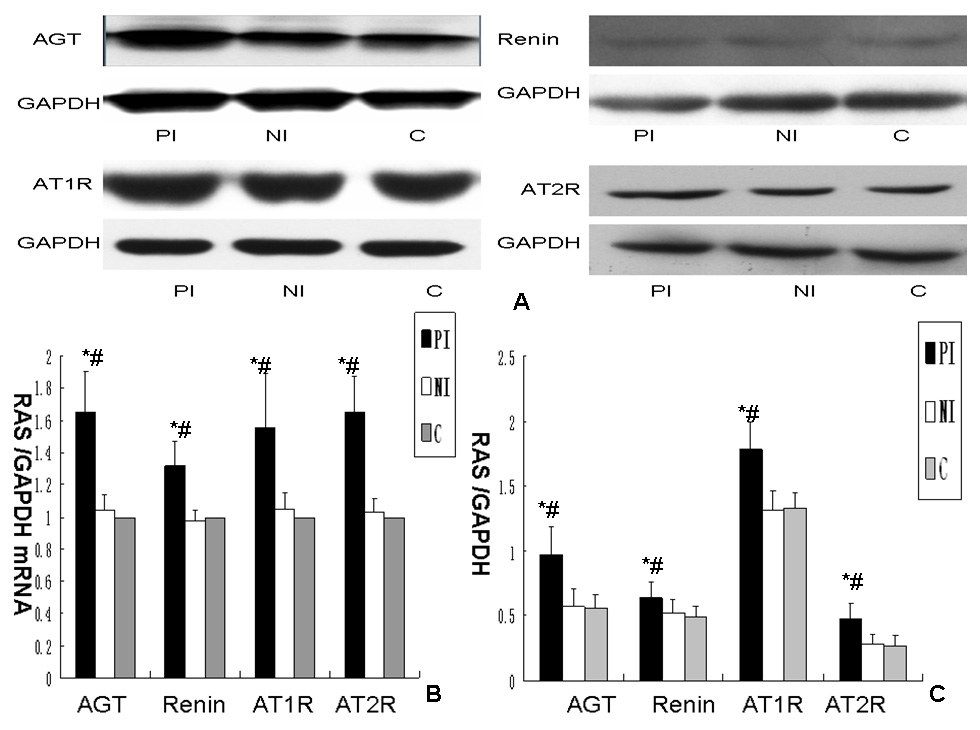
Figure 1
Effect of different media on renin angiotensin system expression in podocyte cultures.
A. Expression of RAS protein expression in podocytes exposed to different media. B. Comparsion of RAS mRNA expression in podocytes exposed to different media. Data were normalised to GAPDH mRNA and controls, column c (controls) were arbitrarily set to 1. Error bars represent the SD. C. Comparison of RAS protein expression in podocytes exposed to different media. Data were normalised to GAPDH, column c (controls) were arbitrarily set to 1. Error bars represent the SD. Column PI: podocytes exposed to culture medium from mesangial cells cultured with aIgA1 (100 µg/mL) isolated from IgA nephropathy mixed 1:9 (v/v) with RPMI 1640 containing 0.5% FBS. Column NI: podocytes exposed to culture medium from mesangial cells cultured with aIgA1 (100 µg/mL) isolated from healthy controls mixed 1:9 (v/v) with RPMI 1640 containing 0.5% FBS. Column C: podocytes exposed to RPMI 1640 containing 0.5% FBS. (P<0.05 vs. column C; &P<0.05 vs. column NI, n = 3).
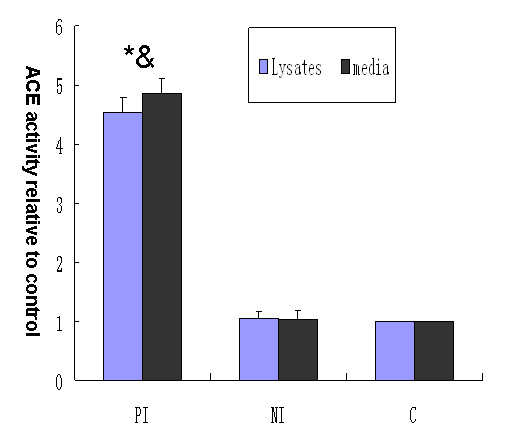
Figure 2
Effect of different media on angiotensin-converting enzyme activity in podocyte lysates and conditioned culture media. Column PI: podocytes exposed to culture medium from mesangial cells cultured with aIgA1 (100 µg/mL) isolated from IgA nephropathy mixed 1:9 (v/v) with RPMI 1640 containing 0.5% FBS. Column NI: podocytes exposed to culture medium from mesangial cells cultured with aIgA1 (100 µg/mL) isolated from healthy controls mixed 1:9 (v/v) with RPMI 1640 containing 0.5% FBS. Column C: podocytes exposed to RPMI 1640 containing 0.5% FBS. (P<0.05 vs. column C; &P<0.05 vs. column NI, n = 3).
Purification of IgA1 by jacalin-agarose affinity chromatography
IgA1 was isolated from serum pooled for IgAN patients and for healthy donors separately by jacalin affinity chromatography as previously described [10, 11]. Because the amount of pIgA1 recovered from the purification process was not sufficient for further analyses, we incubated the purified mIgA1 at 63 °C for 150 min to obtain aIgA1 as described previously [10, 11]. The transition from mIgA1 to aIgA1 was monitored using a Sephacryl S-200 column, and a single peak was observed after incubation at 63 °C. The purified IgA1 was identified by Western blotting and stored at –70 °C for further analysis.
Cell cultures
Mouse podocytes and mesangial cells were provided and cultured as previously described [10, 11]. In the following experiments, cells were first cultured to 80% confluence and then growth was arrested with RPMI-1640 culture medium containing 0.5% FBS for 18–24 h.
Experimental design
Growth-arrest podocytes were first pre-treated for 4 h (1) with enalaprilat (angiotensin-converting enzyme inhibitor, ACEI, 10–5 M ) or (2) with chymostatin (chymase inhibitor, 20 μM, Sigma, St. Louis, MO, USA) alone or (3) in combination, then medium from arrested mesangial cells exposed to aIgA1 from IgAN patients and RPMI 1640 medium containing 0.5% FBS at a 1:9 (v/v) ratio were added; the ratio and time were determined in a pilot study. Podocytes incubated with medium from mesangial cells incubated with aIgA1 from healthy controls and RPMI 1640 medium containing 0.5% FBS in a 1:9 (v/v) ratio are denoted as NI group, and podocytes cultured in RPMI 1640 medium containing 0.5% FBS were used as basal group.
Adhesion assay
Podocytes were harvested with trypsin and suspended in mesangial cell-conditioned medium. In some wells, aIgA1 from IgAN patients or healthy donors was added to podocytes incubated with serum-free medium. An equal number of cells (4×103/well) were seeded in 96-well plates precoated with rat-tail type I collagen (Sigma). After the above treatments at 37 °C for 6 h, two methods were used to evaluate podocyte adhesive capacity. All experiments were repeated three times to ensure reproducibility.
For cell counting, the total number of cells in each well was measured by manual counting. Then cells were washed twice with ice-cold PBS and the number of adherent cells was counted and expressed as a percentage of the total number of cells.
For the hexosaminidase assay, non-adherent cells were removed by three washes with ice-cold PBS. Then 3.75 mM p-nitrophenol-N-acetyl-D-glucosaminide (Sigma, St. Louis, MO, USA) in 50 mM citrate buffer (pH 5.0) containing 0.25%Triton X-100 was added to each well for 1 h at 37 °C. The enzyme was then deactivated by adding 50 mM glycine and 5 mM EDTA (pH 10.4). The absorbance was read at 405 nm using a microplate reader [12].
RNA extraction, cDNA synthesis and real-time PCR
Total RNA was extracted from cells using Trizol reagent (Invitrogen, Carlsbad CA, USA) according to the manufacturer’s instructions. Total RNA (500 ng) from each group was reverse transcribed using the reverse transcriptase (RT) provided in a SYBRPremix Ex Taq kit (Perfect Real Time; TaKaRa, Otsu, Shiga, Japan). Reactions were carried out at 37 °C for 15 min and then 85 °C for 5 s.
The primers used in the experiments are listed in table 1. Using an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA, USA) and a SYBRPremix Ex Taq Perfect Real Time kit, PCR was performed as previously reported [10, 11].
Western blot analysis
Cell proteins were extracted by addition of a lysis buffer (Cell Signaling Technology, Beverley, MA, USA) at 4 °C. The suspension was centrifuged at 14000 g and media containing cellular proteins were collected. For Western blotting, sodium dodecyl sulfate polyacrylamide gels were run under standard conditions for a total protein loading of 80 µg in each lane. The gel was placed in transfer buffer for 15 min and set up for transfer to a polyvinylidene fluoride membrane at 200 mA for 1 h. The membrane was rinsed in Tris-buffered saline and then blocking buffer (5% milk powder) for 5 min. The membrane was then immersed in blocking buffer for 1 h before incubation with primary antibodies (angiotensinogen, renin, angiotensin II type 1 receptor and angiotensin II type 2 receptor rabbit polyclonal antibodies, dilution 1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA; and GAPDH rabbit polyclonal antibody, dilution 1:2000, Cell Signalling Technology) at appropriate dilutions. After rinsing in wash buffer, horseradish peroxidase-conjugated secondary antibody (goat antirabbit, Pierce, New York, USA) was used for 1 h. After final washing, the membrane was developed using ECL Chemiluminescence Reagent (Pierce).
|
Table 1: Sequences of primers. |
| |
|
Sequence(5’→3’)
|
| AGT |
Sense
Antisence |
TGACCCAGTTCTTGCCACTGAG
ACACCGAGATGCTGTTGTCCAC |
| Renin |
Sense
Antisence |
CTCCTGGCAGATCACGATGAAG
GGAGCTCGTAGGAGCCGAGATA |
| AT1R |
Sense
Antisence |
GGTGGCTGAAGCCAGTACCA
TGAGTTGGTCTCAGACACTGTTCAA |
| AT2R |
Sense
Antisence |
CTCCAGGTTTAGACTGCTGCCTTC
GGTTGACACCGAGTTTGTCATTTG |
| AGT: angiotensinogen; AT1R: angiotensin II type 1 receptor; AT2R: angiotensin II type 2 receptor |
Angiotensin II concentrations in podocyte lysates and conditioned culture media
Angiotensin II (Ang-II) levels were determined in podocyte lysates and conditioned culture media. After seeding podocytes on 100-mm dishes and subjecting them to serum restriction for 24 h, the medium was changed as described above. Six hours later the media were collected and cells were washed with ice-cold PBS, scraped from the dishes in the presence of extraction buffer (20 mM Tris-HCl, pH 7.4, 10 mM EDTA, 5 mM EGTA, 5 mM β-mercaptoethanol, 50 mg/mL phenylmethyl sulfonylfluoride, 10 mM benzamidine and 0.1 mg/mL aprotinin), and homogenised. Cell lysates and conditioned media were centrifuged at 15 000 rpm for 10 min at 4 °C and the supernatants were collected.
Commercially available ELISA kits were used (USCN Life Science & Technology, Missouri City, TX, USA) to measure Ang-II in podocyte lysates and conditioned culture media.
Angiotension converting enzyme activity in podocyte lysates and conditioned culture media
Angiotension converting enzyme activity in culture media and cell lysates was determined as previously described [13] using a commercial kit (Navy General Hospital, Beijing, China). The principle of the assay is that hippuryl-histidyl-leucine is hydrolysed to hippuric acid and histidyl-leucine by angiotension converting enzyme in vitro. Hippuric acid is extracted with ethyl acetate, distilled and then dissolved in 1 M sodium chloride. The mixture is monitored by UV spectrophotometry at 228 nm. One unit of angiotension converting enzyme activity is defined as the amount of enzyme required to release 1 nmol of hippuric acid per minute, per millilitre of sample.
Statistical analysis
All data are expressed as mean±SD unless otherwise specified. Statistical differences were assessed using multivariate ANOVA for repeated measures. All P-values quoted are two-tailed, and significance was defined as P<0.05.
Results
Angiotensinogen and renin expression in podocytes incubated with medium of mesangial cells incubated with aIgA1 from IgAN patients
Angiotensinogen and renin mRNA and protein levels were higher in podocytes incubated with medium of mesangial cells incubated with aIgA1 from IgAN patients compared to podocytes exposed to mesangial culture medium with aIgA1 from healthy controls (P <0.05) and podocytes exposed to mesangial culture medium with RPMI 1640 containing 0.5% FBS (P<0.05; fig. 1).
Ang-II type 1 and 2 receptor expression in podocytes incubated with medium of mesangial cells incubated with aIgA1 from IgAN patients
Medium of mesangial cells incubated with aIgA1 from IgAN patients significantly enhanced Ang-II type 1 and 2 receptor mRNA and protein expression in podocytes compared with mesangial culture medium with aIgA1 from healthy controls (P<0.05; fig. 1).
Angiotensin converting enzyme activity in podocyte lysates and conditioned culture media
Angiotensin converting enzyme activity in cell lysates and conditioned culture media from podocytes incubated with medium of mesangial cells incubated with aIgA1 from IgAN patients was more than 4.5-fold higher than in those from podocytes exposed to mesangial culture medium with aIgA1 from healthy controls and from podocytes incubated with RPMI 1640 containing 0.5% FBS (P <0.05; fig. 2).
Ang-II levels in podocyte lysates and conditioned culture media
Ang-II levels in lysates and conditioned culture media from podocytes were 2.05 and 2.18-fold separately higher for exposure to medium of mesangial cells incubated with aIgA1 from IgAN patients than for exposure to mesangial culture medium with aIgA1 from healthy controls (P<0.05). When podocytes were pre-treated with enalaprilat, Ang-II levels in cell lysates and conditioned culture media were lower compared to podocytes without enalaprilat (1.34 ± 0.35 vs 2.05 ± 0.55; 1.45 ± 0.3 vs 2.18 ± 0.45, P <0.05). When podocytes were pre-treated with chymostatin, Ang-II levels in cell lysates and conditioned culture media were lower compared to podocytes without chymostatin (1.75 ± 0.34 vs 2.05 ± 0.55; 1.85 ± 0.4 vs 2.18 ± 0.45, P<0.05). Ang-II levels in cell lysates and conditioned culture media were lower for podocytes exposed to enalaprilat than for podocytes pre-treated with chymostatin (P<0.05). When podocytes were pre-treated with enalaprilat and chymostatin together, Ang-II levels in cell lysates and conditioned culture media were much lower compared to podocytes without chymostatin and enalaprilat(P<0.05), or with enalaprilat(P<0.05) or chymostatin(P<0.05) alone, but were higher than in the control group(P<0.05, fig. 3).
Effect of enalaprilat or chymostatin on podocyte adhesive capacity
The adhesive capacity was better for podocytes pre-treated with enalaprilat exposed to medium of mesangial cells incubated with aIgA1 from IgAN patients than for podocytes incubated with medium of mesangial cells incubated with aIgA1 from IgAN patients alone (P<0.05), but was only slightly improved when cells were pre-treated with chymostatin compared with podocytes pre-treated with enalaprilat (P<0.05). The capacity was greatly improved when cells were pre-treated with both enalaprilat and chymostatin, but it was still lower than in the control group (P<0.05) (fig. 4)
Discussion
In this study we show for the first time that Ang-II levels concurrently increased with angiotensinogen, renin, and Ang-II type 1 and 2 receptor mRNA and protein expression, and especially angiotensin converting enzyme activity in podocytes exposed to mesangial culture medium from IgAN patients, which indicates that the podocyte renin angiotensin system was activated by mesangial culture medium from IgAN patients. When podocytes were pre-treated with the angiotensin converting enzyme innibitor enalaprilat, Ang-II levels significantly decreased. When podocytes were pre-treated with the chymase inhibitor chymostatin, Ang-II levels only slightly decreased. These results suggest that both angiotensin converting enzyme and non-angiotensin converting enzyme pathways are involved in mesangial culture medium from IgAN patients-induced Ang-II production in podocytes, but angiotensin converting enzyme pathways are likely to play a greater role. Similar results were obtained for chymostatin and enalaprilat effects on the adhesive capacity of podocytes exposed to mesangial culture medium from IgAN patients, which implies that renin angiotensin system activation induced by the special mesangial medium contributed to inhibition of podocyte adhesive capacity.
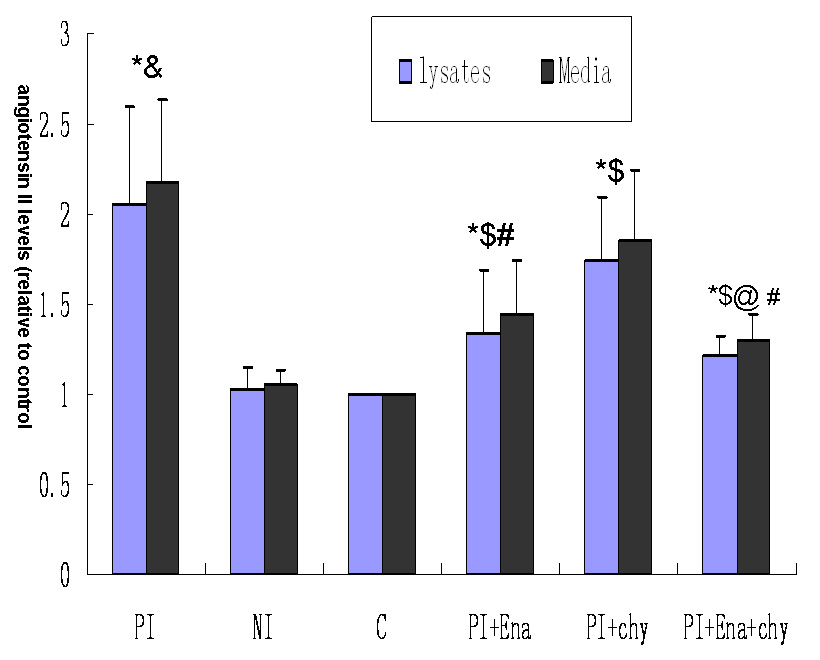
Figure 3
Effect of different media on angiotensin II levels in podocyte lysates and conditioned culture media. Column PI: podocytes exposed to culture medium from mesangial cells cultured with aIgA1 (100 µg/mL) isolated from IgA nephropathy mixed 1:9 (v/v) with RPMI 1640 containing 0.5% FBS. Column NI: podocytes exposed to culture medium from mesangial cells cultured with aIgA1 (100 µg/mL) isolated from healthy controls mixed 1:9 (v/v) with RPMI 1640 containing 0.5% FBS. Column C: podocytes exposed to RPMI 1640 containing 0.5% FBS. Column PI+Ena:Podocytes were pre-treated with enalaprilat (10–5 M) and exposed to supernatant from mesangial cells cultured with aIgA1 (100 µg/mL) isolated from IgAN patients diluted 1:9 (v/v) with RPMI 1640 containing 0.5% FBS. Column PI+chy:Podocytes were pre-treated with chymostatin (20 μM) and exposed to supernatant from mesangial cells cultured with aIgA1 (100 µg/mL) isolated from IgAN patients diluted 1:9 (v/v) with RPMI 1640 containing 0.5% FBS. Column PI+Ena+chy: Podocytes were pre-treated with enalaprilat (10–5 M) and chymostatin (20 μM) and exposed to supernatant from mesangial cells cultured with aIgA1 (100 µg/mL) isolated from IgAN patients diluted 1:9 (v/v) with RPMI 1640 containing 0.5% FBS (P<0.05 vs. column C; &P<0.05 vs. column NI; $P<0.05 vs. column PI; #P<0.05 vs. column PI+chy; @P<0.05 vs. column PI+Ena, n = 3). Data were normalised to controls (column c), which were arbitrarily set to 1. Error bars represent the SD.
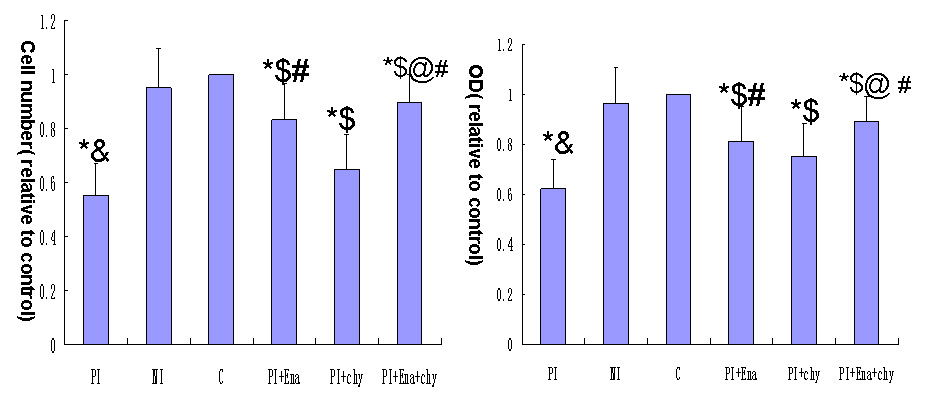
Figure 4
Effect of different media on podocyte adhesive capacity.
(A) Cell counting and (B) hexosaminidase assay.
Column PI: podocytes exposed to culture medium from mesangial cells cultured with aIgA1 (100 µg/mL) isolated from IgA nephropathy mixed 1:9 (v/v) with RPMI 1640 containing 0.5% FBS. Column NI: podocytes exposed to culture medium from mesangial cells cultured with aIgA1 (100 µg/mL) isolated from healthy controls mixed 1:9 (v/v) with RPMI 1640 containing 0.5% FBS. Column C: podocytes exposed to RPMI 1640 containing 0.5% FBS. Column PI+Ena:Podocytes were pre-treated with enalaprilat (10–5 M) and exposed to supernatant from mesangial cells cultured with aIgA1 (100 µg/mL) isolated from IgAN patients diluted 1:9 (v/v) with RPMI 1640 containing 0.5% FBS. Column PI+chy:Podocytes were pre-treated with chymostatin (20 μM) and exposed to supernatant from mesangial cells cultured with aIgA1 (100 µg/mL) isolated from IgAN patients diluted 1:9 (v/v) with RPMI 1640 containing 0.5% FBS. Column PI+Ena+chy: Podocytes were pre-treated with enalaprilat (10–5 M) and chymostatin (20 μM) and exposed to supernatant from mesangial cells cultured with aIgA1 (100 µg/mL) isolated from IgAN patients diluted 1:9 (v/v) with RPMI 1640 containing 0.5% FBS (P<0.05 vs. column C; &P<0.05 vs. column NI; $P<0.05 vs. column PI; #P<0.05 vs. column PI+chy; @P<0.05 vs. column PI+Ena, n = 3). Data were normalised to controls (column c), which were arbitrarily set to 1. Error bars represent the SD.
Previous IgAN clinical studies have demonstrated that renin angiotensin system blockade by angiotensin converting enzyme inhibitors or Ang-II receptor blockers reduces proteinuria and nephropathy progression that cannot be explained merely by their antihypertensive effect [14]. These findings suggest that renin angiotensin system blockade may have direct effects on various renal cells. Many investigators have focused on podocytes because they not only serve as a filtration barrier to protein, but also play an important role in the pathogenesis of glomerulosclerosis. Liebau first demonstrated functional expression of key renin angiotensin system components in differentiated human podocytes by Western blotting and immunostaining [15]. Ang-II promoted podocyte autophagy caused rearrangement of cortical F-actin, reduced α-actinin-4 and focal adhesion expression and induced a migratory phenotype switch in cultured mouse podocytes [16, 17]. All these studies imply that RAS activation in podocytes plays a role in the overall pathophysiology of renal disease.
In our in vitro study we first demonstrated that angiotensinogen, renin, angiotensin converting enzyme, and Ang-II type 1 and 2 receptor expression increased in podocytes concomitantly with Ang-II levels on exposure to mesangial culture medium from IgAN patients, which implies that RAS activation in podocytes was induced by the special medium. We observed two interesting phenomena. First, angiotension converting enzyme-dependent pathways for Ang-II formation are of major significance, as evidenced by the stimulation of angiotension converting enzyme activity and greater inhibition of Ang-II formation by angiotension converting enzyme inhibition, which differs from a previous study suggesting that angiotensin converting enzyme-independent pathways for Ang-II formation are of major significance in disease progression [18]. Second, Ang-II type 1 and 2 receptor expression in podocytes was enhanced by mesangial culture medium from IgAN patients, which may be related to a balance of cell physiological behaviour since Ang II receptor subtypes play opposite roles in regulating the barrier function of the glomerular capillary wall: AT1R-mediated action hampers the expression of slit diaphragm molecules, whereas AT2R-mediated action enhances it [19].
We have reported that aIgA1 (more than 100 ug/ml) can directly induce apoptosis of podocytes [20], which indicated that aIgA1 might affect podocyte biological behaviour. In this in vitro study, the concentration of special medium incubated with podocytes is 10% of original mesangial medium, which means that the concentration of aIgA1 in the special mesangial medium is no more than 10 ug/mL, and we have reported that 10 μg/ml aIgA1 from IgAN patients or healthy control subjects did not show any effect on adhesive capacity of podocytes [10]. We can believe that it is soluble mediators released from mesangial cells rather than aIgA1 in the conditioned medium that affect podocyte adhesive capacity.
IgAN is characterised by polymeric IgA1 deposition in the mesangium, but the tiny amount of polymeric IgA1 in serum limits its use in an in vitro study. It is necessary to use aggregated IgA1 by heating monomeric IgA1, which is similar to IgA immune complex and commonly used in vitro studies [10, 20–22]. IgA1 can activate mesangial cells and stimulate the secretion of many cytokines such as interleukin-6, transforming growth factor-β1 and Ang-II. These cytokines are detected in mesangial cell medium during culture in the presence of aIgA1. We have reported that a medium of mesangial cells incubated with aIgA1 from IgAN patients can induce podocyte apoptosis and inhibit nephrin expression and secretion of transforming growth factor-β1 [10, 11]. Taken together with inhibition of podocyte adhesive capacity in the present study, the results suggest that glomerulo-podocytic communication exists in IgAN, which could be one mechanism for podocyte injury in IgAN. However, we could not restore podocyte adhesive capacity, even when cells were pre-treated with enalaprilat and chymostatin, possibly because of cytokines in the medium, such as transforming growth factor-β1, which can decrease podocyte adhesive capacity [23]. Further study is required to identify the underlying mechanism.
In conclusion, medium from mesangial cells incubated with aIgA1 from IgAN patients can inhibit podocyte adhesive capacity through renin angiotensin system activation. This information provides further insight into the mechanism of podocyte damage in IgAN.
References
1 Li Ls, Liu Zh. Epidemiologic data of renal diseases from a single unit in China: analysis based on 13,519 renal biopsies. Kidney Int. 2004;66:920–3.
2 Nartia I, Gejyo F. Pathogenetic significance of aberrant glycosylation of IgA1 in IgA nephropathy. Clin Exp Nephrol. 2008;12:332–8.
3 van der Boog PJ, van Kooten C, de Fijter JW , de Fijter JW, Daha MR. Role of macromolecular IgA in IgA nephropathy. Kidney Int. 2005;67:813–21.
4 Ding Jx, Xu Lx, Lv Jc, Zhao Mh, Zhang H, Wang Hy. Aberrant sialylation of serum IgA1 was associated with prognosis of patients with IgA nephropathy. Clin Immunol. 2007;125:268–74.
5 Peng Ai, Gu Y, Xiao T, Zhu Ky, Zhang M, Yang Hc, et al. Clinicopathological characteristics of IgA nephropathy with urinary podocyte excretion. Chin J Nephrol. 2007;23:283–5.
6 Shanklang SJ. The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–47.
7 Arant T. Petermann, Ron Krofft, Mary Blonski, et al. Podocytes that detach in experimental membranous nephropathy are viable. Kidney Int. 2003,64:1222–31.
8 Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol. 2002;13:3005–15.
9 Steanie U. Vogelmann, W. James Nelson, Bryan D. Myers, et al. Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol. 2003,285:F40-48.
10 Ye Z, Wang C, Tang Y, et al. Serum IgA1 from patients with IgA nephropathy up-regulates integrin-linked kinase synthesis and inhibits adhesive capacity in podocytes through indirect pathways. Clin Invest Med. 2009;32:E20-7.
11 Wang C, Liu X, Ye Z,Zhang J, Tang H, Chen Z, Zhang H, Lou T. Mesangial medium with IgA1 from IgA nephropathy inhibit nephrin expression in podocytes Eur J Clin Invest. 2009;39:561–7.
12 Landegren U. Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Applications to detection of lymphokines and cell surface antigens. J Immunol Methods. 1984;67:379–88.
13 Cushman DW, Cheung HS. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol. 1971;20:1637–48.
14 Jennings DL, Kalus JS, Coleman CL, et al. Combination therapy with an ACE inhibitor and an angiotensin receptor blocker for diabetic nephropathy: a meta-analysis. Diabet Med. 2007;24:486–93.
15 Max CL, Lang D, Bohm J, et al. Functional expression of the renin-angiotensin system in human podocytes. Am J Physiol Renal Physiol. 2006;290:F710–F719.
16 Yadav A, Vallbu S, Arora S, et al. ANG II promotes autophagy in podocytes. Am J Physiol Cell Physiol. 2010;299:C488–96.
17 Hsu HH, Hoffmann S, Endlich N, et al. Mechanisms of angiotensin II signaling on cytoskeleton of podocytes. 2008;86:1379–94.
18 Sungmi P, Benjamin JB, Hiroyuki K, et al. Major role for ACE-independent intrarenal ANG II formation in type II diabetes. Am J Physiol Renal Physiol. 2010;298:F37–48
19 Koichi S, Gi DH, Naoko M, et al. Angiotensin II Type 1 and Type 2 Receptors Play Opposite Roles in Regulating the Barrier Function of Kidney Glomerular Capillary Wall. Am J Pathol. 2007;170:1841–53.
20 Wang Ch, Peng H, Tang H, et al. Serum IgA1 from IgA nephropathy induces apoptosis in podocytes through direct and indirect pathway. Clin Invest Med. 2007;30:E240–9.
21 Wang Y, Zhao MH, Zhang YK, et al. Binding capacity and pathophysiological effects of IgA1 from patients with IgA nephropathy on human glomerular mesangial cells. Clin Exp Immunol. 2004;136:168–75.
22 Wang Y, Zhao MH, Zhang Y, et al. Serum IgA(1) from patients with IgA nephropathy induces phosphorylation of extracellular signal-regulated kinase and proliferation of human mesangial cells. Zhonghua Yi Xue Za Zhi. 2002;82:1406–9.
23 Cecile D, Marc OB, Anthea H, et al. Mechanical forces and TGFβ1 reduce podocyte adhesion through α3β1 integrin downregulation. Nephrol Dial Transplant. 2009;24:2645–55.



