Ex vivo expansion of hematopoietic stem cells: mission accomplished?
DOI: https://doi.org/10.4414/smw.2011.13316
Summary
A small number of hematopoietic stem cells (HSCs) with self-renewal and multi-lineage repopulation capacity maintain hematopoiesis during the lifetime of an individual. Moreover, HSCs and their potential exist in excess as one individual can share its HSCs with another leading to creation of a genetically identical hematopoietic system. For over half a century this property of HSCs has been utilised by successful allogeneic clinical HSC transplantation for treatment of patients with inherited or acquired genetic and neoplastic diseases of the hematopoietic and immune system. There are now more than twenty thousand allogeneic HSC transplants per year worldwide [1]. However, although more than 17.5 million potential HSC donors are registered and additional 500,000 cord bloods are stored for potential allogeneic HSC transplantation [2], timely availability of appropriately human leukocyte antigen (HLA)-compatible HSCs with sufficient quality for patients still poses a problem in the field. Even if a donor is available, toxicity of the procedure could be reduced by increasing HSC numbers in transplants. One way to solve these issues would be by generation of quality-controlled, off the shelf HSC products via in vitro HSC expansion, a “holy grail” procedure many have been hunting for. Here, we discuss accumulating knowledge on signalling pathways involved in HSC maintenance as well as recent achievements to apply the findings to ex vivo HSC expansion for clinical use. Although the specific issue concerns only highly specialised medicine today, newly generated knowledge will be critical for the whole field of stem cell transplantation and regenerative medicine in the future.
Introduction
HSC biology
Hematopoiesis is a paradigmatic stem cell-sustained organ system where most mature cells are short lived and need to be continuously replenished by high-throughput cell production. It is calculated that in an adult human being about 1011-1012 mature blood cells need to be produced per day for the life of the individual [3]. All differentiated cells are, via developmental intermediates with high proliferation potential, ultimately derived from HSCs, which themselves are homeostatically maintained in a special bone marrow (BM) microenvironment, the so-called “niche” that provides availability of all nutrients sufficient and essential for HSC maintenance as well as protection from HSC-harming agents. Recent studies have shown that there are two different types of BM HSC niches, one in the osteoblastic and another in the perivascular region [4]. However, because of the difficulty in anatomically dissecting these niches [5, 6], the identity and function of all cells involved in forming the niche has not yet been fully defined. In contrast to the environment and the necessary factors for HSC maintenance, HSCs themselves have been isolated using antibodies against characteristic surface marker protein expression sets that allow high enrichment of HSCs by fluorescence-activated cell sorting (FACS), and were tested for their biology and function in subsequent in vitro and in vivo settings [7]. In mice, one of the laboratory animals broadly used for predictive medical research, HSCs represent 0.003% of total BM cells, i.e., an adult mouse contains about 15,000 HSCs. These sustain blood production for the lifetime of the mouse and can be used for several serial HSC transplantations into subsequent recipient mice to rescue them from lethal irradiation [8]. Furthermore, it has been demonstrated that even a single HSC can engraft and provide long-term multi-lineage repopulation of lethally irradiated recipients, indicating robust self-renewal and homeostatic expansion capacity of HSCs in vivo [9–12]. However, although many mechanisms contributing to sustained self-renewal in vivo have been identified [13], use of this knowledge to achieve HSC expansion in vitro remains a challenge as detailed below.
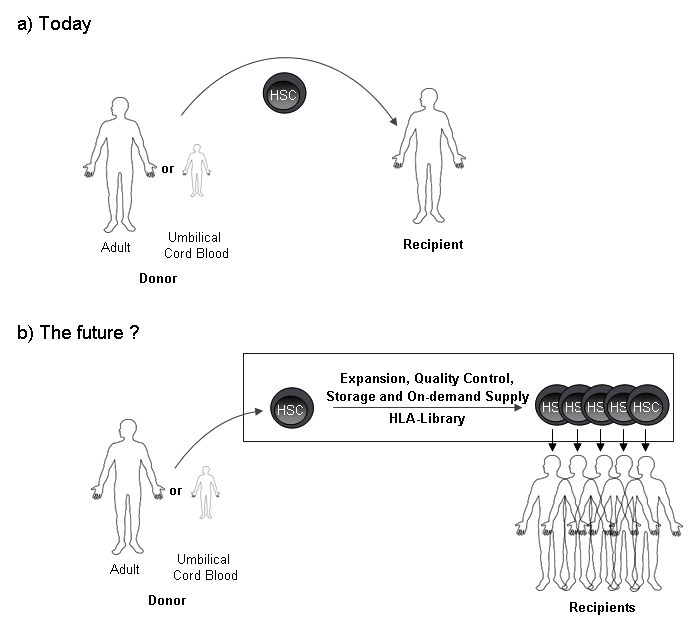
Figure 1
Hematopietic stem cell transplantation: Today and in the future?(a) Today HSC containing transplants are transferred from a donor to a recipient in a 1:1 ratio. (b) Given that HSC expansion is achieved, the future might allow storage of HLA-typed, expanded and quality controlled HSC grafts, ready to be used for recipients upon need.
Clinical relevance
Because of the fluid character of the hematopoietic system, the naturally occurring migration of hematopoietic cells through the blood, and the tropism of hematopoietic precursor cells to home to their bone marrow niches, it is technically easy to perform hematopoietic cell transplantation by simple infusion of the graft into the venous system [14, 15]. Indeed, hematopoietic stem cell transplantation (HSCT) (formerly called and executed bone marrow transplantation – BMT) has been utilised in clinical medicine for over half a century, in order to cure congenital genetic disorders or hematopoietic cancers [14, 16]. While initial attempts of BMT were associated with unacceptable side-effects, the fast growing knowledge of the major histocompatibility complex (in humans also called HLA system) and the necessity to closely match recipient and donor to avoid high rates of rejection, graft failure, and graft versus host disease (GvHD), led to the success of the procedure [17]. Equally important, growing knowledge on appropriate conditioning regimens, and how to deal with rejection and side effects such as GvHD and infection, and finally, establishment of data-bases for available volunteer donors was key to acceptance, availability and success of allogeneic HSCT [18, 19]. Since it has been found that part of HSCs can be mobilised into peripheral blood with recombinant granulocyte-colony stimulating factor (G-CSF) injection and subsequently be collected from peripheral blood (PB) by apheresis with minor side effects [20], mobilised PB HSC donation and transplantation has become the major procedure. Given this progress and the increasing availability of registered potential donors, the annual numbers of allogeneic HSCT have dramatically increased during the last decades, now with annually more than 20,000 allogeneic-HSCTs performed world-wide [1, 14].
However, several major challenges remain regarding quality and timely availability of allogeneic HSC grafts for patients in need: a) patients with rare HLA-types (e.g., rare allele frequency and combination, minority populations) rarely find a donor; b) potential donors might not be available when needed, e.g., due to so far undetected co-morbidities as infection or cancer; c) donor search and confirmation of availability consumes time prohibitive for adequate control of the disease. Thus, immediately available, HLA-matched, quality controlled and off the shelf produced HSC transplants would greatly serve these needs.
Close to these requirements of an alternative source comes donated and stored umbilical cord blood, (UCB) that is, compared to adult blood, highly enriched in migrating HSC (2011 about 500,000 samples stored worldwide and so far about 20,000 transplants performed [21]). However, broad application of UCB transplants to most recipients is limited by the low cell number recovered from a single UCB unit which is not sufficient for adult recipients in most of cases and thus has been mainly used for treatment of pediatric or young adult patients [21–23]. One approach to overcome the limitation of low numbers of HSCs is the use of two combined UCB units [24]. However, once a UCB is used, there is, in contrast to adult volunteer donors, no further availability in case of need for a second graft.
Thus, a more broadly applicable strategy to increase HSC availability would be achievement of ex vivo HSC expansion from any HSC source, allowing even multiple transplants from one HSC type. However, for a first proof of principle, achievement of at least a two-fold in vitro expansion would allow use of UCB for many adult recipients [25, 26] (fig. 1).
Biology of and achievements in HSC expansion
The principal idea to develop a method for ex vivo HSC expansion was inspired from studies using gene targeted animals. From the phenotypical and functional analysis of those animals, it was demonstrated that self-renewal, proliferation and apoptosis of HSC are tightly regulated both by intrinsic, HSC expressed factors, as e.g., signalling molecules, transcription factors, and also by environmental cues, as e.g., cytokines and adhesion molecules [13]. Many groups have been attempting to expand HSC in vitro via the modification of signalling pathways by forced gene expression or suppression (enhancement of positive signals or inhibition of negative signals), addition of recombinant proteins in culture, or by co-culture with stromal cells that might supply those signals. Prominent examples of ex vivo or in vivo expansion of human hematopoietic stem/progenitor cells (HSPCs) are summarized in table 1.
|
Table 1:Examples of ex vivo or in vivo expansion of human hematopoietic stem/progenitor cells (HSPC).
|
|
Method
|
Supplements
|
Input cells
|
Treatment period
|
Absolute number (population)
|
Fold expansion
|
Reference
|
| |
|
|
|
|
In vitro (assay)
|
In vivo(xenograft model)
|
|
| Co-culture system |
Mouse stromal cell expressing HoxB4 protein |
UCB CD34+CD38lo cells |
4–5 weeks |
2.0 (CD34+) |
3.0 (CFUs)
2.9 (LTC-ICs) |
2.5 fold increase in RCs (NOD/SCID) |
S Amsellem et al., Nat Med 2003 |
| Co-culture system |
Rat mammary epithelium expressing Wnt5a, Wnt2b, or Wnt10b |
ABM Lin- CD34+ cells |
7 days |
|
10-20 (CFUs) |
|
David J. Van Den Berg et al., Blood 1998 |
| Liquid culture |
SCF, Flt3L, G-CSF, IL-3, IL-6 |
UCB CD34+CD38- cells |
4 days |
4.0 (CD34+CD38-) |
15.4 (CFUs) |
4.0 fold increase in RCs (NOD/SCID) |
M Bhatia et al., JEM 1997 |
| Liquid culture |
TPO, SCF, Flt3L, Pleiotrophin |
Lin- CD34+CD38- cells |
7 days |
2.0 (Lin-CD34+CD38-) |
4.0 (CFUs) |
Increased frequency of RCs and engraftment level (NOD/SCID) |
HA Himburg et al., Nat Med 2010 |
| Liquid culture |
TPO, SCF, IL-3, IL-6, Flt3L, Delta-1, fibronectin fragment-coated plate |
UCB CD34+CD38- cells |
5 weeks |
100 (CD34+) |
34 (CFUs) |
20 fold increase in engraftment level (29 day-culture) (NOD/SCID/β2m
–/–) |
K Ohishi et al., JCI 2002 |
| Liquid culture |
SCF, Flt3L, G-CSF, IL-3, IL-6, Jagged-1-IgG |
UCB CD34+CD38- cells |
9 days |
3.0 (CD34+CD38-) |
3.0 (CFUs) |
Increased frequency of RCs and engraftment level (NOD/SCID) |
FN Karanu et al., JEM 2000 |
| Liquid culture |
SCF, Flt3L, G-CSF, IL-3, IL-6, Sonic hedgehog |
UCB Lin- CD34+CD38- cells |
12 days |
1.6–1.8 (CD34+CD38-) |
3.5–4.5 (CFUs) |
Increased frequency of RCs and engraftment level (NOD/SCID) |
G Bhardwaj et al., Nat Immunol 2001 |
| Liquid culture |
SCF, Flt3L, G-CSF, IL-3, IL-6, BMP-4 |
UCB Lin- CD34+CD38- cells |
3 days |
1.5 (total cells) |
1.5 (CFUs) |
Increased frequency of RCs (NOD/SCID) |
M Bhatia et al., JEM 1999 |
| Liquid culture |
TPO, SCF, Flt3L, IL-6, StemRegenin 1 |
UCB or mobilized PB derived CD34+ cells |
3 weeks |
47 (CD34+) |
65 (CFUs) |
17 fold increase in RCs (NOD/SCID) |
AE Boitano et al. Science 2010 |
| In vivo treatment |
Wnt5a conditioned medium |
UCB Lin- CD34+CD38- cells |
2 weeks starting at 2–3 weeks after transplantation |
|
|
3 fold higher engraftment level and 1.5 fold increased number of CD34+CD38- cells engrafted (NOD/SCID) |
B Murdoch et al., PNAS 2003 |
| In vivo treatment |
GSK-3 inhibitor |
UCB Lin- or mobilized PB derived MNCs |
Starting on the same day of transplantation |
|
|
5 fold increased number of CD34+ cells engrafted (NOD/SCID) |
JJ Trowbridge et al., Nat Med 2006 |
| CFU, colony-forming unit; ABM, adult bone marrow; MNCs, mononuclear cells; LTC-IC, long-term culture-initiating cells; RCs, repopulating cells; β2m, β-2 microbloblin |
Example for manipulation of intrinsic factors
One important breakthrough that suggested the full potential of ex vivo HSC expansion was retrovirus-mediated introduction of the homeobox B4 (HoxB4) gene which is a member of a large family of transcription factors with DNA-binding homeodomain, known to be involved in cell fate determination [27]. Overexpression of HoxB4 in murine HSC permitted about 1000-fold in vivo [28] and 40-fold in vitro [29] expansion. Many studies revealed that the HoxB4 gene regulates HSC self-renewal [30], although HoxB4-deficient mice only show mild reduction of hematopoietic cellularity with normal HSC generation and homeostasis in steady-state [31]. Of critical importance, HoxB4-transduced HSCs were expanded in vitro and serially transplantable without leading to development of malignancies or hematopoietic dysfunction [28]. However, as virus-mediated gene transfer by itself has the risk of insertional mutagenesis, i.e., activation of potential oncogenes [32], it would be preferable to achieve expansion without manipulation of the HSC genome, but simply by addition of extrinsic factors. Indeed, two independent groups developed a method for ex vivo HSC expansion using direct delivery recombinant HoxB4 protein, and achieved 4–6 fold expansion of in vivo functional mouse HSC [33] and 2.5 fold of human CD34+ cells containing HSC, capable of long-term repopulation of immunodeficient mice, a gold standard xenograft model currently available to test in vivo human HSC potential [34]. Since recombinant HoxB4 protein has a short half-life of less than an hour and is very unstable in culture [28], this technological limitation would need to be resolved before clinical application.
Examples for supplementation of environmental factors
During the last decades, many hematopoietic growth factors and their receptors were identified and tested regarding their efficacy on amplification and maintenance of HSC in vitro alone or in combination as e.g., Interleukin (IL)-3 [35], IL-6 [36-38], IL-11 [39], Flt3-ligand (Flt3L) [40], stem cell factor (SCF) [41], thrombopoietin (TPO) [37], fibroblast growth factor (FGF)-1 [42], Angiopoietin (Ang)-1 [43], Pleiotrophin [44]. Although the in vitroexperimental conditions and subsequent in vivo read out varied largely, the net increase of HSC during short–term liquid cultures ranged from about 2–8 fold for mouse cells and 2–4 fold for human cells. One of the highest HSC amplifications achieved to date is an about 30-fold net increase of functionally defined mouse HSC in serum free medium supplemented with Angiopoietin-like proteins (Angptls), secreted proteins with sequence homology to Angiopoietin [45]. Angptls were identified by comparative gene profiling analysis between mouse fetal liver CD3+ cells, which support HSCex vivo, and non-supporter populations, adult CD3+ or fetal liver Gr-1+ cells. Since a substantial HSC expansion was only observed when Angptls were used in combination with other hematopoietic cytokines, Angptls appear to activate a signalling pathway that cannot be activated by other growth factors. To understand the mechanism of action, the physiological function of Angptls and the cloning of the respective receptors need to be done.
HSCs express Notch family receptors which have three trans-membrane ligands, Jagged-1, Jagged-2, and Delta [46], that are expressed on certain cell types in hematopoietic system, as e.g., bone marrow stromal cells [47], endothelial cells [47] and osteoblasts [48]. However, mice with inactivation of Notch-1 and Jagged-1 showed normal hematopoiesis and survived as long as control mice after myeloablation, indicating a dispensable role of Jagged-1 in HSC maintenance [49]. A significant role of Notch-1 signalling pathways in regulation of HSCs has been suggested by studies on HSC niches. Mice lacking either the bone morphogenic protein receptor (BMP) or the parathyroid hormone receptor showed an increase in HSC numbers, associated with an increased number of osteoblasts [50, 51]. Interestingly, Jagged-1 secretion from osteoblasts was enhanced and led to increased activation of Notch signals in HSCs. Consistently, overexpression of constitutive active Notch-1 resulted in immortalisation of a cytokine-dependent mouse hematopoietic cell line with in vivo self-renewal and multi-lineage reconstitution capacity [52]. The same group subsequently engineered a Delta-1 protein fusion with the Fc portion of human immunoglobulin (Delta-1 Fc), and succeeded to increase mouse hematopoietic progenitors capable of short-term lymphoid and myeloid lineage repopulation several log-fold [38]. Moreover, a 100-fold increase of human CD34+ cells with enhanced ability to repopulate non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice was achieved after several weeks serum-free culture with Delta-1 Fc in combination with cytokines [53, 54]. Also, it was shown that addition of Jagged-1 to human cell culture did not increase recovery of total cell numbers but induced survival and maintenance of human cells with NOD/SCID multi-lineage repopulating capacity [47]. A subsequent clinical phase I trial evaluating immobilised Delta-1 mediated cord blood-derived ex vivo HSC expansion and transplantation suggested an enhanced rate of myeloid engraftment, rapid neutrophil recovery and no signs for chronic GVHD [53]. However, long-term risks and benefits remain to be evaluated.
The Hedgehog gene has been discovered from screenings of mutants with disrupted body patterning in Drosophila [55]. Hedgehog gene family members were shown to be implicated in modulation of hematopoietic differentiation [56], thymic development [57] and early hemato-vascular development [58]. Although deficiency of hedgehog signals do not have any impact on in vivo HSC maintenance in mice [59], it was found that one hedgehog gene, the corresponding receptors and the related downstream transcriptional factors are expressed in both human hematopoietic progenitors and BM-derived stromal cells [60]. Moreover, the modulation of hedgehog signalling with neutralising antibodies or the addition of recombinant protein changed cytokine-dependent self-renewal and proliferation in highly purified human HSPCs. Further analysis revealed that the effect of sonic hedgehog on human HSPCs may be in part mediated through regulation of BMP4 signalling pathway [60]. Consistently, addition of BMP4 to hematopoietic cytokines cocktails can extend the culture period of UBC-derived human HSPCs without losing in vivo repopulation, which indicates the potential role of BMP4 in ex vivo HSPC survival [61].
Another pathway which is involved in developmental processes as well as HSC function is the Wnt pathway. Murine fetal liver cells in conditioned medium from cells transfected with Wnt proteins [62], and co-culture of human BM cells with human stromal cell line expressing Wnt proteins [63] showed 7–11 fold increase in total cell number and 10–20 fold increase in number of mix colony-forming cells for mouse and human cells, respectively. Moreover, in vivo injection of Wnt5a-conditioned medium increased human cell engraftment in NOD/SCID mice, whereas in vitro proliferation was unaffected [64]. Further studies provided evidence that Wnt signalling regulates HSC self-renewal by increasing HoxB4 and Notch-1 expression [65]. Wnt-3a might induce self-renewal of mouse HSC in single cell cultures along with low concentration of SCF [66], however, these studies need to be specified as the constitutive activation of Wnt signalling in HSCs leads to blocked differentiation, loss of repopulation and down-regulation of self-renewal gene expression [67, 68]. Also, no impairment in HSC function and lymphopoiesis was observed in mice deficient for β- and γ-catenin, important in Wnt signalling [69, 70]. These conflicting findings might be explained by the evidence that Wnt-5a, involved in a non-canonical Wnt pathway, can promote murine HSC quiescence and in vivo repopulation by suppressing the Wnt-3a mediated canonical Wnt pathway [71]. Another possible explanation might be functional redundancy of other signalling pathways. Indeed, in vivo administration of a glycogen synthase kinase (GSK)-3 inhibitor improved in vivolong term repopulation of mouse and human HSPCs, possibly by modulating Notch, Hedgehog and Wnt signals [72].
While previous studies relied on observation and hypothesis-driven identification of factors and pathways for HSC self-renewal and expansion, it is now possible to achieve unbiased, hypothesis-free, efficient data collection by large-scale screening. In the zebrafish, a model system to study key genetic regulators in hematopoiesis [73], Prostaglandin E2 (PGE2) was identified as a regulator of HSC function [74]. Treatment with a long-acting derivative of PGE2, 16, 16-dimethyl-PGE2 (dmPGE2) increased HSC numbers in zebrafish, enhanced the production of colony-forming progenitors from mouse embryonic stem cells and increased the frequency of biologically functional adult mice HSC [74]. Prostaglandins are known to play a major role in inflammatory and immune responses [75]. Although steady-state hematopoiesis is normal in mice lacking cyclooxygenase (COX)-2, one of two PG synthases, hematopoietic recovery after myeloablation was markedly impaired [76]. In vivo competitive transplantation demonstrated enhanced frequency and competitive advantage of ex vivo short-term dmPGE2-treated mouse BM cells which was stably maintained in serial transplants [74]. This effect could be in part explained by increased homing through CXCR4 up-regulation, induced proliferation and prolonged survival [77]. In addition, studies on zebrafish and mouse HSC revealed that PGE2 is required for Wnt-mediated HSC generation and can increase HSC numbers synergistically with Wnt signalling, suggesting signal cross talks between those two pathways [78]. However, in vivo challenge of PGE2 to mice preferentially expanded hematopoietic progenitors but not HSC, and thus augmented only short-term engraftment [79]. To test the efficacy of PGE2 on human HSC, clinical trials on UCB transplantation are ongoing in which one of two UCB is ex vivo treated with dmPGE2 [80].
Recent advances in ex vivo HSC expansion
A similar approach to search for small molecules that expand HSCs during ex vivo culture was recently reported by Boitano et al. [81]. Mobilised Human PB CD34+ cells were cultured in vitro with serum-free medium containing TPO, SCF, Flt3L and IL-6 in a screening system using a drug library of 100,000 small molecules and the expression level of human HSPCs markers, CD34 and CD133 [82] were monitored. A purine derivative named as StemRegenin 1 (SR1) was identified as a most potential and promising candidate molecule increasing the number of CD34+ cells after 5-7 days. Of note, SR1 without hematopoietic cytokines did not induce CD34+ cell expansion and in a high concentration SR1 showed an anti-proliferative effect, suggesting that SR1 enhanced cytokine-mediated signals within a narrow dosing range. Also, SR1 did not expand mouse HSC but CD34+ cells from bone marrow of humans, monkeys and dogs as well as CD34+ cells from human UCB and mobilised PB CD34+ cells, indicating a potentially broad application in higher organisms. Serial transplantation into NOD/SCID mice revealed that 3 week culture of human UCB-derived CD34+ cells with SR1 resulted in 17-fold net expansion of cells with in vivo repopulation capacity for 7–8 months. Further analysis demonstrated that SR1 antagonised acryl hydrocarbon receptor (AHR)-mediated signalling by direct binding to the receptor. In line with this, it was previously documented that upon AHR agonist challenge, the repopulating activity of mouse HSC was significantly diminished and ex vivo treatment of BM cells with AHR agonist inhibited the proliferation of immature cells [83]. Thus, AHR signalling seems to control regulators of HSC function which include the cell cycle, self-renewal and homing [83]. It will be important to test the clinical potential and safety of SR1 alone or in combination with other molecules on clinical outcomes after transplantation.
Outlook
Gene targeting technology has greatly contributed to understanding the signalling pathways that maintain HSC homeostasis, i.e. self-renewal, differentiation and apoptosis, and modification of such signals has revealed first possibilities to ex vivo HSCs expansion and delineated avenues for its future application in clinical transplantation. However, artificial manipulation of HSC function remains a challenging subject. The difficulty of in vitro HSC maintenance/expansion but not differentiation appears to be closely related to HSC dormancy, an in vivo HSC characteristic that is thought to be associated with self-renewal capacity and unresponsiveness to growth stimulants. It has been suggested that mouse HSCs are divided into two pools, dormant and actively cycling HSCs, and quiescent HSCs have more repopulating potential than cycling ones [84, 85]. However, a recent study by our laboratory revealed that both dormant and cycling HSCs have equivalent lifelong self-renewal potential with fluctuating repopulation capacity, and that the HSC cycling status is dynamically switched over time leading to similar turnover rate in all HSCs at end of life [12]. Given that the same holds true for human HSC, induction of HSC proliferation by exogenous factors might not affect their critical lifelong repopulating potential (not measured in surrogate NOD/SCID assays), and thus HSC expansion would be achievable. However, experimental evidence suggests that HSC repopulating ability decreases with divisions and longer culture periods [37], and that overexpression of Bcl-2, an anti-apoptotic protein enhanced colony forming capacity in the presence of cytokines [86]. Thus, it is crucial to determine the optimal dose of stimulants for balancing proliferation with self-renewal. In fact some of the reagents described above have a narrow range in the effective concentration and show different outcomes on HSC function. Although almost all of liquid culture systems that have been developed to expand HSC in vitro require supplement of hematopoietic cytokines, in vitro culture conditions with only cytokines seem suboptimal to block apoptotic pathways in HSCs and to promote proliferation without reducing self-renewal. Thus, it will be critical to search for further factors that can compensate for diminished self-renewal during cytokine-dependent proliferation [81]. Also, as current in vitro systems lack three-dimensional environments that embed HSCs, the use of respective scaffold structures might help to enhance the chances for success [87]. Another way to provide a hematopoietic environment in vitro is co-culture of hematopoietic cells with mesenchymal stromal cells (MSCs) which have been shown to possess a potential for ex vivo UCB CD34+ cell expansion and immunomodulatory activity against GVHD [88–91]. Clinical trials to expand UCB with related donor MSCs are currently underway [92].
Continuous activation of self-renewal and lack of differentiation potential might lead to unexpected oncogenic events resulting in tumourigenesis as most of genes involved in self-renewal have also oncogenic potential [93]. Therefore, whenever a new method to amplify HSC ex vivo is developed, it would be essential to test functionality and safety of the expanded HSC, and thus it would be important to develop preclinical validation systems before stepping forward to human clinical trials. For now, most of studies relied on in vivo functional testing of human HSPCs in xenograft mouse models. However, because of no or only limited crossreactivity of mouse differentiation and survival factors to human receptors, human HSCs are, in contrast to mouse HSCs, not homeostatically expanded in current models [94]. To overcome these limitations, many groups have tried to supplement factors that are not supplied by mouse environment or the transplanted human cells themselves. First proof of principle that faithful expression of human factors through genetic modification or injection of recombinant human factors can significantly enhance reconstitution of human hemato-lymphoid system in mice has been achieved [95–101]. Thus, the identification of critical factors for HSC expansion in screens and the selective provision of these in non-perfectly cross-reactive xeno-models for homeostatic expansion of HSCs to test biology might hold a key to final success for clinically applicable human HSC expansion in the future.
References
1 Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A, et al. Hematopoietic Stem Cell Transplantation A Global Perspective. Jama-J Am Med Assoc. 2010;303:1617–24.
2 Champlin R. Now everyone has a donor for HSCT. Blood. 2011;118:218.
3 Gordon MY, Lewis JL, Marley SB. Of mice and men... and elephants. Blood. 2002;100:4679–80.
4 Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301.
5 Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–6.
6 Xie Y, Yin T, Wiegraebe W, He XC, Miller D, Stark D, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457:97–101.
7 Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806.
8 Allsopp RC, Morin GB, DePinho R, Harley CB, Weissman IL. Telomerase is required to slow telomere shortening and extend replicative lifespan of HSCs during serial transplantation. Blood. 2003;102:517–20.
9 Dick JE, Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333:218–21.
10 Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–21.
11 Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–5.
12 Takizawa H, Regoes RR, Boddupalli CS, Bonhoeffer S, Manz MG. Dynamic variation in cycling of hematopoietic stem cells in steady state and inflammation. J Exp Med. 2011.
13 Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106.
14 Appelbaum FR. Hematopoietic-cell transplantation at 50. N Engl J Med. 2007;357:1472–5.
15 Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–6.
16 Appelbaum FR. Hematopoietic cell transplantation from unrelated donors for treatment of patients with acute myeloid leukemia in first complete remission. Best Pract Res Clin Haematol. 2007;20:67–75.
17 Krenger W, Hollander GA. The role of the thymus in allogeneic hematopoietic stem cell transplantation. Swiss Med Wkly. 2010;140:w13051.
18 Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–101.
19 Passweg J, Baldomero H, Stern M, Bargetzi M, Ghielmini M, Leibundgut K, et al. Hematopoietic stem cell transplantation in Switzerland: a comprehensive quality control report on centre effect. Swiss Med Wkly. 2010;140:326–34.
20 To LB, Haylock DN, Simmons PJ, Juttner CA. The biology and clinical uses of blood stem cells. Blood. 1997;89:2233–58.
21 Gluckman E. Milestones in umbilical cord blood transplantation. Blood Rev. 2011.
22 Thomson BG, Robertson KA, Gowan D, Heilman D, Broxmeyer HE, Emanuel D, et al. Analysis of engraftment, graft-versus-host disease, and immune recovery following unrelated donor cord blood transplantation. Blood. 2000;96:2703–11.
23 Wagner JE, Rosenthal J, Sweetman R, Shu XO, Davies SM, Ramsay NK, McGlave PB, et al. Successful transplantation of HLA-matched and HLA-mismatched umbilical cord blood from unrelated donors: analysis of engraftment and acute graft-versus-host disease. Blood. 1996;88:795–802.
24 Sideri A, Neokleous N, Brunet de la Grange P, Guerton B, Le Bousse Kerdiles MC, Uzan G, et al. An overview of the progress on double umbilical cord blood transplantation. Haematologica. 2011.
25 Chen YB, Spitzer TR. Current status of reduced-intensity allogeneic stem cell transplantation using alternative donors. Leukemia. 2008;22:31–41.
26 Rubinstein P, Dobrila L, Rosenfield RE, Adamson JW, Migliaccio G, Migliaccio AR, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci U S A 1995;92:10119–22.
27 McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302.
28 Sauvageau G, Thorsteinsdottir U, Eaves CJ, Lawrence HJ, Largman C, Lansdorp PM, et al. Overexpression of HOXB4 in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes Dev. 1995;9:1753–65.
29 Antonchuk J, Sauvageau G, Humphries RK. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell. 2002;109:39–45.
30 Sauvageau G, Iscove NN, Humphries RK. In vitro and in vivo expansion of hematopoietic stem cells. Oncogene. 2004;23:7223–32.
31 Brun AC, Bjornsson JM, Magnusson M, Larsson N, Leveen P, Ehinger M, Nilsson E, Karlsson S. Hoxb4-deficient mice undergo normal hematopoietic development but exhibit a mild proliferation defect in hematopoietic stem cells. Blood 2004; 103:4126-33.
32 Baum C, von Kalle C, Staal FJ, Li Z, Fehse B, Schmidt M, et al. Chance or necessity? Insertional mutagenesis in gene therapy and its consequences. Mol Ther. 2004;9:5–13.
33 Krosl J, Austin P, Beslu N, Kroon E, Humphries RK, Sauvageau G. In vitro expansion of hematopoietic stem cells by recombinant TAT-HOXB4 protein. Nat Med. 2003;9:1428–32.
34 Amsellem S, Pflumio F, Bardinet D, Izac B, Charneau P, Romeo PH, et al. Ex vivo expansion of human hematopoietic stem cells by direct delivery of the HOXB4 homeoprotein. Nat Med. 2003;9:1423–7.
35 Rennick DM, Lee FD, Yokota T, Arai KI, Cantor H, Nabel GJ. A cloned MCGF cDNA encodes a multilineage hematopoietic growth factor: multiple activities of interleukin 3. J Immunol. 1985;134:910–4.
36 Bhatia M, Bonnet D, Kapp U, Wang JC, Murdoch B, Dick JE. Quantitative analysis reveals expansion of human hematopoietic repopulating cells after short-term ex vivo culture. J Exp Med. 1997;186:619–24.
37 Ema H, Takano H, Sudo K, Nakauchi H. In vitro self-renewal division of hematopoietic stem cells. J Exp Med. 2000;192:1281–8.
38 Varnum-Finney B, Brashem-Stein C, Bernstein ID. Combined effects of Notch signaling and cytokines induce a multiple log increase in precursors with lymphoid and myeloid reconstituting ability. Blood. 2003;101:1784–9.
39 Lemieux ME, Chappel SM, Miller CL, Eaves CJ. Differential ability of flt3-ligand, interleukin-11, and Steel factor to support the generation of B cell progenitors and myeloid cells from primitive murine fetal liver cells. Exp Hematol. 1997;25:951–7.
40 Miller CL, Eaves CJ. Expansion in vitro of adult murine hematopoietic stem cells with transplantable lympho-myeloid reconstituting ability. Proc Natl Acad Sci. U S A 1997;94:13648–53.
41 Brandt J, Briddell RA, Srour EF, Leemhuis TB, Hoffman R. Role of c-kit ligand in the expansion of human hematopoietic progenitor cells. Blood. 1992;79:634–41.
42 de Haan G, Weersing E, Dontje B, van Os R, Bystrykh LV, Vellenga E, et al. In vitro generation of long-term repopulating hematopoietic stem cells by fibroblast growth factor-1. Dev Cell. 2003;4:241–51.
43 Nakamura Y, Yahata T, Muguruma Y, Uno T, Sato T, Matsuzawa H, et al. Angiopoietin-1 supports induction of hematopoietic activity in human CD34- bone marrow cells. Exp Hematol. 2007;35:1872–83.
44 Himburg HA, Muramoto GG, Daher P, Meadows SK, Russell JL, Doan P, et al. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat Med. 2010;16:475–82.
45 Zhang CC, Kaba M, Ge G, Xie K, Tong W, Hug C, et al. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med. 2006;12:240–5.
46 Kojika S, Griffin JD. Notch receptors and hematopoiesis. Exp Hematol. 2001;29:1041–52.
47 Karanu FN, Murdoch B, Gallacher L, Wu DM, Koremoto M, Sakano S, et al. The notch ligand jagged-1 represents a novel growth factor of human hematopoietic stem cells. J Exp Med. 2000;192:1365–72.
48 Pereira RMR, Delany AM, Durant D, Canalis E. Cortisol regulates the expression of Notch in osteoblasts. J Cell Biochem. 2002;85:252–8.
49 Mancini SJ, Mantei N, Dumortier A, Suter U, MacDonald HR, Radtke F. Jagged1-dependent Notch signaling is dispensable for hematopoietic stem cell self-renewal and differentiation. Blood. 2005;105:2340–2.
50 Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003; 425:836-41.
51 Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–6.
52 Varnum-Finney B, Xu L, Brashem-Stein C, Nourigat C, Flowers D, Bakkour S, et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med. 2000;6:1278–81.
53 Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16:232–6.
54 Ohishi K, Varnum-Finney B, Bernstein ID. Delta-1 enhances marrow and thymus repopulating ability of human CD34(+)CD38(-) cord blood cells. J Clin Invest. 2002;110:1165–74.
55 Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801.
56 Detmer K, Walker AN, Jenkins TM, Steele TA, Dannawi H. Erythroid differentiation in vitro is blocked by cyclopamine, an inhibitor of hedgehog signaling. Blood Cells Mol Dis. 2000;26:360–72.
57 Outram SV, Varas A, Pepicelli CV, Crompton T. Hedgehog signaling regulates differentiation from double-negative to double-positive thymocyte. Immunity. 2000;13:187–97.
58 Dyer MA, Farrington SM, Mohn D, Munday JR, Baron MH. Indian hedgehog activates hematopoiesis and vasculogenesis and can respecify prospective neurectodermal cell fate in the mouse embryo. Development. 2001;128:1717–30.
59 Gao J, Graves S, Koch U, Liu S, Jankovic V, Buonamici S, et al. Hedgehog signaling is dispensable for adult hematopoietic stem cell function. Cell Stem Cell. 2009;4:548–58.
60 Bhardwaj G, Murdoch B, Wu D, Baker DP, Williams KP, Chadwick K, et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001;2:172–80.
61 Bhatia M, Bonnet D, Wu D, Murdoch B, Wrana J, Gallacher L, et al. Bone morphogenetic proteins regulate the developmental program of human hematopoietic stem cells. J Exp Med. 1999;189:1139–48.
62 Austin TW, Solar GP, Ziegler FC, Liem L, Matthews W. A role for the Wnt gene family in hematopoiesis: expansion of multilineage progenitor cells. Blood. 1997;89:3624–35.
63 Van Den Berg DJ, Sharma AK, Bruno E, Hoffman R. Role of members of the Wnt gene family in human hematopoiesis. Blood. 1998;92:3189–202.
64 Murdoch B, Chadwick K, Martin M, Shojaei F, Shah KV, Gallacher L, et al. Wnt-5A augments repopulating capacity and primitive hematopoietic development of human blood stem cells in vivo. Proc Natl Acad Sci. U S A 2003;100:3422–7.
65 Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–14.
66 Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–52.
67 Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol. 2006;7:1048–56.
68 Scheller M, Huelsken J, Rosenbauer F, Taketo MM, Birchmeier W, Tenen DG, et al. Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat Immunol. 2006;7:1037–47.
69 Cobas M, Wilson A, Ernst B, Mancini SJ, MacDonald HR, Kemler R, et al. Beta-catenin is dispensable for hematopoiesis and lymphopoiesis. J Exp Med. 2004;199:221–9.
70 Koch U, Wilson A, Cobas M, Kemler R, Macdonald HR, Radtke F. Simultaneous loss of beta- and gamma-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2008;111:160–4.
71 Nemeth MJ, Topol L, Anderson SM, Yang Y, Bodine DM. Wnt5a inhibits canonical Wnt signaling in hematopoietic stem cells and enhances repopulation. Proc Natl Acad Sci. U S A 2007;104:15436–41.
72 Trowbridge JJ, Xenocostas A, Moon RT, Bhatia M. Glycogen synthase kinase-3 is an in vivo regulator of hematopoietic stem cell repopulation. Nat Med. 2006;12:89–98.
73 Bertrand JY, Traver D. Hematopoietic cell development in the zebrafish embryo. Curr Opin Hematol. 2009;16:243–8.
74 North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–11.
75 Miller SB. Prostaglandins in health and disease: an overview. Semin Arthritis Rheum. 2006;36:37–49.
76 Lorenz M, Slaughter HS, Wescott DM, Carter SI, Schnyder B, Dinchuk JE, et al. Cyclooxygenase-2 (COX-2) is essential for normal recovery from 5-fluorouracil (5-FU) induced myelotoxicity in mice. Blood. 1996;88:1358–8.
77 Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444–55.
78 Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136:1136–47.
79 Frisch BJ, Porter RL, Gigliotti BJ, Olm-Shipman AJ, Weber JM, O’Keefe RJ, et al. In vivo prostaglandin E2 treatment alters the bone marrow microenvironment and preferentially expands short-term hematopoietic stem cells. Blood. 2009;114:4054–63.
80 Durand EM, Zon LI. Newly emerging roles for prostaglandin E2 regulation of hematopoiesis and hematopoietic stem cell engraftment. Curr Opin Hematol. 2010;17:308–12.
81 Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–8.
82 de Wynter EA, Buck D, Hart C, Heywood R, Coutinho LH, Clayton A, et al. CD34+AC133+ cells isolated from cord blood are highly enriched in long-term culture-initiating cells, NOD/SCID-repopulating cells and dendritic cell progenitors. Stem Cells. 1998;16:387–96.
83 Singh KP, Casado FL, Opanashuk LA, Gasiewicz TA. The aryl hydrocarbon receptor has a normal function in the regulation of hematopoietic and other stem/progenitor cell populations. Biochem Pharmacol. 2009;77:577–87.
84 Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–29.
85 Foudi A, Hochedlinger K, Van Buren D, Schindler JW, Jaenisch R, Carey V, et al. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat Biotechnol. 2009;27:84–90.
86 Domen J, Weissman IL. Hematopoietic stem cells need two signals to prevent apoptosis; BCL-2 can provide one of these, Kitl/c-Kit signaling the other. J Exp Med. 2000;192:1707–18.
87 Dellatore SM, Garcia AS, Miller WM. Mimicking stem cell niches to increase stem cell expansion. Curr Opin Biotechnol. 2008;19:534–40.
88 Kobune M, Kawano Y, Kato J, Ito Y, Chiba H, Nakamura K, et al. Expansion of CD34+ cells on telomerized human stromal cells without losing erythroid-differentiation potential in a serum-free condition. Int J Hematol. 2005;81:18–25.
89 Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–41.
90 Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–6.
91 McNiece I, Harrington J, Turney J, Kellner J, Shpall EJ. Ex vivo expansion of cord blood mononuclear cells on mesenchymal stem cells. Cytotherapy. 2004;6:311–7.
92 Kelly SS, Sola CB, de Lima M, Shpall E. Ex vivo expansion of cord blood. Bone Marrow Transplant. 2009;44:673–81.
93 Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11.
94 Manz MG. Human-hemato-lymphoid-system mice: opportunities and challenges. Immunity. 2007;26:537–41.
95 O’Connell RM, Balazs AB, Rao DS, Kivork C, Yang L, Baltimore D. Lentiviral vector delivery of human interleukin-7 (hIL-7) to human immune system (HIS) mice expands T lymphocyte populations. PLoS One. 2010;5:e12009.
96 Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med. 2009;206:25–34.
97 van Lent AU, Dontje W, Nagasawa M, Siamari R, Bakker AQ, Pouw SM, et al. IL-7 enhances thymic human T cell development in “human immune system” Rag2-/-IL-2Rgammac-/- mice without affecting peripheral T cell homeostasis. J Immunol. 2009;183:7645–55.
98 Billerbeck E, Barry WT, Mu K, Dorner M, Rice CM, Ploss A. Development of human CD4+FoxP3+ regulatory T cells in human stem cell factor, GM-CSF and interleukin 3 expressing NOD SCID IL2R{gamma}NULL humanized mice. Blood. 2011.
99 Willinger T, Rongvaux A, Takizawa H, Yancopoulos GD, Valenzuela DM, Murphy AJ, et al. Human IL-3/GM-CSF knock-in mice support human alveolar macrophage development and human immune responses in the lung. Proc Natl Acad Sci. U S A 2011;108:2390–5.
100 Rongvaux A, Willinger T, Takizawa H, Rathinam C, Auerbach W, Murphy AJ, et al. Human thrombopoietin knockin mice efficiently support human hematopoiesis in vivo. Proc Natl Acad Sci. U S A 2011;108:2378–83.
101 Willinger T, Rongvaux A, Strowig T, Manz MG, Flavell RA. Improving human hemato-lymphoid-system mice by cytokine knock-in gene replacement. Trends Immunol. 2011;32:321–7.
