Improved health of hospitality workers after a Swiss cantonal smoking ban
DOI: https://doi.org/10.4414/smw.2011.13317
AD
Durham, S
Bergier, X
Morisod, I
Locatelli, JP
Zellweger, CK
Huynh
Summary
QUESTIONS UNDER STUDY: Hospitality workers are a population particularly at risk from the noxious effects of environmental tobacco smoke (ETS). The Canton of Vaud, Switzerland banned smoking in public places in September 2009. This prospective study addresses the impact of the ban on the health of hospitality workers.
METHODS: ETS exposure was evaluated using a passive sampling device that measures airborne nicotine; lung function was assessed by spirometry; health-related quality of life, ETS exposure symptoms and satisfaction were measured by questionnaire.
RESULTS: 105 participants (smokers and non-smokers) were recruited initially and 66 were followed up after one year. ETS exposure was significantly lower after the ban. Hospitality workers had lower pre-ban forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) values than expected. FEV1 remained stable after the ban, with a near-significant increase in the subgroup of asthmatics only. FVC increased at one year follow-up from 90.42% to 93.05% (p = 0.02) in the entire cohort; women, non-smokers and older participants gained the greatest benefit. The health survey showed an increase in physical wellbeing after the ban, the greatest benefit being observed in non-smokers. ETS exposure symptoms were less frequent after the ban, especially red and irritated eyes and sneezing. The new law was judged useful and satisfactory by the vast majority of employees, including smokers.
CONCLUSION: The recent cantonal ban on smoking in public places brought about an improvement in lung function, physical well-being and ETS symptoms of hospitality workers, including smokers.
Introduction
Exposure to environmental tobacco smoke (ETS) has been associated with a multiplicity of ailments, including respiratory symptoms [1, 2], lung cancer [3–5], myocardial infarction [6–9] and stroke [10, 11]. ETS is considered a group 1 carcinogen according to the International Agency for Research on Cancer classification [12]. The World Health Organization Framework Convention on Tobacco Control strongly recommends the implementation of nationwide smoke-free policies in order to improve the indoor air quality of hospitality premises and workplaces. Thus, in recent years, smoking has been banned in public places in several countries and regions, with improvements in respiratory symptoms and function in hospitality workers [13–21], as well as a significant fall in hospital admissions for myocardial infarction [22–26]. Workers in hospitality venues are more at risk than the rest of the population, being exposed daily to higher doses of ETS; airborne nicotine concentrations are up to 18.5 times higher in hospitality venues than in offices or households [4]. Unfortunately, however, hospitality workers are often the last to benefit from a smoke-free work environment.
In Switzerland the health costs related to ETS have been estimated at CHF 330,000,000 a year [27], and it had no countrywide smoke-free policy before 2010. The citizens of the Swiss Canton of Vaud voted for a law banning smoke in public places that took effect on 15September 2009.
The objective of our prospective study (BASTA study: Before and After Secondhand Tobacco Act) was to evaluate the impact of the smoke-free policy on the health of hospitality workers (restaurants, bars, tearooms, discotheques) in the Canton of Vaud. As opposed to most studies, in this one our wish was to assess the ban’s impact in non-smokers as well as smokers. For this purpose we assessed ETS exposure by passive sampling of airborne nicotine and lung functions by spirometric measurements; health-related quality of life, ETS exposure symptoms and the perceived impact of the law were measured by questionnaire.
Methods
Objectives
The Canton of Vaud is situated in the French-speaking region of Switzerland and has a population of 701,526 inhabitants, representing 9% of the Swiss population (Federal statistics, 31.12.2009). On 30 November 2008 the citizens of this canton voted for a law banning smoke in all public places but allowing the creation of service-free isolated smoking areas. The law took effect on 15 September 2009. The aim of this prospective study was to determine the impact of the ban on the health of hospitality workers.
Participants
Eligible subjects were all adult hospitality workers, smokers and non-smokers, working in the Canton of Vaud during the period from 30 April to 10 September 2009, before the cantonal smoke-free law took effect. A letter inviting hospitality workers to participate in the study, but offering no reward or incentive, was mailed to the owners of all the 1,798 hospitality venues (restaurants, bars, tea rooms, discotheques) in the Canton of Vaud phone directory, and was met with an affirmative response rate of just 2%. Subjects were recruited in the 36 establishments which responded affirmatively on a voluntary basis after it was explained that the study’s purpose was to investigate the health impact of the upcoming cantonal smoke-free law. The participants received no financial compensation. The response rate of participants at the venues that agreed to take part in the study was not assessed, nor were the reasons for non-participation.
The first set of measurements took place on the day of recruitment. A second set was taken after six months, and the final set was undertaken from 31 May to 26 September 2010, representing a time point one year after initial recruitment (and approximately 1 year after the ban), which enabled us to avoid any seasonal effect bias. Results at 6 months are not presented as they contribute little to the present article.
Description of procedures
ETS exposure was measured using a passive sampling device [28]. These personal Monitors of NICotine (MoNIC badges) were given to all participants at the start of their workshift, after proper instructions, and were worn pinned to their shirts throughout the entire shift. At the end of their shifts the badge was removed and placed in an airtight container before analysis. The badges were analysed by a method adapted from that of Hammond and Ogden [29, 30]. Nicotine is analysed by gas phase chromatography with a nitrated product-specific detector. Physiological respiratory data were used to calculate the number of cigarettes inhaled passively, or cigarette equivalents (CE), during the period of exposure. To simplify, we adopted an average ventilation rate of 10 L/min, corresponding to 1,000 times the speed of uptake of the MoNIC badge. Once the quantity of nicotine was known, we could calculate the CE inhaled passively by taking into account a nicotine rate of 0.2 mg/cigarette (corresponding to a “light” cigarette). For smokers the inhaled CE per day was calculated by adding the passive smoking CE measured by the MoNIC badges to the number of self-reported cigarettes consumed by active smoking.
Lung function tests were performed with an EasyOne portable spirometer (ndd Medizintechnik AG, Switzerland) as they were shown to produce highly reproducible measurements [31, 32]. Each participant was shown the proper technique and measures were taken in a standing position. Participants were asked to perform the technique at the start of their workshift for practice and then again at the end of their workday at the workplace; only this last measure was taken into consideration for the analysis. Each participant underwent at least three forced expiratory manoeuvres and the resulting graphs were analysed by a trained pneumologist (Jean-Pierre Zellweger) for validity. Graphs judged invalid were rejected. Lung function tests, i.e. forced expiratory volume in one second (FEV1) and forced vital capacity (FVC), are expressed as the percentage of expected values for sex, age and height using a sample population of Switzerland [33] and by multiplying the values of participants of African ascent by 0.88. The expected values are recalculated at each visit in order to use the age at the time of measurement.Lung function measurements were excluded from analysis if participants had a cold on the day of measurement. 4 outliers were also excluded as they exhibited too wide a variation between measurements to be considered valid, suggesting either a technical problem or insufficient cooperation. The “lung age” was determined using the formula developed by Morris and Temple [34] as follows: in men, lung age = 2.87 × height (in inches) − (31.25 × observed FEV1 (in litres)) − 39.375; in women, lung age = 3.56 × height (in inches) − (40 × observed FEV1(in litres)) − 77.28. “Δ Lung age” is the difference between the lung age and the chronological age in years.
Participants completed a survey containing the SF-36v2 Health Survey [35], a short-form, multipurpose health survey with 36 items. It is a generic instrument and has been widely used in a variety of populations. It includes 8 health concepts (physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, mental health, and reported health transition) as well as physical and mental health summary scores. Norm-based scoring is used with higher scores indicating better overall functioning with the mean set as 50. The survey also included questions regarding the participants’ characteristics, including smoking habits and working status as well as questions regarding ETS symptoms and the perceived impact of the law.
Asthmatic participants are defined as having a self-reported doctor’s diagnosis of asthma, or as currently taking asthma medications. Never-smokers are defined as never having smoked at least 20 packs of cigarettes or 360 g of tobacco in their lifetime. Ex-smokers are defined as having quit smoking at least 6 months before study enrolment. Participants who report smoking at any time during the study period are considered to be smokers throughout. Non-smokers represent the combination of never-smokers and ex-smokers. Body mass index (BMI) was established with self-reported height and weight using the following formula: BMI = weight (in kilograms) / (height [in metres])2.
Ethics
The study protocol was submitted to and approved by the University of Lausanne’s clinical research ethical committee (protocol 176/07 and addendum). All participants gave written informed consent.
Statistical methods
To analyse the FEV1, FVC, “Δ Lung age” and results of the SF-36v2 survey, we used a longitudinal model which is well suited to the analysis of repeated measures for each participant. This allowed us to treat missing values without having biased results, provided our dropouts can be considered to be missing at random [36, 37].
We used Stata/IC statistical software (v 11.1, StataCorp LP, College Station, TX, USA) for baseline data description, ETS exposure analysis (1-sided Fisher’s exact test) and perceived impact of the law (χ2 test). We used the R system for statistical computation and graphics for all longitudinal models (v 2.11.1, www.r-project.org/ http://www.r-project.org , function “lme”, library “nlme”). All statistical analyses for exposure data were performed with the SYSTAT statistical software package and SigmaPlot v11 (SYSTAT Software Inc., USA; release 12), using two group t-test for comparisons.
Results
105 participants were recruited before the smoking ban took effect in the 36 venues taking part in the study. The second assessment, done after the ban, took place 1 year after the first (average of 357.9 days post-recruitment) with 66 participants followed-up (dropout rate of 37%). The characteristics of the remaining 66 participants were similar to the initial 105 (table 1). It was noteworthy, however, that a slightly increased loss of smokers, younger participants and women occurred during follow-up. Participants were on average in their forties and had been working 40-hour weeks for an average of 15 years. A majority of the participants were smokers and had a “lung age” that was 5 years older than their chronological age; their non-smoking colleagues did not present this difference (table 1).
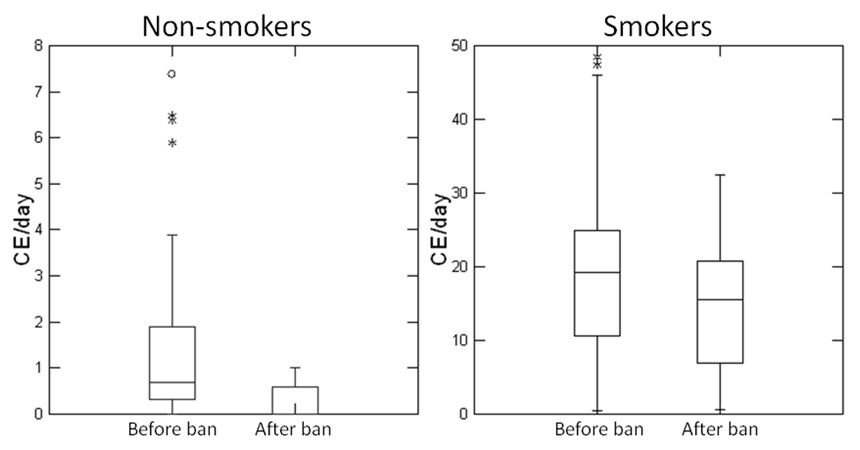
Figure 1
Decreased ETS exposure after the ban.
Box plot density displaying the inhaled cigarette equivalents (CE) per day of non-smokers (left panel) and smokers (right panel) before and at one year follow-up after the ban. The horizontal lines represent from bottom to top the 10th, 25th, 50th (median), 75th, and 90th percentiles. The whiskers show the range of observed values falling within the inner fences (1.5x inter-quartile range). Outside values between the inner and outer fences are plotted with asterisks. Far outside values (3x inter-quartile range), are plotted with empty circles.
ETS exposure assessment
Exposure to ETS declined significantly in hospitality venues after the introduction of the new smoke-free law (fig. 1). Before the ban non-smokers inhaled the equivalent of 1.4 cigarettes per day, with a maximum of 7.4, this relatively low exposure being nonetheless significantly reduced at one-year follow-up (p <0.05). Smokers’ cigarette equivalents (CE) were obtained by adding the passive CE acquired from the MoNIC badges to the self-reported active cigarette consumption. Before the ban the inhaled CE of smokers averaged 19, with a maximum of 48.5, and was almost significantly reduced to 15.3 (maximum 32.5) at follow-up after the ban (p = 0.07).
Lung functions
Both baseline FEV1 and FVC are reduced to approximately 90% of the predicted value compared to the reference Swiss population of never smoking adults [33] (table 2). FEV1 values were lower in men (87.84%) than in women (91.76%), in smokers (88.68%) than in non-smokers (91.58%) and in participants over 35 years old (87.3%) than in younger participants (93.12%), with similar values for FVC.
At the one-year control there was a significant increase in FVC from 90.42% to 93.05% (p = 0.02) after the ban. This increase was especially marked in women (+3.07%, p = 0.05), non-smokers (+3.91%, p = 0.04) and older participants (+4.22%, p = 0.004). Moreover, the small subgroup of asthmatic participants had an almost significant increase in FEV1 from 85.8% to 88.43% (p = 0.07).
Health survey
Physical functioning, as assessed with the SF-36v2, was significantly increased after the ban from 50.58 to 52.65(p = 0.04), translating into a higher global physical score (52.5 to 54.07, p = 0.05). This improvement in global physical score was especially marked in the subgroup of non-smokers (52.36 to 55.51, p = 0.02) (table 3). None of the other scores was significantly altered after the ban.
ETS exposure symptoms
Over 61% of surveyed hospitality employees reported being bothered by ETS at work before the ban (75.86% of non-smokers and 52.27% of smokers). Typical ETS exposure symptoms reported by the participants in the four weeks prior to questioning were generally reduced at follow-up (table 4). Red and irritated eye symptoms decreased from 26.79% and 31.48% to 12.5% and 11.11% respectively (p = 0.047 and 0.009), sneezing also decreased significantly from 23.53% prior to the ban to 7.84% afterwards (p = 0.027).
Perceived impact of the law
Prior to the ban, 84.88% of participants believed the new law would be beneficial for their health, with an increase to 90.32% at follow-up (p = 0.329). Smokers shared this conviction as 81.48% before the ban and 85.29% after the ban judged it useful as well (p = 0.643). 77.5% of surveyed employees were satisfied with the law before the ban took effect, increasing to 85.25% 1 year later (p = 0.247). Smoking participants showed a similar profile (70% to 76.47%, p = 0.514). Finally, employees were asked to estimate what impact this law would have on the establishment’s sales figures. 53% of employees estimated that the venue where they were working would see a reduction of over 5% in sales figures due to the new law before it took effect. One year later, after the law took effect, only 44% still believed that the reduction was over 5% (p = 0.322). Smokers tended to be more pessimistic than non-smokers, as 56.41% vs 48% imagined this reduction before the ban, with a decrease to 54.29% of smokers (p = 0.854) compared to 30.77% of non-smokers (p = 0.208) one year later.
|
Table 1: Demographic characteristics of the participants. |
| |
At baseline (n = 105)
|
At follow-up (n = 66)
|
| Age, mean (SD), y |
37.4 (12.5) |
41.3 (12.1) |
| Sex, No. (%), M |
47 (44.8) |
31 (47) |
| Work venues, No. (%) |
Restaurant |
69 (65.7) |
47 (71.2) |
| |
Bar |
17 (16.2) |
10 (15.2) |
| |
Tea Room |
8 (7.6) |
2 (3) |
| |
Discotheque |
5 (4.8) |
4 (6.1) |
| |
Other |
6 (5.7) |
3 (4.6) |
| Work, mean (SD), h/w* |
43.4 (19.5) |
39.1 (15.1) |
| Work, mean (SD), years* |
14.4 (13.5) |
16.6 (14.2) |
| Asthmatic, No. (%) |
13 (12.4) |
10 (15.1) |
| BMI, mean (SD), kg/m2
|
22.6 (3) |
22.8 (3.1) |
| Smoking status, No. (%) |
Smoker |
64 (61) |
36 (54.6) |
| |
Non-smoker |
41 (39) |
30 (45.4) |
| |
– Ex-smoker |
18 (17.1) |
14 (21.2) |
| Smoker’s consumption, mean (SD), cig/d |
14.8 (9.8) |
14.5 (9.2) |
| Age when started smoking, mean (SD), y* |
18.4 (5.3) |
18.5 (5.3) |
| No of years smoked, mean (SD), y* |
15.3 (8.8) |
17.9 (8.9) |
| Δ Lung age, y** |
Entire cohort |
3 |
2.5 |
| |
Smokers |
5.6 |
4.4 |
| |
Non-smokers |
–0.8 |
–0.2 |
| * Data missing from several participants (questions unanswered), not accounted for. ** Calculated using data from the same participants as for lung function tests |
|
Table 2: Lung functions before and after the ban. |
|
|
FEV1, mean, %
|
|
|
Before ban
|
After ban
|
P-value
|
| Entire cohort |
89.86 |
89.94 |
0.9 |
| Men |
87.84 |
86.94 |
0.35 |
| Women |
91.76 |
92.43 |
0.38 |
| Non-smokers |
91.58 |
90.8 |
0.41 |
| Smokers |
88.68 |
89.35 |
0.38 |
| Age <35 years |
93.12 |
93.05 |
0.95 |
| Age >35 years |
87.3 |
87.48 |
0.8 |
| Asthmatics |
85.8 |
88.43 |
0.07 |
|
|
FVC, mean, %
|
|
|
Before ban
|
After ban
|
P value
|
| Entire cohort |
90.42 |
93.05 |
0.02 |
| Men |
87.34 |
89.1 |
0.35 |
| Women |
93.18 |
96.25 |
0.05 |
| Non-smokers |
91.46 |
95.37 |
0.04 |
| Smokers |
89.66 |
91.42 |
0.26 |
| Age <35 years |
93.47 |
92.63 |
0.7 |
| Age >35 years |
88.04 |
92.26 |
0.004 |
| Asthmatics |
89.76 |
93.39 |
0.23 |
| Values obtained from a longitudinal model taking into account all the observations (n = 86). Observations from participants claiming to have a “cold” on the day of the exam were removed, as well as 4 clear outliers. In bold: P values ≤0.05 |
|
Table 3: 36-item short form health survey before and after the ban. |
| |
Global
|
Non-smoker
|
Smoker
|
| |
Before |
After |
p-value |
Before |
After |
p-value |
Before |
After |
p-value |
| PF |
50.58
|
52.65
|
0.04
|
50.61 |
54.39 |
0.13 |
50.58 |
51.26 |
0.6 |
| RP |
50.77 |
52.43 |
0.13 |
49.9 |
52.5 |
0.13 |
51.31 |
52.32 |
0.48 |
| BP |
50.4 |
51.29 |
0.4 |
50.64 |
52.56 |
0.23 |
50.25 |
50.3 |
0.97 |
| GH |
50.29 |
49.88 |
0.6 |
52.16 |
51.54 |
0.6 |
49.13 |
48.79 |
0.74 |
| VT |
50.61 |
49.82 |
0.67 |
50.27 |
49.32 |
0.54 |
50.82 |
50.19 |
0.64 |
| SF |
45.72 |
45.86 |
0.91 |
45.95 |
47.5 |
0.39 |
45.58 |
44.58 |
0.53 |
| RE |
47.57 |
47.78 |
0.87 |
46.83 |
46.97 |
0.95 |
48.03 |
48.4 |
0.84 |
| MH |
44.69 |
44.31 |
0.76 |
45.75 |
45.64 |
0.95 |
44.03 |
43.34 |
0.68 |
| Summary |
|
|
|
|
|
|
|
|
|
| GPS |
52.5
|
54.07
|
0.05
|
52.36
|
55.51
|
0.02
|
52.67 |
52.98 |
0.55 |
| GMS |
45.13 |
44.08 |
0.41 |
45.47 |
44.17 |
0.51 |
44.93 |
44.04 |
0.6 |
| Abbreviations: BP, bodily pain; GH, general health; GMS, global mental score; GPS, global physical score; MH, mental health; PF, physical functioning; RE, role emotional; RP, role physical; SF, social functioning; VT, vitality. In bold: P values ≤0.05 |
|
Table 4: Reported symptoms before and after the ban. |
| |
Before ban %
|
After ban %
|
p-value
|
| Cough (n = 50) |
14 |
16 |
0.5 |
| Wheezing (n = 51) |
7.84 |
7.84 |
0.646 |
| Chest oppression (n = 52) |
5.77 |
1.92 |
0.309 |
| Shortness of breath (n = 51) |
9.8 |
7.84 |
0.5 |
| Red eyes (n = 56) |
26.79
|
12.5
|
0.047
|
| Irritated eyes (n = 54) |
31.48
|
11.11
|
0.009
|
| Irritated throat (n = 52) |
13.46 |
9.62 |
0.38 |
| Irritated nose (n = 52) |
13.46 |
9.62 |
0.38 |
| Runny nose (n = 50) |
20 |
10 |
0.131 |
| Headache (n = 53) |
35.85 |
28.3 |
0.266 |
| Sneezing (n = 51) |
23.53
|
7.84
|
0.027
|
| Frequent/very frequent reported symptoms in the 4-week period prior to survey. All participants having answered the questions at both surveys. In bold: p-values ≤0.05 |
Discussion
Decreased ETS exposure
To asses ETS exposure we chose a method allowing measurement of airborne nicotine using an individual passive sampling device [28]. Among the different components of ETS, such as small particulate matter, CO or tar, nicotine is an ideal candidate to evaluate exposure. Its levels can be easily correlated with the official indications on cigarette packets to establish cigarette equivalents.
The smoke-free law that took effect in September 2009 was followed, as expected, by a significant decrease in ETS exposure. Exposure after the ban is not of course non-existent in non-smokers, as one can imagine that nicotine particles from smoking patrons, colleagues or the exterior contribute to this very low level of exposure. Another point is that smoking outdoors on terraces is not forbidden and leads to a minimal amount of ETS exposure as well.
The levels of ETS were low compared to a similar study [28]. We believe this is due to the fact that the measurements were taken during the spring and summer months when windows were opened and many customers remained on terraces outside. Due to time constraints between acceptance of the law by the population and its implementation we were unable to start the study earlier to assess for winter ETS exposure, which would certainly have been higher. This makes the difference observed between ‘before’ and ‘after’ the ban even more interesting.
A potential bias may derive from the fact that employers who are sensitive to the topic of ETS may have been more likely to accept that their workers participate in the study. These employers may offer better ventilated work spaces for their employees, which could also lead to the low levels of initial ETS exposure we witnessed. Nonetheless, we show that a decrease in this relatively small amount of ETS exposure leads to a significant health impact.
Hospitality workers have inferior lung function to the general population
The participants in this study have approximately 10% reduced lung function (FEV1 and FVC) compared to the predicted values, which is in agreement with studies in similar populations in Ireland [18] and Argentina [21], but less so in Scotland [20], where the reduction was more limited. The predicted values used for our study are derived from a representative sample of various Swiss regions [33], albeit in never-smokers, and is therefore ideal for our situation. The higher proportion of smokers in our study population (61% at baseline) compared to the citizens of Lausanne, the largest city in the Canton of Vaud (27%), as well as from Switzerland in general (32% of men, 23.8% of women) [38, 39], does not completely explain this observation, as non-smokers also exhibit this decrease; the difference between smokers and non-smokers is relatively minimal (table 2). This difference however is more striking when “lung age” is assessed; smokers present lungs that are 5 years older than their chronological age, a circumstance not observed in non-smokers (table 1). The spirometric measures were performed at the workplace, and it can therefore be argued that this is a suboptimal setting and may account for the lower performance. However, the criteria for valid spirometry were strict and the results were checked and validated by a trained pneumologist. The population we studied has worked in high ETS exposure settings for many years (table 1) which is the most likely cause for this finding. It is known that increased exposure to air pollutants results in a more rapid decline in lung function [40]. It will be interesting to analyse a future generation of hospitality workers not exposed to ETS to validate this point.
Improvement of lung functions after the secondhand tobacco act
One year after the smoking ban there is a clear improvement in FVC but not in FEV1. This is in agreement with previous studies where the most marked changes after smoking bans occur in FVC [15, 18, 21]. Interestingly, this improvement seems to benefit smokers as well. The largest benefit is in non-smokers, as is to be expected, in women and even more in older participants (over 35 years old). It has been demonstrated that women smokers who quit recover lung function faster than men [41], the hypothetical grounds being differences in lung characteristics and particle distribution. However, the better recovery of older participants is more surprising and to our knowledge has not been observed previously.
To avoid a “learning effect” bias, spirometric technique was performed for practice by the participants at the beginning of their workday and was then repeated for measurement at the end of the workday. As the second assessment took place one year after the first measurements we were able to avoid the seasonal effect bias. Due to the length and difficult logistics of the study (workplace measurements at the beginning and end of workshifts) we had several field workers participate in this study, which however introduces an additional bias.
Additional follow-up would be necessary to address the long term lung function benefits of the smoking ban.
Improved physical wellbeing after the ban
The ban was followed by an improvement in physical health, as demonstrated by the quality of life survey (SF-36v2). This improvement is even more significant in non-smokers. It may be speculated that this is in part attributable to the improved lung function and to a decrease in ETS exposure symptoms. This is an effect directly perceived by the workers themselves and is even more encouraging for further smoking bans in public places worldwide.
Benefits for smokers as well
Many of the previous studies addressing the effects of ETS only look at the status of non-smokers. However, smokers are obviously also in contact with secondhand smoke when at work, thereby increasing their overall exposure. We show that there is a trend towards improvement in this sub-group, albeit to a lesser extent than in non-smokers. This trend does not seem due to decreased consumption of cigarettes, as the participants’ consumption is the same at baseline and follow-up (table 1).
Ban well perceived among workers
The vast majority of employees, including smokers, found the ban beneficial to their health. This can be explained by improved physical wellbeing (table 3) and reduced ETS symptoms (table 4). The hospitality workers were on the whole satisfied with the new ban without major opposition from smokers, who also benefit from the law.
Limitations
Our dropout rate of 37% was consistent with previous studies at one year [13, 16–19], as hospitality venues have an oft-changing workforce. The main reasons for participants dropping out were changes in profession or untraceability. A self-selection bias is inevitable as workers with symptoms are more likely to be interested to participate in such a study. However, our study was performed at the workplace during the participants’ working hours, with the owners’ prior approval, thus limiting the personal time participants have to give up for the study. The majority of the workers in the different venues that accepted participation were willing to be part of the study, thus limiting this bias at the cost of losing out on many subjects with less enthusiastic employers.
The study was insufficiently powered for sub-groups such as asthmatics, which showed near significant improvements in lung function despite their small numbers (13 of the initial 105 participants, i.e. 12.38%) and also for different smoker statuses. Every venue in the phone directory was contacted and the answer rate was abysmally low at a little over 2%.
Conclusion
We confirm that smoke free laws benefit all hospitality workers, not just the non-smoking population, with a perceptible improvement in physical wellbeing. These workers have a reduced lung capacity which starts to recover after the ban. Participants, including smokers, judged the ban useful for their health and are satisfied with the law. We support enactment of new laws to protect workers in regions where they are currently left exposed.
Acknowledgements: We would like to thank Martha Guimaraes and Valentine Rozat for help in setting up the study and for data acquisition, Karine Giraudon for statistical expertise, Pierre-Olivier Bridevaux for help with the survey, Sylvie Payot for setting up the electronic database, Christine Arnoux for ETS exposure measurements, Samia Benraïs, Frédéric Cornuz, Alessandra Dosch, Alexandre Emery, Luc K.S. Ho, Gilles Lambelet, Etienne Revelly, Marianne Rey, Rosalie Tran and Sara Vonaesch for data acquisition.
References
1 Leuenberger P, Schwartz J, Ackermann-Liebrich U, Blaser K, Bolognini G, Bongard JP, et al. Passive smoking exposure in adults and chronic respiratory symptoms (SAPALDIA Study). Swiss Study on Air Pollution and Lung Diseases in Adults, SAPALDIA Team. Am J Respir Crit Care Med. 1994;150(5 Pt 1):1222–8.
2 Zemp E, Elsasser S, Schindler C, Kunzli N, Perruchoud AP, Domenighetti G, et al. Long-term ambient air pollution and respiratory symptoms in adults (SAPALDIA study). The SAPALDIA Team. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1257–66.
3 Brennan P, Buffler PA, Reynolds P, Wu AH, Wichmann HE, Agudo A, et al. Secondhand smoke exposure in adulthood and risk of lung cancer among never smokers: a pooled analysis of two large studies. Int J Cancer. 2004;109(1):125–31.
4 Siegel M, Skeer M. Exposure to secondhand smoke and excess lung cancer mortality risk among workers in the “5 B’s”: bars, bowling alleys, billiard halls, betting establishments, and bingo parlours. Tob Control. 2003;12(3):333–8.
5 Zhong L, Goldberg MS, Parent ME, Hanley JA. Exposure to environmental tobacco smoke and the risk of lung cancer: a meta-analysis. Lung Cancer. 2000;27(1):3–18.
6 He J, Vupputuri S, Allen K, Prerost MR, Hughes J, Whelton PK. Passive smoking and the risk of coronary heart disease – a meta-analysis of epidemiologic studies. N Engl J Med. 1999;340(12):920–6.
7 McElduff P, Dobson AJ, Jackson R, Beaglehole R, Heller RF, Lay-Yee R. Coronary events and exposure to environmental tobacco smoke: a case-control study from Australia and New Zealand. Tob Control. 1998;7(1):41–6.
8 Pitsavos C, Panagiotakos DB, Chrysohoou C, Skoumas J, Tzioumis K, Stefanadis C, et al. Association between exposure to environmental tobacco smoke and the development of acute coronary syndromes: the CARDIO2000 case-control study. Tob Control. 2002;11(3):220–5.
9 Rosenlund M, Berglind N, Gustavsson A, Reuterwall C, Hallqvist J, Nyberg F, et al. Environmental tobacco smoke and myocardial infarction among never-smokers in the Stockholm Heart Epidemiology Program (SHEEP). Epidemiology. 2001;12(5):558–64.
10 Bonita R, Duncan J, Truelsen T, Jackson RT, Beaglehole R. Passive smoking as well as active smoking increases the risk of acute stroke. Tob Control. 1999;8(2):156–60.
11 Iribarren C, Darbinian J, Klatsky AL, Friedman GD. Cohort study of exposure to environmental tobacco smoke and risk of first ischemic stroke and transient ischemic attack. Neuroepidemiology. 2004;23(1-2):38–44.
12 IARC. Tobacco Smoke and Involuntary Smoking. 2004.
13 Allwright S, Paul G, Greiner B, Mullally BJ, Pursell L, Kelly A, et al. Legislation for smoke-free workplaces and health of bar workers in Ireland: before and after study. BMJ. 2005;331(7525):1117.
14 Eagan TM, Hetland J, Aaro LE. Decline in respiratory symptoms in service workers five months after a public smoking ban. Tob Control. 2006;15(3):242–6.
15 Eisner MD, Smith AK, Blanc PD. Bartenders’ respiratory health after establishment of smoke-free bars and taverns. JAMA. 1998;280(22):1909–14.
16 Farrelly MC, Nonnemaker JM, Chou R, Hyland A, Peterson KK, Bauer UE. Changes in hospitality workers' exposure to secondhand smoke following the implementation of New York's smoke-free law. Tob Control. 2005;14(4):236–41.
17 Fernandez E, Fu M, Pascual JA, Lopez MJ, Perez-Rios M, Schiaffino A, et al. Impact of the Spanish smoking law on exposure to second-hand smoke and respiratory health in hospitality workers: a cohort study. PLoS One. 2009;4(1):e4244.
18 Goodman P, Agnew M, McCaffrey M, Paul G, Clancy L. Effects of the Irish smoking ban on respiratory health of bar workers and air quality in Dublin pubs. Am J Respir Crit Care Med. 2007;175(8):840–5.
19 Larsson M, Boethius G, Axelsson S, Montgomery SM. Exposure to environmental tobacco smoke and health effects among hospitality workers in Sweden – before and after the implementation of a smoke-free law. Scand J Work Environ Health. 2008;34(4):267–77.
20 Menzies D, Nair A, Williamson PA, Schembri S, Al-Khairalla MZ, Barnes M, et al. Respiratory symptoms, pulmonary function, and markers of inflammation among bar workers before and after a legislative ban on smoking in public places. JAMA. 2006;296(14):1742–8.
21 Schoj V, Alderete M, Ruiz E, Hasdeu S, Linetzky B, Ferrante D. The impact of a 100% smoke-free law on the health of hospitality workers from the city of Neuquen, Argentina. Tob Control. 2010;19(2):134–7.
22 Barone-Adesi F, Vizzini L, Merletti F, Richiardi L. Short-term effects of Italian smoking regulation on rates of hospital admission for acute myocardial infarction. Eur Heart J. 2006;27(20):2468–72.
23 Pell JP, Haw S, Cobbe S, Newby DE, Pell AC, Fischbacher C, et al. Smoke-free legislation and hospitalizations for acute coronary syndrome. N Engl J Med. 2008;359(5):482–91.
24 Sargent RP, Shepard RM, Glantz SA. Reduced incidence of admissions for myocardial infarction associated with public smoking ban: before and after study. BMJ. 2004;328(7446):977–80.
25 Trachsel LD, Kuhn MU, Reinhart WH, Schulzki T, Bonetti PO. Reduced incidence of acute myocardial infarction in the first year after implementation of a public smoking ban in Graubuenden, Switzerland. Swiss Med Wkly. 2010 Jan 12.
26 Bonetti PO, Trachsel LD, Kuhn MU, Schulzki T, Erne P, Radovanovic D, et al. Incidence of acute myocardial infarction after implementation of a public smoking ban in Graubunden, Switzerland: Two year follow-up. Swiss Med Wkly. 2011;141:w13206.
27 Hauri DD, Lieb CM, Rajkumar S, Kooijman C, Sommer HL, Roosli M. Direct health costs of environmental tobacco smoke exposure and indirect health benefits due to smoking ban introduction. Eur J Public Health. 2011;21(3):316–22.
28 Huynh CK, Moix JB, Dubuis A. Development and application of the passive smoking monitor MoNIC. Rev Med Suisse. 2008;4(144):430–3.
29 Hammond SK, Leaderer BP, Roche AC, Schenker M. Collection and analysis of Nicotine as a marker for environmental tobacco smoke. Atmospheric Environment (1967). 1987;21(2):457-62.
30 Ogden MW, Maiolo KC. Comparative Evaluation of Diffusive and Active Sampling Systems for Determining Airborne Nicotine and 3-Ethenylpyridine. Environmental Science & Technology. 1992;26(6):1226–34.
31 Barr RG, Stemple KJ, Mesia-Vela S, Basner RC, Derk SJ, Henneberger PK, et al. Reproducibility and validity of a handheld spirometer. Respir Care. 2008;53(4):433–41.
32 Walters JA, Wood-Baker R, Walls J, Johns DP. Stability of the EasyOne ultrasonic spirometer for use in general practice. Respirology. 2006;11(3):306–10.
33 Brandli O, Schindler C, Kunzli N, Keller R, Perruchoud AP. Lung function in healthy never smoking adults: reference values and lower limits of normal of a Swiss population. Thorax. 1996;51(3):277–83.
34 Morris JF, Temple W. Spirometric “lung age” estimation for motivating smoking cessation. Preventive medicine. 1985;14(5):655–62.
35 Ware JE, Jr. SF-36 health survey update. Spine (Phila Pa 1976). 2000;25(24):3130–9.
36 Mallinckrodt CH, Clark WS, David SR. Accounting for dropout bias using mixed-effects models. J Biopharm Stat. 2001;11(1-2):9–21.
37 Weiss RE. Modeling longitudinal data. New York; London: Springer; 2005.
38 Firmann M, Mayor V, Vidal PM, Bochud M, Pecoud A, Hayoz D, et al. The CoLaus study: a population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc Disord. 2008;8:6.
39 Marques-Vidal P, Cerveira J, Paccaud F, Cornuz J. Smoking trends in Switzerland, 1992–2007: a time for optimism? J Epidemiol Community Health. 2011;65(3):281–6.
40 Downs SH, Schindler C, Liu LJ, Keidel D, Bayer-Oglesby L, Brutsche MH, et al. Reduced exposure to PM10 and attenuated age-related decline in lung function. N Engl J Med. 2007;357(23):2338–47.
41 Downs SH, Brandli O, Zellweger JP, Schindler C, Kunzli N, Gerbase MW, et al. Accelerated decline in lung function in smoking women with airway obstruction: SAPALDIA 2 cohort study. Respir Res. 2005;6:45.
