Optimal reperfusion in ST-elevation myocardial infarction – the role of the coronary microcirculation
DOI: https://doi.org/10.4414/smw.2011.13313
Caterina
De, RK
Kharbanda, AP
Banning
Summary
Coronary microcirculation plays a crucial role for the outcomes of patients with STEMI. Although PPCI improves outcomes compared to thrombolysis, a substantial amount of STEMI patients do not achieve optimal myocardial reperfusion. Angiographic methods for assessment of reperfusion like TIMI Flow and MBG are easy to use but new, catheter laboratory based techniques to assess reperfusion have a lot of potential to assess and potentially guide management of patients with STEMI.
Introduction
ST-elevation myocardial infarction (STEMI) results from occlusion of a major epicardial artery leading to myocardial ischemia and cell death [1].
It is clear that the amount of irreversible cell death directly determines prognosis and it is now established that primary percutaneous coronary intervention (PPCI), delivered in a timely manner, should be the treatment of choice [2, 3].
Optimal reperfusion of the coronary microcirculation (CM) is not achieved in a substantial amount of STEMI patients and this negatively affects their prognosis [4].
Although some aspects discussed in this manuscript might be similar in Non-ST Elevation acute coronary syndrome (ACS), the aim of this review article is to highlight the role of the CM in acute STEMI and discuss its assessment and treatment in patients undergoing PPCI.
Assessing infarct size in STEMI using biomarkers
The most common way to assess myocardial damage in clinical practice is related to serial measurement of biomarkers of myocardial necrosis. The activity of different enzymes such as Lactate Dehydrogenase (LDH) and Creatine Kinase (CK) have been used historically to assess the extent of myocardial damage [5] but currently Creatine Kinase Myocardial-Band (CK-MB) and Troponins (I and T) are the most frequently used markers for diagnosis and quantification of myocardial infarction. The use of high-sensitive troponin assays has clearly improved the diagnosis of ACS [6]. The quantification of myocardial infarction is important and single-point Troponin measurements at 24, 48 and 72 hours have been suggested by different studies [7, 8]. New markers are currently being evaluated for the diagnosis and prognosis of myocardial infarction [9], but more studies are needed before they can be introduced into clinical practice. Of note, the status of the coronary microcirculation in STEMI is closely linked to infarct size and prognosis of these patients [10].
Non-invasive assessment of the coronary microcirculation
The primary objective of reperfusion therapies is not only restoration of blood flow in the epicardial coronary artery, but also complete and sustained reperfusion of the infarcted myocardium [11]. Prolonged occlusion of a coronary artery leads to loss of anatomic integrity of the microcirculation [12]. After infarct-related artery recanalisation, areas with severe microvascular damage sustain no reflow, whereas normal reperfusion is achieved in areas with anatomically preserved microcirculation. A number of additional factors are thought to contribute to the no reflow phenomenon: e.g., leucocyte accumulation within capillaries [12, 13], oxygen radicals [14], vasoconstriction mediated by thrombotic material [15].
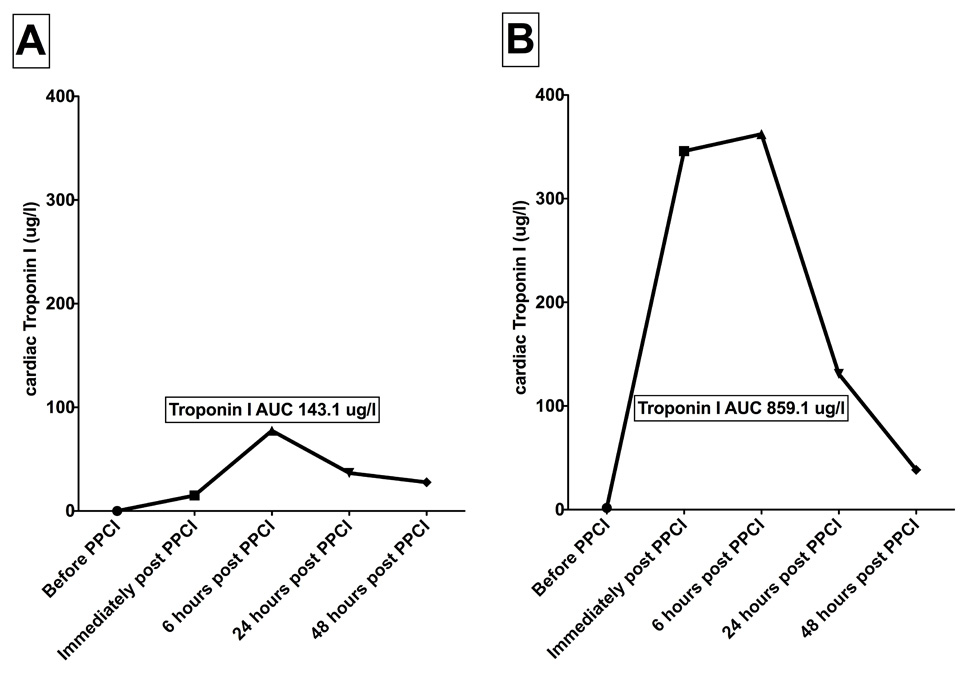
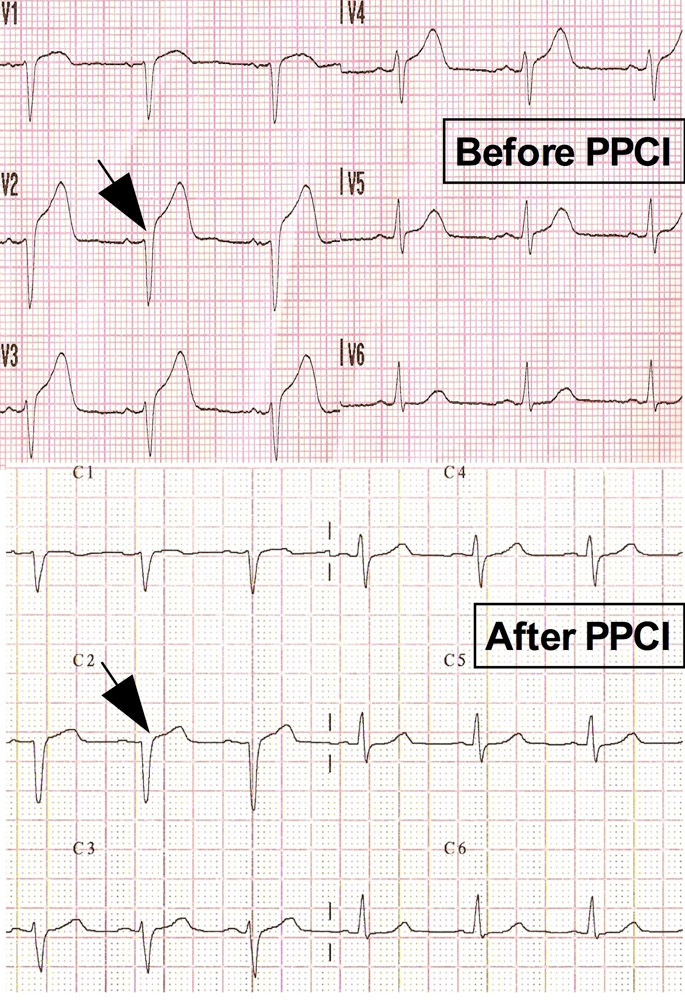
Figure 1
Troponin I curves of two patients with anterior ST-elevation myocardial infarction
Both patients had occlusion of the proximal left anterior descending artery and similar symptom-to-balloon times. The ECGs of patient A are presented in figure 2A and the ECGs of patient B are presented in figure 2B.
Electrocardiography (ECG)
Early resolution of the ST-segment elevation on the ECG after revascularisation is a powerful positive prognostic sign [16]. Cardiac enzymes release (Troponin I) and ECGs of two patients who underwent PPCI at our institution are shown in figure 1 and 2. Both patients had occlusion of the proximal left anterior descending artery and similar symptom-to-PPCI times. ST-segment elevation resolved almost completely after PPCI in patient A, while ST-segment elevation persisted in patient B. Lack of myocardial reperfusion, indicated by the lack of ST-segment normalisation in patient B, was associated with more myocardial injury compared to patient A (fig. 1). The left ventricular ejection fraction at 6 months was 55% for patient A and 40% for patient B. This example illustrates the importance of ST-segment resolution, which relates to outcome and prognosis [17].
It is important to realise that a small proportion of STEMI patients will present without ST-elevation [18] or will have left-bundle-branch block. Interpretation of ST-resolution is difficult or impossible in these patients and other measures of successful reperfusion have to be used.
Myocardial contrast echocardiography (MCE)
MCE uses appearance of micro-bubbles, which remain exclusively within the intravascular space. Hence, their presence within a myocardial territory denotes the status of microvascular perfusion within that region [19]. MCE is readily available and studies have demonstrated that extent of microvascular damage detected and quantified by MCE is a powerful independent predictor of LV remodelling after STEMI compared to MBG [20].
Alternative techniques to assess myocardial reperfusion using echocardiography include measurement of wall thickness, which corresponds to myocardial oedema [21, 22].
Absence of radiation exposure and wide availability make echocardiography an ideal bedside technique but as with other echocardiographic techniques it is dependent on echo quality and inter-observer variability.
Cardiac magnetic resonance imaging (CMR)
CMR reliably assesses left- and right ventricular function and is a sensitive method to assess myocardial oedema and microvascular obstruction (MVO) in the setting of acute STEMI [23].
CMR is superior to electrocardiography and MBG regarding assessment of myocardial perfusion [24, 25] and recently published animal studies have established the histological correlate of MVO [26]. The prognostic importance of MVO on CMR has been demonstrated in various studies and holds true even after correction for infarct size [27, 28].
The disadvantage of CMR is that it is not readily available in many centres performing PPCI. Additionally, presence of metal implants, pacemakers, defibrillators, claustrophobia and severe renal failure, which precludes use of gadolinium-based contrasts, limit the usage of CMR.
Assessment of the coronary microcirculation in the catheterisation laboratory
Angiographic assessment of CM
The most commonly used method to angiographically quantify coronary flow is the Thrombolysis In Myocardial Infarction (TIMI) flow grade (table 1 A) [29, 30]. The disadvantage of this method is that it mainly describes epicardial instead of myocardial blood flow. In 1998 the group of Ziljstra established myocardial blush grade (MBG, table 1) as a simple method to assess myocardial reperfusion and demonstrated that this was an independent predictor of mortality in acute STEMI [11]. TIMI Flow and MBG are both simple methods readily available after a coronary angiogram has been performed but they are both semi-quantitative. In 1996 the corrected TIMI frame count (CFTC) was introduced as a simple, quantitative method to compare coronary flow [31]. Although this method is reproducible, CFTC does not discriminate between epicardial and myocardial flow.
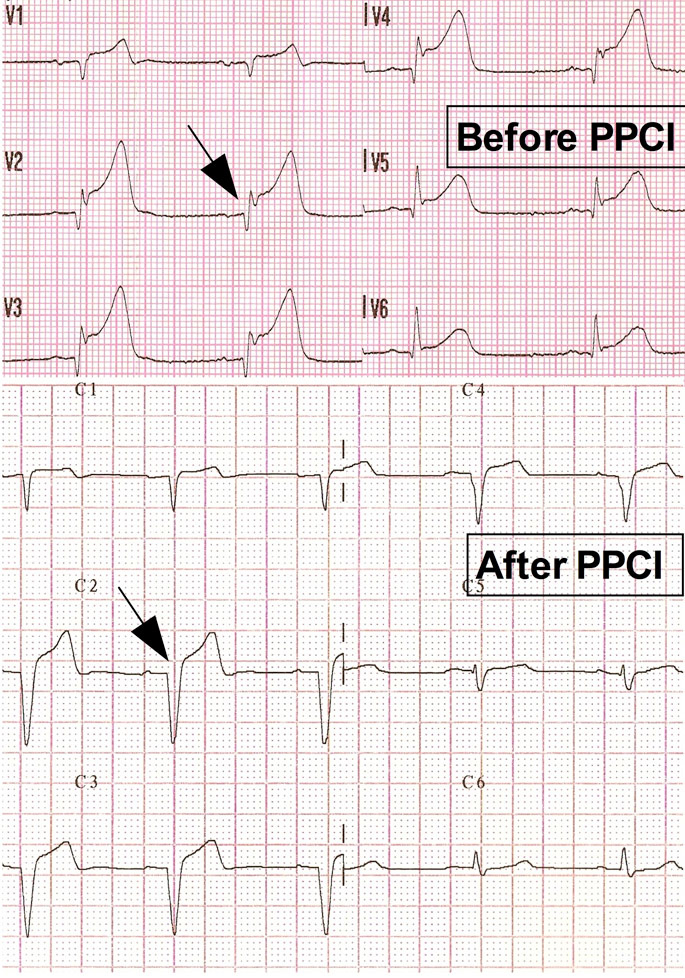
Figure 2 B
Pre- and post-procedural ECGs of two patients with anterior ST-Elevation myocardial infarction (Troponin curves presented in fig. 1).
Patient A had good and patient B had poor ST-segment resolution (arrows) post-procedure.
The direct quantitative assessment of the status of coronary microcirculation at the end of PPCI represents an attractive way to assess the extent of coronary microvascular dysfunction. Doppler flow wires or combined pressure and temperature-tipped guidewires allow assessment of the CM in the catheterisation laboratory. In the absence of epicardial stenosis, coronary flow reserve (CFR) reflects the ability of the microvasculature to respond to a vaso-dilatatory stimulus.
Doppler wire
Using the FloWire (Volcano Corporation) CFR is determined by the ratio of blood flow velocity at maximal hyperaemia, usually achieved with an intracoronary or intravenous infusion of adenosine, to basal value, expressed as the ratio of blood flow during hyperaemia to blood flow at rest. A CFR of less than 2.0 is usually considered abnormal and healthy individuals can have a CFR of 5-6. Previous studies have demonstrated good correlations of invasively assessed CFR using the FloWire and MCE [32] in patients with STEMI.
Pressure wire (Certus, SJM)
Using thermodilution, the pressure- and temperature tipped wire has been validated against the Doppler flow wire, and now used as a novel method to assess the status of coronary microcirculation [33, 34]. Proper guide catheter engagement is necessary in order to get reliable thermodilution curves. In our institution pressure wires are mainly used with 6F catheters and we have found it difficult to generate reproducible curves with 5F catheters.
The index of microcirculatory resistance (IMR), defined as the distal coronary pressure divided by the inverse of the hyperaemic mean transit time at maximal hyperaemia, provides a quantitative measurement of coronary microvasculature function that is independent of epicardial stenosis or hemodynamic condition [35].
Thermodilution curves of two STEMI patients with good and rather poor microcirculation are presented in figure 3.
Recently published studies have demonstrated that IMR assessed immediately after PPCI shows very good correlation with recovery of left ventricular function after STEMI [36, 37].
|
Table 1: Thrombolysis In myocardial infarction (TIMI) flow grading. |
|
TIMI flow grade
|
Description
|
|
TIMI 0
|
Absence of any antegrade flow beyond a coronary occlusion |
|
TIMI I
|
Faint antegrade flow beyond the occlusion, with incomplete filling of the distal coronary bed |
|
TIMI II
|
Delayed or sluggish antegrade flow with complete filling of the distal territory |
|
TIMI III
|
Normal flow which fills the distal coronary bed completely |
|
Table 2: Myocardial blush grading (MBG). |
|
MBG
|
Description
|
|
MBG 0
|
Failure of dye to enter the microvasculature: minimal or no ground glass appearance (“blush”) or opacification of the myocardium. |
|
MBG 1
|
Dye slowly enters but fails to exit the microvasculature: ground glass appearance that fails to clear from the microvasculature, and dye staining is present on >30 seconds later. |
|
MBG 2
|
Delayed entry and exit of dye from the microvasculature: dye is strongly persistent after 3 cardiac cycles of the washout phase and either does not or only minimally diminishes in intensity during washout. |
|
MBG 3
|
Normal entry and exit of dye from the microvasculature. Blush of the myocardium that is either gone or only mildly persistent at the end of the washout phase. |
Incidence and prognostic importance of no reflow
The incidence of no-reflow strictly depends on its definition as well as on the way and on the time of its assessment. Generally, according to different techniques, its reported incidence ranges about 40% of STEMI patients [38]. However, on the basis of the analysis of the CADILLAC (Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications) population, the combination of angiographic (TIMI flow less than 3 of TIMI 3 flow but MBG <2) and ECG-based (ST-segment resolution >70%) criteria resulted in a 65% rate of no reflow among patients presenting without cardiogenic shock [39].
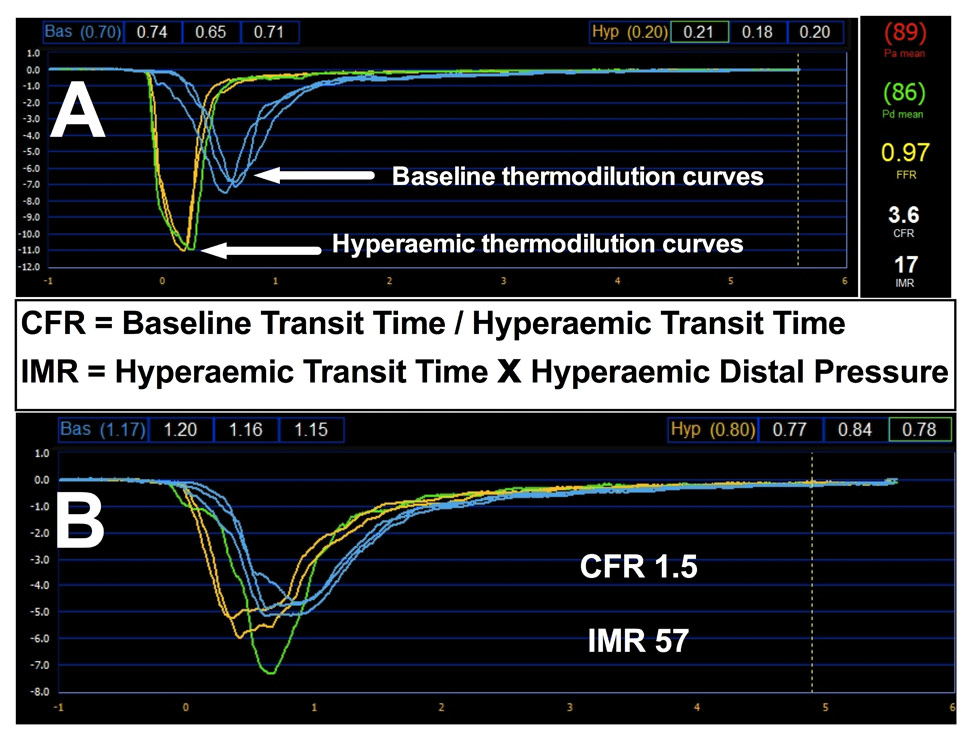
Figure 3
Thermodilution curves of two patients with STEMI.
Coronary microcirculation is preserved in patient A, while it is moderately impaired in patient B. Coronary Flow Reserve (CFR) is calculated as a ratio of mean baseline transit time (blue) and mean hyperaemic transit time (yellow). The index of microcirculatory resistance (IMR) is calculated as hyperaemic transit time x mean distal coronary pressure during hyperaemia.
Irrespectively of how it is defined, consistent data have clearly shown that no-reflow has a strong negative impact on outcome, negating or quenching the potential benefit of PPCI [20]. Indeed, the occurrence of no-reflow translates into a higher prevalence of early post-infarction complications (arrhythmias, pericardial effusion, cardiac tamponade, early congestive heart failure) as well as long-term left adverse ventricular remodelling, late hospital readmission for heart failure and mortality.
Does implantation of coronary stents improve or aggravate noreflow?
Distal embolisation of plaque material is common in PCI of stable patients and is associated with the occurrence of periprocedural myocardial infarction [40–42] and impairment of myocardial perfusion [43]. Coronary stents are routinely used nowadays in PPCI and have advantages with regard to acute vessel closure and rates of in-stent restenosis compared to balloon angioplasty [44].
However, it is not clear whether stenting with or without use of glycoprotein IIb/IIIa improves myocardial perfusion in patients undergoing PPCI [45]. Downstream embolisation of plaque and thrombotic material can occur spontaneously in STEMI and concerns have been raised that stenting might worsen coronary flow [44] possibly by aggravating distal embolisation.
Thrombus aspiration
Removal of thrombotic material using manual aspiration catheters can usually be safely undertaken once the guide wire has been crossed into the distal infarct artery and several studies and meta-analysis demonstrated a benefit in terms of better myocardial perfusion and reduced mortality [46, 47].
Use of GP IIb/IIIa antagonists and bivalirudin
GP IIb/IIIa antagonists (GPA)
These were introduced into clinical practice more than 15 years ago. Initially they were used for the majority of percutaneous interventions but their use has declined recently. Three GPA are currently used in clinical practice: abciximab, tirofiban and eptifibatide.
Abciximab has a IIa A, tirofiban a IIb B and eptifibatide a IIb C indication for use in patients undergoing PPCI [1]. In a recently published study pre-catheterisation laboratory administration of abciximab resulted in higher rates of infarct-artery patency at baseline coronary angiography compared with no pre-treatment but post-procedural angiographic and microcirculatory variables were unaffected by facilitation therapy [48]. The BRAVE-3 trial, which randomised PPCI patients pre-treated with clopidogrel to abciximab or placebo did not demonstrate a reduction of infarct size with use of GPA [49]. Despite these rather negative results, several meta-analysis indicate a benefit from the usage of GPA especially in high-risk patients [50–52]. With the introduction of newer more powerful antiplatelet agents (e.g., prasugrel and ticagrelor) the role of GPA in treating patients with STEMI is likely to change in the future.
Bivalirudin
Anticoagulation with bivalirudin alone, as compared with heparin plus glycoprotein IIb/IIIa inhibitors, significantly reduced 30-day rates of major bleeding and net adverse clinical events in the HORIZONS-AMI trial [53]. Importantly, the benefit was sustained over a longer period [54]. Whether bivalirudin offers the same advantage for the coronary microcirculation compared to GPA remains to be proven.
Vasodilators
The endothelium plays an important role in outcomes of patients with stable coronary artery disease [55]. The rationale for the use of vasodilators in STEMI relies on the pharmacological improvement of the patency of microcirculation once no-reflow has occurred.
1. Adenosine exerts a potent vasodilator effect at microvascular level, where it also increases platelet nitric oxide synthase activity [56]. In addition, adenosine is a known mediator of cardio-protection, since it replenishes high-energy phosphate stores in both myocytes and endothelial cells and inhibits oxygen free radical formation and neutrophil-related myocardial damage [57–59]. If tolerated, high intracoronary doses up to 4 mg have been shown to be safely administered even intracoronary, although conflicting data regarding its effective prognostic benefit are currently available [60, 61].
2. Sodium nitroprusside is a potent endothelium independent vasodilator known to relax both arterial and venous smooth muscle withouteffects on myocardial contractility. Maximal coronary hyperaemia, equivalentin magnitude but longer in length compared to intracoronary adenosine, can be safely achievedwith intracoronary nitroprusside in doses of 0.3–0.9 µg/kg [62]. However, its clinical efficacy in management of no-reflow has not been properly tested yet.
3. Similarly to sodium nitroprusside, verapamil has a direct action on arteriolar smooth muscle. Intracoronary verapamil (50–900 µg total dose) has been shown to improve TIMI flow grade in the majority of intraprocedural no-reflow [63] and to increase myocardial salvage in first AMI patients [64]. Endothelin antagonists, diltiazem and nicorandil are also available in the clinical arena, but their effective role in improving myocardial perfusion in the management of no reflow has yet to be convincingly demonstrated.
Preconditioning
Besides pharmacological agents, the interest surrounding mechanical strategies aiming at preconditioning myocardium, that is rendering it more resistant to a subsequent ischemic event, have constantly grown over the last 25 years. The issue of cardioprotection might be of great relevance, as it basically acts against ischemia-reperfusion injury and might increase the proportion of patients achieving an effective reperfusion, and thus a lower rate of no-reflow, after infarct-related artery recanalisation. Several pharmacological strategies have been tested to this aim, with overall conflicting results [65].
In the setting of STEMI the protective effect of cardiac preconditioning has been inferred by the observation that, in patients with pre-infarction angina, thrombolytic therapy resulted in more rapid reperfusion and smaller infarcts, thus suggesting that episodes of angina just before the occlusion of a coronary artery might condition both vessels and myocytes, thus allowing a more effective reperfusion and increased myocardial salvage [66]. However, since the delivery of local ischemic preconditioning is technically challenging in humans and ischaemia is often an unpredictable event, as it is always the case of STEMI, this field of research was really close to fail to translate into clinical practice. The discovery that the protective stimulus can be applied at a distance from the target organ (remote ischemic conditioning, RIP) shed new light on cardio-protection techniques [67]. In the experimental setting, several studies have highlighted that transient ischaemia of a wide range of tissues induces a systemic effect at distance, providing multi-organ protection against subsequent extended ischaemia-reperfusion injury [68]. Clinically, RIP can be easily applied to patients by alternating consecutive cycles of inflation and deflation of a pressure cuff to the upper arm. RIP has been shown to be effective in reducing myocardial necrosis in coronary heart surgery [69] and during elective PCI [70]. More importantly, Botker et al have shown that its application during transport to hospital in patients with STEMI undergoing PPCI was associated with significant increase in myocardial salvage [71]. Although its efficacy, and mechanism deserves further clinical confirmation in the setting of STEMI, RIP represents a cheap, safe and promising strategy to render the heart more resistant to ischaemia-reperfusion injury in the clinical setting.
References
1 Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29(23):2909–45.
2 Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, et al. Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2010;31(20):2501–55.
3 Stolt Steiger V, Goy JJ, Stauffer JC, Radovanovic D, Duvoisin N, Urban P, et al. Significant decrease in in-hospital mortality and major adverse cardiac events in Swiss STEMI patients between 2000 and December 2007. Swiss Med Wkly. 2009;139(31-32):453–7.
4 Ndrepepa G, Tiroch K, Keta D, Fusaro M, Seyfarth M, Pache J, et al. Predictive factors and impact of no reflow after primary percutaneous coronary intervention in patients with acute myocardial infarction. Circ Cardiovasc Interv. 2010;3(1):27–33.
5 Ahumada G, Roberts R, Sobel BE. Evaluation of myocardial infarction with enzymatic indices. Prog Cardiovasc Dis. 1976;18(5):405–20.
6 Twerenbold R, Reichlin T, Reiter M, Muller C. High-sensitive cardiac troponin: friend or foe? Swiss Med Wkly. 2011;141:w13202.
7 Hallen J, Buser P, Schwitter J, Petzelbauer P, Geudelin B, Fagerland MW, et al. Relation of cardiac troponin I measurements at 24 and 48 hours to magnetic resonance-determined infarct size in patients with ST-elevation myocardial infarction. Am J Cardiol. 2009;104(11):1472–7.
8 Younger JF, Plein S, Barth J, Ridgway JP, Ball SG, Greenwood JP. Troponin-I concentration 72 h after myocardial infarction correlates with infarct size and presence of microvascular obstruction. Heart. 2007;93(12):1547–51.
9 Katan M, Christ-Crain M. The stress hormone copeptin: a new prognostic biomarker in acute illness. Swiss Med Wkly. 2010;140:w13101.
10 Schwartz BG, Kloner RA. Coronary no reflow. J Mol Cell Cardiol. 2011.
11 van ’t Hof AW, Liem A, Suryapranata H, Hoorntje JC, de Boer MJ, Zijlstra F. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation. 1998;97(23):2302–6.
12 Kloner RA, Ganote CE, Jennings RB. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest. 1974;54(6):1496–508.
13 Ambrosio G, Weisman HF, Mannisi JA, Becker LC. Progressive impairment of regional myocardial perfusion after initial restoration of postischemic blood flow. Circulation. 1989;80(6):1846–61.
14 Przyklenk K, Kloner RA. “Reperfusion injury” by oxygen-derived free radicals? Effect of superoxide dismutase plus catalase, given at the time of reperfusion, on myocardial infarct size, contractile function, coronary microvasculature, and regional myocardial blood flow. Circ Res. 1989;64(1):86–96.
15 Kleinbongard P, Bose D, Baars T, Mohlenkamp S, Konorza T, Schoner S, et al. Vasoconstrictor potential of coronary aspirate from patients undergoing stenting of saphenous vein aortocoronary bypass grafts and its pharmacological attenuation. Circ Res. 2011;108(3):344–52.
16 Schroder R. Prognostic impact of early ST-segment resolution in acute ST-elevation myocardial infarction. Circulation. 2004;110(21):e506–510.
17 De Luca G, Maas AC, Suryapranata H, Ottervanger JP, Hoorntje JC, Gosselink AT, et al. Prognostic significance of residual cumulative ST-segment deviation after mechanical reperfusion in patients with ST-segment elevation myocardial infarction. Am Heart J. 2005;150(6):1248–54.
18 Francois SJ, Erne P, Urban P, Maggiorini M, Seifert B, Gutzwiller F, Radovanovic D. Impact of a normal or non-specific admission ECG on the treatment and early outcome of patients with myocardial infarction in Swiss hospitals between 2003 and 2008. Swiss Med Wkly. 2010;140:w13078.
19 Hayat SA, Senior R. Myocardial contrast echocardiography in ST elevation myocardial infarction: ready for prime time? Eur Heart J. 2008;29(3):299–314.
20 Galiuto L, Garramone B, Scara A, Rebuzzi AG, Crea F, La Torre G, et al. The extent of microvascular damage during myocardial contrast echocardiography is superior to other known indexes of post-infarct reperfusion in predicting left ventricular remodeling: results of the multicenter AMICI study. J Am Coll Cardiol. 2008;51(5):552–9.
21 Streb W, Marciniak M, Claus P, Marciniak A, McLaughlin M, D’Hooge J, et al. Full or pressure limited reperfusion of an acute myocardial infarct results in a different wall thickness and deformation of the distal myocardium – implications for clinical reperfusion strategies. Eur J Echocardiogr. 2008;9(4):458–65.
22 Turschner O, D’Hooge J, Dommke C, Claus P, Verbeken E, De Scheerder I, et al. The sequential changes in myocardial thickness and thickening which occur during acute transmural infarction, infarct reperfusion and the resultant expression of reperfusion injury. Eur Heart J. 2004;25(9):794–803.
23 Beek AM, van Rossum AC. Cardiovascular magnetic resonance imaging in patients with acute myocardial infarction. Heart. 2010;96(3):237–43.
24 Vicente J, Mewton N, Croisille P, Staat P, Bonnefoy-Cudraz E, Ovize M, Revel D. Comparison of the angiographic myocardial blush grade with delayed-enhanced cardiac magnetic resonance for the assessment of microvascular obstruction in acute myocardial infarctions. Catheter Cardiovasc Interv. 2009;74(7):1000–7.
25 Nijveldt R, Beek AM, Hirsch A, Stoel MG, Hofman MB, Umans VA, et al. Functional recovery after acute myocardial infarction: comparison between angiography, electrocardiography, and cardiovascular magnetic resonance measures of microvascular injury. J Am Coll Cardiol. 2008;52(3):181–9.
26 Driesen RB, Zalewski J, Driessche NV, Vermeulen K, Bogaert J, Sipido KR, et al. Histological correlate of a cardiac magnetic resonance imaged microvascular obstruction in a porcine model of ischemia-reperfusion. Cardiovasc Pathol. 2011.
27 Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97(8):765–72.
28 Bekkers SC, Yazdani SK, Virmani R, Waltenberger J. Microvascular obstruction: underlying pathophysiology and clinical diagnosis. J Am Coll Cardiol. 2010;55(16):1649–60.
29 Chesebro JH, Knatterud G, Roberts R, Borer J, Cohen LS, Dalen J, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: A comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation. 1987;76(1):142–54.
30 Anabitarte P, Kurz DJ, Stettler I, Naegeli B, Bertel O, Frielingsdorf J, et al. Long-term survival and functional outcome of unselected patients undergoing percutaneous coronary intervention for acute myocardial infarction. Swiss Med Wkly. 2009;139(43-44):636–41.
31 Gibson CM, Cannon CP, Daley WL, Dodge JT, Jr., Alexander B, Jr., Marble SJ, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93(5):879–88.
32 Lepper W, Hoffmann R, Kamp O, Franke A, de Cock CC, Kuhl HP, et al. Assessment of myocardial reperfusion by intravenous myocardial contrast echocardiography and coronary flow reserve after primary percutaneous transluminal coronary angioplasty [correction of angiography] in patients with acute myocardial infarction. Circulation. 2000;101(20):2368–74.
33 Fearon WF, Farouque HM, Balsam LB, Caffarelli AD, Cooke DT, Robbins RC, et al. Comparison of coronary thermodilution and Doppler velocity for assessing coronary flow reserve. Circulation. 2003;108(18):2198–200.
34 Cuculi F, De Caterina AR, Banning AP. Large perfusion defect on scintigraphy explained by severe microcirculatory dysfunction. J Invasive Cardiol. 2011;23(10):E247–248.
35 Fearon WF, Balsam LB, Farouque HM, Caffarelli AD, Robbins RC, Fitzgerald PJ, et al. Novel index for invasively assessing the coronary microcirculation. Circulation. 2003;107(25):3129–32.
36 Fearon WF, Shah M, Ng M, Brinton T, Wilson A, Tremmel JA, et al. Predictive value of the index of microcirculatory resistance in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2008;51(5):560–5.
37 Lim HS, Yoon MH, Tahk SJ, Yang HM, Choi BJ, Choi SY, et al. Usefulness of the index of microcirculatory resistance for invasively assessing myocardial viability immediately after primary angioplasty for anterior myocardial infarction. Eur Heart J. 2009;30(23):2854–60.
38 Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol. 2009;54(4):281–92.
39 McLaughlin MG, Stone GW, Aymong E, Gardner G, Mehran R, Lansky AJ, et al. Prognostic utility of comparative methods for assessment of ST-segment resolution after primary angioplasty for acute myocardial infarction: the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial. J Am Coll Cardiol. 2004;44(6):1215–23.
40 Cuculi F, Lim CC, Banning AP. Periprocedural myocardial injury during elective percutaneous coronary intervention: is it important and how can it be prevented? Heart. 96(10):736–40.
41 Selvanayagam JB, Porto I, Channon K, Petersen SE, Francis JM, Neubauer S, et al. Troponin elevation after percutaneous coronary intervention directly represents the extent of irreversible myocardial injury: insights from cardiovascular magnetic resonance imaging. Circulation. 2005;111(8):1027–32.
42 Porto I, Selvanayagam JB, Van Gaal WJ, Prati F, Cheng A, Channon K, et al. Plaque volume and occurrence and location of periprocedural myocardial necrosis after percutaneous coronary intervention: insights from delayed-enhancement magnetic resonance imaging, thrombolysis in myocardial infarction myocardial perfusion grade analysis, and intravascular ultrasound. Circulation. 2006;114(7):662–9.
43 Selvanayagam JB, Cheng AS, Jerosch-Herold M, Rahimi K, Porto I, van Gaal W, et al. Effect of distal embolization on myocardial perfusion reserve after percutaneous coronary intervention: a quantitative magnetic resonance perfusion study. Circulation. 2007;116(13):1458–64.
44 Grines CL, Cox DA, Stone GW, Garcia E, Mattos LA, Giambartolomei A, et al. Coronary angioplasty with or without stent implantation for acute myocardial infarction. Stent Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med. 1999;341(26):1949–56.
45 Costantini CO, Stone GW, Mehran R, Aymong E, Grines CL, Cox DA, et al. Frequency, correlates, and clinical implications of myocardial perfusion after primary angioplasty and stenting, with and without glycoprotein IIb/IIIa inhibition, in acute myocardial infarction. J Am Coll Cardiol. 2004;44(2):305–12.
46 Bavry AA, Kumbhani DJ, Bhatt DL. Role of adjunctive thrombectomy and embolic protection devices in acute myocardial infarction: a comprehensive meta-analysis of randomized trials. Eur Heart J. 2008;29(24):2989–3001.
47 De Luca G, Dudek D, Sardella G, Marino P, Chevalier B, Zijlstra F. Adjunctive manual thrombectomy improves myocardial perfusion and mortality in patients undergoing primary percutaneous coronary intervention for ST-elevation myocardial infarction: a meta-analysis of randomized trials. Eur Heart J. 2008;29(24):3002–10.
48 Prati F, Petronio S, Van Boven AJ, Tendera M, De Luca L, de Belder MA, et al. Evaluation of infarct-related coronary artery patency and microcirculatory function after facilitated percutaneous primary coronary angioplasty: the FINESSE-ANGIO (Facilitated Intervention With Enhanced Reperfusion Speed to Stop Events-Angiographic) study. JACC Cardiovasc Interv. 2010;3(12):1284–91.
49 Mehilli J, Kastrati A, Schulz S, Frungel S, Nekolla SG, Moshage W, et al. Abciximab in patients with acute ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention after clopidogrel loading: a randomized double-blind trial. Circulation. 2009;119(14):1933–40.
50 De Luca G, Gibson CM, Bellandi F, Murphy S, Maioli M, Noc M, et al. Early glycoprotein IIb-IIIa inhibitors in primary angioplasty (EGYPT) cooperation: an individual patient data meta-analysis. Heart. 2008;94(12):1548–58.
51 Labinaz M, Ho C, Banerjee S, Martin J, Chen S, Mensinkai S. Meta-analysis of clinical efficacy and bleeding risk with intravenous glycoprotein IIb/IIIa antagonists for percutaneous coronary intervention. Can J Cardiol. 2007;23(12):963–70.
52 De Luca G, Navarese E, Marino P. Risk profile and benefits from Gp IIb-IIIa inhibitors among patients with ST-segment elevation myocardial infarction treated with primary angioplasty: a meta-regression analysis of randomized trials. Eur Heart J. 2009;30(22):2705–13.
53 Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, et al. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358(21):2218–30.
54 Mehran R, Lansky AJ, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, et al. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet. 2009;374(9696):1149–59.
55 Flammer AJ, Luscher TF. Three decades of endothelium research: from the detection of nitric oxide to the everyday implementation of endothelial function measurements in cardiovascular diseases. Swiss Med Wkly. 2010;140:w13122.
56 Russo I, Doronzo G, Mattiello L, De Salve A, Trovati M, Anfossi G. The activity of constitutive nitric oxide synthase is increased by the pathway cAMP/cAMP-activated protein kinase in human platelets. New insights into the antiaggregating effects of cAMP-elevating agents. Thromb Res. 2004;114(4):265–73.
57 Cronstein BN, Rosenstein ED, Kramer SB, Weissmann G, Hirschhorn R. Adenosine; a physiologic modulator of superoxide anion generation by human neutrophils. Adenosine acts via an A2 receptor on human neutrophils. J Immunol. 1985;135(2):1366–71.
58 Ely SW, Berne RM. Protective effects of adenosine in myocardial ischemia. Circulation. 1992;85(3):893–904.
59 Jordan JE, Zhao ZQ, Sato H, Taft S, Vinten-Johansen J. Adenosine A2 receptor activation attenuates reperfusion injury by inhibiting neutrophil accumulation, superoxide generation and coronary endothelial adherence. J Pharmacol Exp Ther. 1997;280(1):301–9.
60 Marzilli M, Orsini E, Marraccini P, Testa R. Beneficial effects of intracoronary adenosine as an adjunct to primary angioplasty in acute myocardial infarction. Circulation. 2000;101(18):2154–9.
61 Desmet W, Bogaert J, Dubois C, Sinnaeve P, Adriaenssens T, Pappas C, et al. High-dose intracoronary adenosine for myocardial salvage in patients with acute ST-segment elevation myocardial infarction. Eur Heart J. 2011;32(7):867–77.
62 Parham WA, Bouhasin A, Ciaramita JP, Khoukaz S, Herrmann SC, Kern MJ. Coronary hyperemic dose responses of intracoronary sodium nitroprusside. Circulation. 2004;109(10):1236–43.
63 Piana RN, Paik GY, Moscucci M, Cohen DJ, Gibson CM, Kugelmass AD, et al. Incidence and treatment of “no-reflow” after percutaneous coronary intervention. Circulation. 1994;89(6):2514–8.
64 Taniyama Y, Ito H, Iwakura K, Masuyama T, Hori M, Takiuchi S, et al. Beneficial effect of intracoronary verapamil on microvascular and myocardial salvage in patients with acute myocardial infarction. J Am Coll Cardiol. 1997;30(5):1193–9.
65 Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121–35.
66 Andreotti F, Pasceri V, Hackett DR, Davies GJ, Haider AW, Maseri A. Preinfarction angina as a predictor of more rapid coronary thrombolysis in patients with acute myocardial infarction. N Engl J Med. 1996;334(1):7–12.
67 Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic “preconditioning” protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87(3):893–9.
68 Kharbanda RK, Nielsen TT, Redington AN. Translation of remote ischaemic preconditioning into clinical practice. Lancet. 2009;374(9700):1557–65.
69 Venugopal V, Hausenloy DJ, Ludman A, Di Salvo C, Kolvekar S, Yap J, et al. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold-blood cardioplegia: a randomised controlled trial. Heart. 2009;95(19):1567–71.
70 Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, et al. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: a prospective, randomized control trial. Circulation. 2009;119(6):820–7.
71 Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375(9716):727–34.