Artificial muscle: the human chimera is the future
DOI: https://doi.org/10.4414/smw.2011.13311
Summary
Severe heart failure and cerebral stroke are broadly associated with the impairment of muscular function that conventional treatments struggle to restore. New technologies enable the construction of “smart” materials that could be of great help in treating diseases where the main problem is muscle weakness. These materials “behave” similarly to biological systems, because the material directly converts energy, for example electrical energy into movement. The extension and contraction occur silently like in natural muscles. The real challenge is to transfer this amazing technology into devices that restore or replace the mechanical function of failing muscle. Cardiac assist devices based on artificial muscle technology could envelope a weak heart and temporarily improve its systolic function, or, if placed on top of the atrium, restore the atrial kick in chronic atrial fibrillation. Artificial sphincters could be used to treat urinary incontinence after prostatectomy or faecal incontinence associated with stomas. Artificial muscles can restore the ability of patients with facial paralysis due to stroke or nerve injury to blink. Smart materials could be used to construct an artificial oesophagus including peristaltic movement and lower oesophageal sphincter function to replace the diseased oesophagus thereby avoiding the need for laparotomy to mobilise stomach or intestine. In conclusion, in the near future, smart devices will integrate with the human body to fill functional gaps due to organ failure, and so create a human chimera.
Introduction
Heart failure and cerebral stroke are some of the most dreadful and frequent diseases in high-developed countries and, from the clinical point of view, are both associated with impairment of muscular function. Conventional medical and surgical treatments can barely restore the muscular function in case of muscular necrosis or paralysis.
– Natural muscles self-repair, provide billions of work cycles involving contraction of more than 25%, can increase their strength and change stiffness in response to need, and can even transform to become a source of essential nutritional value for a hungry body. They convert the energy of adenosine triphosphate to mechanical energy with an exceptional efficiency of about 45%. So far, scientists have been unable to create materials or devices that reproduce exactly natural muscle function and their working processes. However, there are, smart materials that are the basis for many technologies used in medicine, manufacturing, and defence that, in specific configurations and with limited applications, could reproduce at least part of natural muscle function. Scientists define these materials as being “smart” because they react to changes in stimuli such as temperature, medium pH, light or magnetic fields and generate movements. The characteristics of the ideal artificial muscle should be similar to those of biological muscle. Biological muscles are optimised systems that are relatively similar in all species and exhibit quick reaction times with large linear forces. Biological muscle contraction, stimulated by electrochemical nerve conduction, is caused by chemically induced reversible hydrogen bonding between actin and myosin. An electroactive polymer acts in the same way because the contraction and expansion of the material follow ionic flows directed by an applied electric field [1–3]. A memory shape alloy reacts to the environmental temperature, quickly reorganising its three-dimensional molecular structure and this process induces shape changes that result in movement [1, 2]. However, even if these smart materials seem to act like natural muscles, it should be clear that we are still very far from being able to reproduce Nature. Such materials could be of great help in treating diseases where the main problem is the weakness of the muscle, because, in specific conditions, smart materials act as natural muscles. They “behave” similarly to biological systems, therefore the definition of Biomimetics materials. Contraction and expansion occur silently, because the material directly converts a given form of energy such as electricity into movement.
Artificial muscle technology for medical applications
Instead of reproducing nature by creating large macroscopic movements by the combined effects of trillions of molecular actuators (the actin-myosin process), the artificial muscle uses material deformations induced by environmental changes.
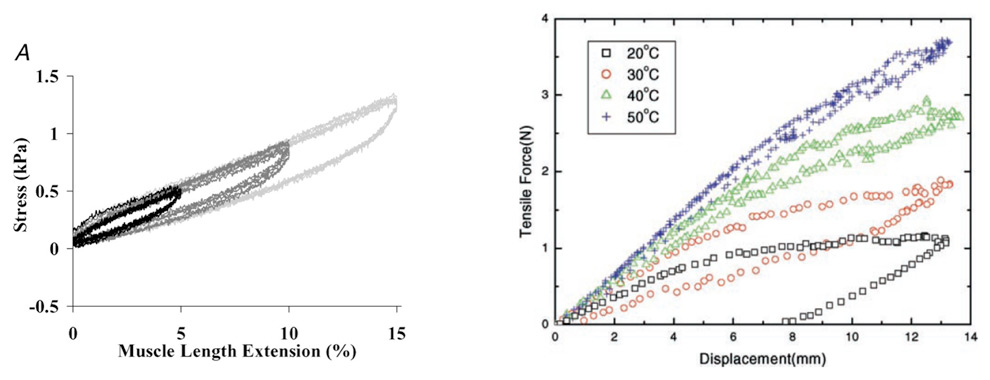
Figure 1
The above curves illustrate the extension relationship of a muscle that is stretched (left) and an SMA spring actuator (right) at different temperatures. The idea in this comparison is to identify a hysteresis loop in both cases. Left: typical stress–muscle length extension relationships, measured during 6 Cycles of 5, 10 and 15% length extension at 25 μm s−1. Right: isothermal force-displacement curves of an SMA spring actuator.
Shape memory alloys (SMAs)
These show properties that make them the ideal key component of artificial muscle for medical application [1]. An SMA is able of remembering a previously memorised shape. It has to be deformed in its Martensitic (low temperature) phase and subsequently heated to the high temperature phase Austenite, e.g., in hot water or with an electrical current [2]. The alloy generates a high force during the phase change. Any system able to convert energy into movement is defined actuator, thus, nitinol is used as actuator in a huge number of applications. The shape change is not restricted to pure bending. The most suitable actuation mode has proven to be the linear contraction of a straight wire actuator.
Nitinol, a nickel titanium (55% Ni, 45% Ti) alloy discovered in 1962 in the United States Naval Ordnance Laboratory, is biocompatible and extensively used for the endovascular treatment of cardiovascular diseases (coronary stents, endoprostheses for the treatment of aortic aneurysms, etc.) because of its superelasticity. One of the key characteristics of the Nitinol wires that could enable its use as actuator is that the wires reduce their length up to 8%, very smoothly or extremely rapidly depending on the way the electric current is applied and the transitional temperature reached. The form of the energy wave applied to heat the wire determines the velocity of the fiber contraction and the length of the contraction as well. Therefore, with the appropriate energy supply the wire acts like natural skeletal muscle (fig. 1). Nitinol suitable for medical application has a transitional temperature below 50 °C and to avoid thermal lesions it has to be insulated from the surrounding tissue.
An important quest is the energy supply. The actuation of Nitinol wires needs a current of 450 mA and a voltage depending on the length and the resistance of the wire, but usually lower than 24 V. Electrically driven actuators rely on batteries, and this could restrict their clinical applications. They operate with a very low efficiency, not exceeding 10%.
Electroactive polymers (EAP)
Electroactive polymer artificial muscles were developed at SRI International (Menlo Park, California) in the 1990s. They are functional materials that can be used as actuators in active structures, in particular when large deformations and low forces are required. Such actuators transform electrical energy directly into mechanical work and produce large strains. In ionic EAPs the activation forces are generated by diffusion of mobile ions under low current and usually only a few volts, which make them suitable for medical applications [3]. Electroactive polymer artificial muscle is composed of an inner core of a dielectric elastomer film (e.g., silicone, acrylic, teflon) with a flexible electrode coating placed on either side, usually made of gold. When a DC voltage is applied to the electrodes, the elastomeric polymer expands in a plane perpendicular to the force due to the attraction between the electrodes. When the electrical current is released, the artificial muscle contracts back to its original shape, thus converting the electrostatic force into mechanical work [4]. Another advantage of EPAs consists in the extreme variability of shapes and structures they can be fashioned: string, spiral or sheet that can also be bended or twisted.
However, actuator performance typically degrades after a few thousand cycles when the actuator is operated at high strains and high mechanical loads. Energy conversion is low (typically less than 1%) but could be improved drastically by avoiding electrolyte electrolysis and harvesting stored electrical energy. An artificial muscle that takes the energy supply directly from the blood stream analogous to natural muscle, represents a major step forward in expanding its clinical application. Nature’s technique of converting the chemical energy of a high-energy-density fuel to mechanical work has advantages, which can be partially achieved by driving electrical actuators with fuel cells. Such a system exists and is called breathing artificial muscle [5], but is still far from clinical applications.
Potential clinical applications
The real challenge is to transfer such amazing technologies into implantable devices that integrate with the human body to fill the functional gaps arising from organ or system failure, therefore creating “human chimera”.
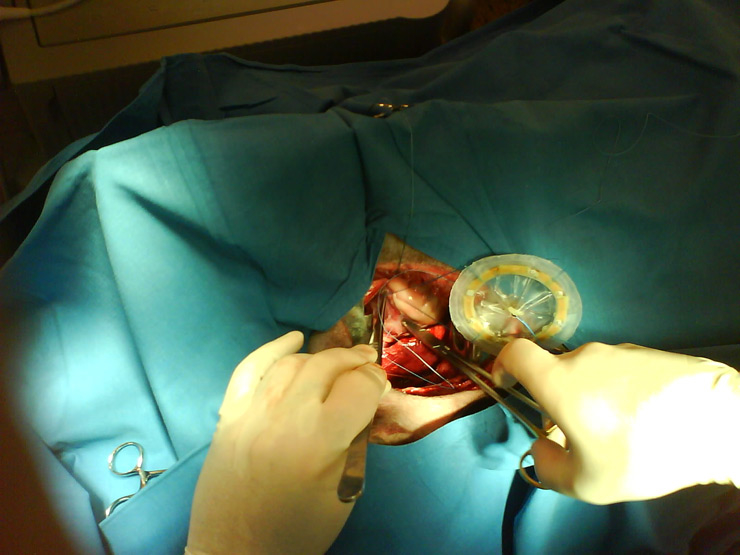
Figure 2
Animal implant of the Atripump. The concave part is assumed to touch atrium’s epicardium. When ON, the dome reduces its depth pushing down the atrial wall. When OFF, the dome returns to its rest position allowing atrial filling.

Figure 3
The external bi-ventricular assist device comprises a skeleton of carbon fibre (black bands) and a mesh (yellow) of aramidic fibres. The carbon fibre follows the interventricular septum, the atrio-ventricular groove, and encircles the cardiac apex. Zig-zagging through the aramidic tissue are interwoven nitinol fibres, which are joined to the carbon skeleton at the apex of the heart and then spiral around the ventricles to the atrio-ventricular groove level.

Figure 4
The urinary sphincter is made of 3 silicon rings (green part) whose diameter decreases when the nitinol fibre (black springs) is activated. The rings are placed around the urethra. Up to 5 rings could be placed to ensure the optimal control of urinary flow reducing the risk of urethra lesions.
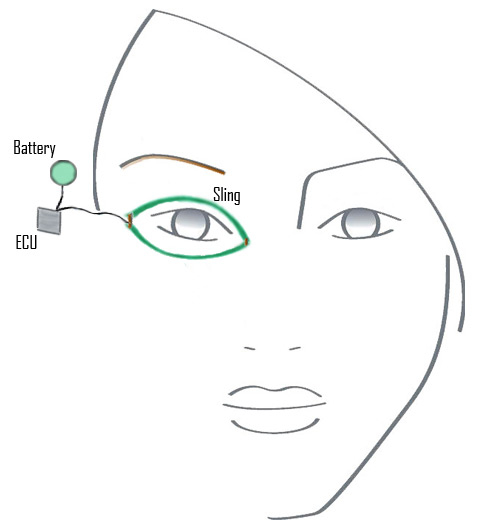
Figure 5
Illustration of a left eyelid sling that is attached to the electroactive polymer artificial muscle device (EPA) after passing through an interpolation unit is implanted in the lateral orbital wall (note screw fixation). The power supply and artificial muscle are implanted in the temporal fossa. Conceptually, when the normal right eyelid blinks, the electrical sensor (green) sends a signal to the battery to activate the EPA.
Cardiac assist devices based on artificial muscles
The first use of Nitinol wires as artificial muscle for an artificial heart has celebrated its 35th anniversary [6]. Nitinol wires were arranged in waves and placed around the ventricles. Animal results were encouraging, but material fatigue, excessively slow contraction and thermal injury proved to be insurmountable problems at that time. Today, we have commercially available Nitinol that has a lifetime of billions of cycles and transitional temperature compatible with human body implants.
Atripump for the treatment of chronic atrial fibrillation
Atrial fibrillation (AF) is the most common cardiac arrhythmia in over 50 year-olds and causes up to a 15% reduction in left ventricular ejection fraction. Moreover, because blood pools in the atria, in about 5% of patients clotted blood dislodges causing stroke, therefore lifelong anticoagulation therapy is recommended [7]. Consequently, patients with chronic AF suffer from cardiac failure and have a high risk of thrombo-embolic events. Treatment of AF consists primarily of palliation, mostly in the form of pharmacological intervention aimed at reducing the risk of stroke using anticoagulant agents. The only surgical intervention that potentially cures AF rather than palliates, is the Maze procedure. However, the rate of restoring atrial contraction is low [8]. Patients continue to require anticoagulation therapy for life and, thus are exposed to the risk of haemorrhagic complications. We first introduced the concept of an Atrial Assist Device and developed the related device called Atripump to definitely restore the pump function of the atrium in patients suffering of chronic AF [9–12]. Atripump is a dome shape silicone coated nitinol actuator 5 mm high, mounted on a plastic ring 55 mm in diameter. Once electrically heated, nitinol wires reduce their length causing a reduction of the dome concavity. Current cut off produces wire elongation and the recovery of the initial concavity of the dome. The changes in dome concavity result in volume displacement. This cycle is controlled by a pacemaker-like unit that senses ventricular activity and gives current to the wires following a dedicated algorithm. The dome is sutured onto the atrium’s epicardium in order to provide the mechanical support to blood circulation (fig. 2). Because there is no contact between the assist device and host blood, no anticoagulation therapy is required. This surgical approach could increase cardiac output up to 10% as measured in several animal studies [11, 12] and should significantly improve the quality of life of patients suffering from chronic AF. Moreover, the device actively washes blood out of atria reducing the risk of clot formation and systemic embolism, making anticoagulation treatment unnecessary. The heating issue that has always been one of the major limitations for human applications of nitinol actuators has considerably lost its importance. It is true that, when the dome is activated in air at room temperature, the silicon membrane reaches temperature above 50 °C making it incompatible with human implantation. However, when the dome is in contact with liquids, such as the blood in cardiac chambers, heat dissipates so quickly that silicon membrane temperature stays above 35 °C. In an elegant study published in 1988, Emoto and co-workers reported the effects on surrounding tissue of heat dissipation associated with thermally powered LVAS implantation [13]. Systemic (affecting the lungs, kidneys, blood tissue, and coagulation processes) and local effects of 20 W heat-dissipation were analysed in 5 calves. Conclusions were that heat promoted neointima proliferation at the interface pump-blood flow and angiogenesis was observed in the tissue capsule adjacent to the heat- dissipating surface. However, no deleterious systemic or local effects of 20 W heat-dissipation were reported. Based on these results, we could speculate that also the heat dissipated by the dome will not cause systemic or local damage. The atrium and the blood circulating in it will work as heat exchange keeping the membrane temperature in a range compatible with human implantation.
External compression of failing heart: artificial cardiomyoplasty
Artificial muscle technology has allowed the construction of a bi-ventricular assist device (BiVAD) that is able to restore ventricular pumping function through external compression. Current ventricular assist devices (VADs) are pneumatic/rotary pulsatile pumps based on non-compliant chamber into which the blood is forced, or magnetically suspended propellers that generate continuous flow once placed into the blood stream [14]. Pulsatile pumps are heavy which could causes surrounding tissue damage, have extensive thrombogenic surfaces exposed to blood making anticoagulant therapy mandatory and are associate with a high incidence of life-threatening infections [18]. Continuous flow pumps are associated with fewer complications than pulsatile ones because they are smaller with less thrombogenic surface exposed to blood, but can be used only to assist the left circulation [14]. None of these devices is able to assist both ventricles, which means that if the patient needs biventricular assistance, two pumps have to be implanted. External ventricular compression provided by cardiomyoplasty is the most physiological way to improve pump function in a diseased heart, particularly in cases of dilated cardiopathy [19, 20]. Any device able to reproduce external cardiac compression (artificial cardiomyoplasty) could potentially overcome the drawbacks of the existing VADs.
The BiVAD consists of Nitinol wires woven in aradimic tissue and connected to a carbon fibre skeleton that reproduces the mechanical function of the interventricular septum (fig. 3). Wire contraction induces the shortening of the aramidic tissue that compresses the ventricles. Increasing the contact surface permits a redistribution of the strength over a larger surface avoiding organ injury. The skeleton enables an asymmetrical operation between left and right ventricle. The number of wires and the amplitude of pumping movement changes according to the severity of right and left heart failure. Each ventricular assist device can be arranged with 1 to 5 separate levels of wires modulating the compressing force. An electronic control unit activates wire contraction in a dedicated mode for each ventricle. The total power consumption of the device is 10W. This device has been tested in a bench model of diseased heart [21] and provided 12% improvement of left ventricular ejection fraction and up to 20% improvement in right ventricular function. Like human muscle fibre, the force of contraction increases when the preload increases following the Frank-Starling law.
The key advantages of artificial cardiomyoplasty could be summarised as follow:
1) The absence of blood exposed thrombogenic surfaces makes the anticoagulant therapy unnecessary avoiding bleeding and embolic events.
2) One device provides independent compression of the right and left ventricle. The control unit can modulate compression force, compression rate and ejection fraction of each ventricle independently, providing the most compliant cardiac assist device ever developed.
3) It is easy to implant and does not require cardiopulmonary bypass in contrast to all other VADs.
4) In case it fails, the residual ventricular function is not affected as would happen if a continuous axial flow pump stopped working, where the equivalent of severe aortic regurgitation arises.
The First planned application of the BiVAD is to help the weaning from extracorporal circulation in paediatric patients with congenital heart disease as an alternative to more invasive Extra Corporal Membrane Oxigenator treatment.
Artificial sphincters and peristaltic conduits
Artificial urinary sphincter
Urinary incontinence is a common and often embarrassing problem and is due to functional impairment of muscles and nerves that help to hold or release urine. Treatment of urinary incontinence depends on severity and the degree of response to a particular treatment. For patients with mild symptoms there are a variety of treatments ranging from pads, exercises to strengthen pelvic floor muscles and pessaries. Anti-cholinergic and Alpha-adrenergic drugs such as pseudoephedrine may be prescribed for advanced incontinence. For patients suffering from more severe symptoms hormone replacement therapy is sometimes prescribed together with anti-depressants and in case of severe incontinence, surgery is necessary. The surgical approach consists of constructing sub-urethral slings from existing muscles, or implantation of an artificial urinary sphincter [14]. For over 30 years, physicians worldwide have implanted the AMS 800 device in more than 100,000 men as a treatment for stress urinary incontinence due to prostatectomy. The AMS 800 is an implantable, fluid-filled, solid silicone elastomeric device used to treat incontinence arising after radical prostatectomy. The system simulates normal sphincter function by opening and closing the urethra, under patient control. The patient opens the sphincter using a remote control following the natural stimulus. However, there are a number of drawbacks including the need for frequent mechanical revisions, strict patient selection, optimal preoperative bladder management and regular follow-ups. Even when these criteria are respected, successful outcomes are regarded as low. In a variety of studies revision of the device occurred on average in 39% of patients, urethral erosion in 5%, infection in 3%, and mechanical failure in 15% [15].
Another medical device for treatment of male stress incontinence after prostatectomy is ProACT (Proactive Adjustable Continence Therapy) (Uromedica). ProACT is a minimally invasive urological implant designed to treat male patients who have stress urinary incontinence arising from intrinsic sphincter deficiency following radical prostatectomy for prostate cancer or transurethral resection of the prostate (TURP) for benign prostatic hyperplasia (BPH). ProACT therapy offers a first-line treatment option for post-prostatectomy incontinence before invasive surgical consideration or implant of an Artificial Urinary Sphincter (AUS) or adjustable sling. Again there are similar disadvantages owing to the need for frequent mechanical revisions. These systems are both designed to exert a constant pressure on a very limited region of the urethra frequently leading to tissue erosion, ischaemia, scarring, necrosis and infection. This is the main reason why their use is rarely indicated.
An innovative approach is to use smart material switching the compressed area of the urethra in order to achieve continence. This is called the “piano” concept and is realised by assembling 3 to 5 shutter units over a predetermined length and position along the urethra. These shutters function in pairs with modulated alternation of their open and closed positions over time, such that the above-mentioned problems due to constant compression are greatly reduced or even eliminated. An implantable control unit having dimensions similar to current cardiac pacemakers drives the system. Figure 4 details the technical aspects of the device. This sphincter has been extensively tested in an animal model [22]. The device was positioned around the urethra of male sheep and the control unit was placed 5 cm away, under the skin, through a second incision. The sphincter was open each hour for a period of 10 minutes to guarantee urination. This study defined the in-bladder leak point pressure and the tissue tolerability of the device. To measure the in-bladder leak point pressure the bladder was filled with saline while just one ring was closed and bladder pressure was monitored. To verify tissue tolerability, animals were sacrificed after 2, 5 and 9 weeks and two biopsies around the cuffs were analysed. Sections of urethra not surrounded by a cuff were taken as controls. Provoked incontinence occurred at a bladder pressure over 1 bar. The closing force of the cuff was approximately 0.7N. No clinical infection occurred. Histological investigation showed no signs of infection, scarring or atrophy. No difference in tissue structure and organisation of the urethra with and without the artificial sphincter was observed. This animal study showed that the artificial urinary sphincter based on smart materials can provide continence and has good tissue tolerability short term [22].
The eyelid sling
Electroactive polymer artificial muscle is an emerging technology that has the potential to be used in rehabilitating facial movement in patients with paralysis. Blinking is an essential part of maintaining a healthy eye. The lid wipes the surface of the eye clean and spreads tears across the cornea. Without this lubrication, the eye is soon at risk of developing corneal ulcers that eventually can cause blindness. Over 40’000 cases of facial paralysis are seen in the United States every year, with most of these being idiopathic (Bell’s palsy) or virally induced [16]. In the few cases of a facial paralysis in which the patients do not recover, a variety of surgical options can be used to maintain facial muscle tone, rehabilitate some function, and protect the globe from exposure keratopathy. These surgical options include facial nerve grafting, cable grafting (sural nerve), cross-face jump grafts, or anastomosis with other cranial nerves (hypoglossal-facial nerve). However, in many cases of permanent facial paralysis, nerve grafting may not be possible owing to, (a) extensive tumour excision, including the facial nerve or distal facial muscles; (b) permanent facial nerve dysfunction after Bell’s palsy or herpes zoster palsy; or (c) congenitally absent facial nerve function with multiple other cranial neuropathies (Mobius syndrome).
Surgeons from UC Davis Medical Center have demonstrated that artificial muscles can restore blinking in patients with facial paralysis [23]. Their first priority was to determine if the stroke and load requirements of the eyelid sling mechanism were within the specifications of artificial muscle. They measured the force required to close the eyelid in cadavers that was approximately 1500 to 1800 mN, and then the force requirement for closing the eyelid with the temporalis fascia sling (627 ± 128 mN) and with the ePTFE eyelid sling (1347 mN ± 318 mN; P < .05). These values were within the range of force developed by EAP. Then, they used an eyelid sling mechanism to create an eyelid blink actuated by an artificial muscle. The technique consists of inserting a sling made of EAP around the eye. Small titanium screws secure the eyelid sling to the small bones of the eye. The sling is connected to a control unit and a battery supply (fig. 5). The artificial muscle device and battery were placed in the natural hollow (fossa temporalis) of the temple to disguise their presence. The displacement of the eyelid sling that was required for complete eyelid closure was 3 mm when both a lower and upper eyelid sling was used, and 6 mm with the solitary upper eyelid sling. In a real setting, a myoelectric sensor could be placed in electrical contact with the actuator to provide a signal from the contralateral orbicularis oculi muscle to coordinate eyelid blinking.
In conclusion, the eyelid sling concept was successful at creating eyelid closure in a cadaver model. The stroke requirements of the proposed artificial muscle device were within the range of available EPAM technology. Future aims include consideration of different sling materials and development of both the EPA device and the articulation component, which is planned for the lateral orbital rim. Biocompatibility and durability studies of EPA in a gerbil model are under way. This technology will be available for patients within the next five years. For people with other types of paralysis, the use of artificial muscles could someday mean regaining the ability to smile.
Artificial peristaltic oesophagus
The oesophagus is not a simple tube for passage of food; it actively transports the food by peristalsis. One can swallow food against gravity, for instance, while doing a handstand. Dysfunction of peristalsis leads to aspiration of food leading to life threatening complications. Without the peristaltic function, an artificial oesophagus has no practical use. Artificial muscles could be used to construct an artificial oesophagus with peristaltic movement and a lower oesophageal sphincter to replace the diseased oesophagus. This approach would also avoid the need for laparotomy to mobilise the stomach or intestine thereby reducing the complexity of the operation.
The artificial oesophagus that was developed by Yambe’s team consisted of a Gore-Tex vascular graft and a nickel-titanium shape memory alloy (SMA) coil [24]. Serial paired SMA coils were placed continuously around the artificial vascular graft in an annular manner. Each pair was connected to an electrical circuit consisting of memory relay switching. The device has been tested in an animal model [24]. After implantation into the neck of a goat, peristaltic movement was generated in the artificial oesophagus by contraction of the NiTi-SMAs, triggered by a pressure sensor in the proximal part of the tube. The simulated peristaltic movement was similar to the peristaltic movement observed in the x-ray barium study performed in humans. However, this device needs more technical improvements and extensive animal studies, before reaching the preclinical phase.
With the availability of an artificial oesophagus, oesophageal carcinoma surgery will become easier and less invasive, and it will be possible to perform the surgery entirely by thoracoscopy. It will widen the indications for an oesophageal operation such that more patients, including the elderly, can benefit from the operative treatment.
|
Table: Clinical applications of artificial muscle technology. |
|
Domain of application
|
Disease or impaired function
|
Device based on artificial muscle technology
|
| Cardiovascular |
Atrial fibrillation |
Atripump |
| |
Heart failure |
BiVAD |
| |
A-V fistula for hemodialysis |
Flow |
| Urinary |
Urinary incontinence |
ATRUS |
| Gastrointestinal |
Oesophageal cancer |
Artificial oesophagus |
| |
Colostomy |
Anal sphincter |
| Neurology |
Facial paralysis |
Eyelid sling |
Conclusions
We reviewed some of the clinical applications of these amazing technologies demonstrating that in the future it will be possible to integrate smart devices into the human body, thereby creating “human chimera”. Artificial organs will eventually avoid some of the problems related to organ transplants like shortage of donors or immunosuppression with the advantage of improving the quality of life even in severely diseased patients, not eligible for transplant. However, to face all the technical challenges of artificial organs, researchers with a diversity of scientific backgrounds have to work closer than ever and this is the real revolution of the future medicine.
References
1 Morgan NB. Medical shape memory alloy applications. The market and its products. Mat Scien Engin. 2003:378(25):16–23.
2 Duerig T, Pelton A, Stockel D. An overview of NiTi medical applications. Mat Sci Engin. 1999;273:149–60.
3 Bar-Cohen Y. Biomimetics using nature to inspire human innovation. Bioinspir Biomim. 2006;1:1–12.
4 Bar-Cohen Y. Electroactive polymer (EAP) actuators as artificial muscles: reality, potential, and challenges. 2nd ed. Bellingham, WA: SPIE Press; 2004:8.
5 Ebron VH, Yang Z, DJ, Kozlov ME, Oh J, Xie H, Razal J, et al. Fuel-powered artificial muscles. Science. 2006;311:1580–3.
6 Sawyer PN, Page M, Baseliust L, Mc Cool C, Lester E, Stanczewsky B, et al. Further studies of nitinol wire as contractile artificial muscle for an artificial heart. Cardiovasc Dis Bulletin Texas Heart Institute. 1976;3(1):65–78.
7 Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task force on Practice Guidelines and the European Society of Cardiology Commettee for practice Guidelines. J Am Coll Cardiol. 2006;48:e149-e246.
8 Yuda S, Nakatani S, Kosakai Y, Yamagishi M, Miyatake K. Long term follow up of atrial contraction after the maze procedure in patients with mitral valve disease. J Am Coll Cardiol. 2001;37(6):1622–7.
9 Tozzi P, Hayoz D, Thévenaz P, Roulet JY, Salchli F, von Segesser LK. Artificial muscles to restore transport function of diseased atria. ASAIO J. 2008;54(1):11–3.
10 Tozzi P, Hayoz D, Siniscalchi G, Salchli F, von Segesser LK. Artificial muscle to wash blood out of fibrillating atrium: an alternative to lifelong anticoagulation. ASAIO J. 2009;55(1):24–7.
11 Abdelnour-Berchtold E, Tozzi P, Siniscalchi G, Hayoz D, von Segesser LK. Atrial assist device, a new alternative to lifelong anticoagulation? Swiss Med Wkly. 2009;139(5-6):82–7.
12 Tozzi P, Hayoz D, Taub S, Muradbegovic M, Rizzo E, von Segesser LK. Biometal muscle to restore atrial transport function in a permanent atrial fibrillation animal model: a potential tool in the treatment of end-stage heart failure. Eur J Cardiothorac Surg. 2010;37(4):870–4.
13 Hemoto H, Harasaki H, Fujimoto LK, Navarro R, White M, Whalen R, et al. Systemic and local effects of heat dissipation in the thermally powered LVAS. ASAIO Trans. 1988;34(3):361–6.
14 Kamdar F, Boyle A, Liao K, Colvin-Adams M, Joyce L, John R. Effects of centrifugal, axial and pulsatile left ventricular assist device support on end-organ function in heart failure patients. J Heart Lung Transplant. 2009;28(4):352–9.
15 Lee R, Te AE, Kaplan SA, Sandhu JS. Temporal trends in adoption of and indications for the artificial urinary sphincter. J Urol. 2009;181;2622–7.
16 Montague DK, Angermeier KW. Postprostatectomy urinary incontinence:the case for artificial urinary sphincter implantation. Urology. 2000;55(2):2–4.
17 Tollefson TT, Senders CW. Restoration of eyelid closure in facial paralysis using artificial muscle. Laryngoscope. 2007;117(11):1907–11.
18 Rogers JG, Aaronson KD, Boyle AJ, Russell SD, Milano CA, Pagani FD, et al.; HeartMate II investigators, continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55(17):1826–34.
19 Chachques JC, Argyriadis PG, Fontaine G, Hebert JL, Frank RA, D'Attellis N, et al. Right ventricular cardiomyoplasty: 10-year follow-up, Ann Thorac Surg. 2003;75(5):1464–8.
20 Suzuki Y, Daitoku K, Minakawa M, Fukui K, Fukuda I. Dynamic cardiomyoplasty using artificial muscle, J Artif Organs. 2008;11(3):160–2. Epub 2008 Oct 5.
21 Muradbegovic M, Taub S, Rizzo E, von Segesser LK, Tozzi P.Ultimate test bench for pediatric biventricular assist device based on artificial muscles. ASAIO J. 2011;57(1):62–7.
22 Müller B, Deyhle H, Mushkolaj S, Wieland M. The challenges in artificial muscle research to treat incontinence. Swiss Med Wkly. 2009;139(41-42):591–5.
23 Senders CW, Tollefson TT, Curtiss S, Wong-Foy A, Prahlad H. Force requirements for artificial muscle to create an eyelid blink with eyelid sling. Arch Facial Plast Surg. 2010;12(1):30–6.
24 Watanabe M, Sekine K, Hori Y, Shiraishi Y, Maeda T, Honma D, et al. Artificial esophagus with peristaltic movement. ASAIO J. 2005;51(2):158–61.




