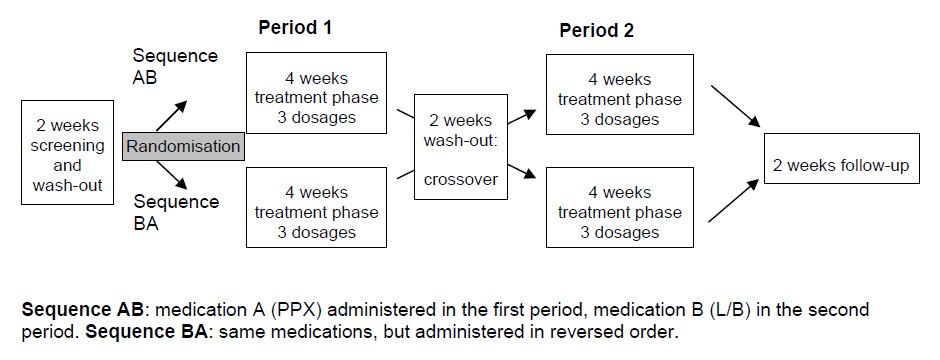
Figure 1
Study design: Randomized, double-blind, comparative crossover study.
DOI: https://doi.org/10.4414/smw.2011.13274
Restless legs syndrome (RLS) is the most common movement disorder and one of the most common sleep-related disorders with a frequency in the general population between 2 to 10% [1]. The International RLS Study Group published an accurate description of diagnostic criteria and a severity scale for this disorder [2]. Periodic limb movements in sleep (PLMS) occur in about 80% of RLS patients [3, 4]. The PLMS index (PLM count per hour sleep) or the PLM index (PLMI), that considers the time spent in bed (during sleep and wakefulness), and the IRLS are commonly used measures to assess the efficacy of a treatment for RLS. Dopaminergic medications are currently considered the treatment of choice for RLS [5, 6]. The efficacy of the dopamine precursor levodopa is well established. Levodopa administered near bedtime was of clear therapeutic value in treating RLS and PLMS in a series of controlled trials [7, 8].
Pramipexole (PPX) is a non-ergotamine dopamine agonist (DA) with selectivity for the D3/2 subtype of the D2 receptor family. Previous clinical trials have demonstrated that PPX is useful in short- and long-term treatment of RLS [9–12]. To our best knowledge, only two studies comparing the clinical efficacy of levodopa and dopamine agonist therapy in RLS have been reported thus far [13, 14]. In a double-blind, cross-over randomised 16-day trial including 11 patients with idiopathic RLS, pergolide (0.125 mg/d) was found to be superior to standard levodopa (250 mg/d) in reduction of both PLMI (as assessed by polysomnography) and RLS symptoms (as assessed by a simple scale: complete, nearly complete and no relief) [13]. In a second double-blind, randomised, active-controlled, parallel-group, multi-centre 30-week long-term study with 361 patients, cabergoline (2–3 mg/d) showed a superior efficacy over standard levodopa (200–300 mg/d) for reduction of the IRLS total score [14]. In this second study, standard levodopa was found to be better tolerated than cabergoline but more often associated with augmentation. The aim of the present study was to test non-inferiority of PPX in a head-to-head comparison with dual-release-levodopa (L/B) in the treatment of RLS by comparing their outcome using objective measurements, standardised rating scales, as well as quality of life measures. The choice of the crossover design and of the efficacy endpoints reflects the experience from earlier studies, where the superiority of both the control and the test drug against placebo were clearly established [9]. The dual release levodopa formulation combines immediate and slow-release levodopa properties and is commonly used in Switzerland. It was chosen for comparison with PPX because of the lower risk of augmentation in slow release levodopa formulations [8]. Dosages of PPX and L/B were chosen based upon clinical practice.
The study was reviewed and approved by the Institutional Review Boards (IRB) and was notified to the Swiss Health Authority.

Figure 1
Study design: Randomized, double-blind, comparative crossover study.
This investigation was performed as a multi-centre, randomised, double-blind, comparative crossover trial (fig. 1) and was conducted in six certified Swiss sleep-centres (Zurich, Bern, Basel (2), Zurzach, Lugano).
Men and women between 25 and 85 years of age were eligible to participate in the study if they fulfilled all clinical criteria for diagnosis of idiopathic RLS [2]. They had to present RLS symptoms almost every day, as judged by the investigator and with more than five PLM/h during bedtime in each of three consecutive screening nights. All patients were “de novo” patients with regard to any previous RLS therapy with PPX, L/B or another DA.
PPX 0.25–0.75 mg (Mirapex/Sifrol®) and dual-release L/B 125–375 mg (Madopar® DR) [15] were administered orally, once daily before bedtime.
Tablets for both treatments were packed in identical capsules for blinding. Treatment was started at the lowest dose (1 capsule), and then adjusted over a period of two weeks (up to 3 capsules). For further details please see figure 1. There was no evidence of any carry-over effect.
Data required for the analysis were recorded and transferred electronically to a central database by means of an electronic data capture system (Tri@l-IT, clinIT Ltd, Freiburg, Germany).
Overall evaluation of a treatment effect on RLS included an objective measure of the frequency of periodic leg movements during the time spent in bed(PLM index, PLMI), as measured by three-night actigraphic recordings (primary endpoint), and clinical evaluation of the severity of RLS, using the IRLS rating scale (total change in IRLS score), and Visual Analogue Scales (VAS) for the assessment of RLS symptoms as a whole during the day, at sleep onset and at night [16]. In addition, outcome measures on quality of life, daytime sleepiness and mood were recorded using the SF-36 scale, Epworth Sleepiness Scale (ESS) [17], Hospital Anxiety and Depression Scale (HADS) [18], and Clinical Global Impression (CGI).
The primary efficacy variable was PLMI, using the PAM-RL monitor system (IM Systems, Baltimore, USA) [19]. Each of the four series of actigraphic measurements were obtained over three consecutive nights from both legs. The analysis only considered the periods when the patient was in bed with the lights turned off. PLM were analysed automatically and checked visually. Periods with clear artefacts were excluded from the analysis [20].
Safetywas assessed with regard to type and frequency of adverse events (WHO body system and preferred terms), including a separate checklist of ten potential adverse drug reactions (PARS), domperidone needs (overall consumption), laboratory analyses and vital signs.
The study followed a two-sequence, two-period crossover design, and the comparison aimed at providing evidence for the non-inferiority of PPX with respect to dual release L/B [21]. The sample size calculation was based on previous placebo-controlled studies of levodopa and PPX [10–12]. Based on previous studies we considered a reduction of the mean PLMI to 10/h or less as being significant. A sample size of n = 20 patients per group completing the study (i.e. a total of n = 40 patients) was chosen for this study. The statistical analyses of the primary and secondary parameters were based on a per-protocol (PP) population analysing all patients who completed both treatment periods. Overall safety was assessed in the intend-to-treat (ITT) population. The hypothesis of non-inferiority of pramipexole compared to dual release levodopa/benserazide was tested for PLMI in the per-protocol patient population at the level α = 2.5%. The analysis was performed using an ANCOVA-model with the factors treatment, sequence, subject sequence and period (randomisation) and the baseline (BL) values as covariate. As the normality distribution assumption for the residuals could not be accepted, a non-parametric crossover test (Wilcoxon rank sums test) was performed.
In order to incorporate covariates, we used an ANCOVA model for IRLS. For other secondary parameters an ANCOVA model (SF 36, CGI), Wilcoxon rank sums test (VAS, ESS) and exact test (HADS) were used.
Out of 113 screened patients, 67 were randomised into this trial (intent-to-treat population). Mainly due to the rigorous criteria for protocol adherence and strict study drug compliance requests, 28 subjects from these had to be excluded from the analysis and 39 patients could be included in the per protocol population. There were 23 females (59%) and 16 males with a mean age of 57 ± 11 (range: 35 to 78). All of them were of Caucasian origin, and mean body mass index (BMI) was 25 ± 3 (range: 18 to 34). There was no difference in drop-out rate due to lack of benefit between the two groups. A summary of the disposition of randomised patients is provided in figure 2.
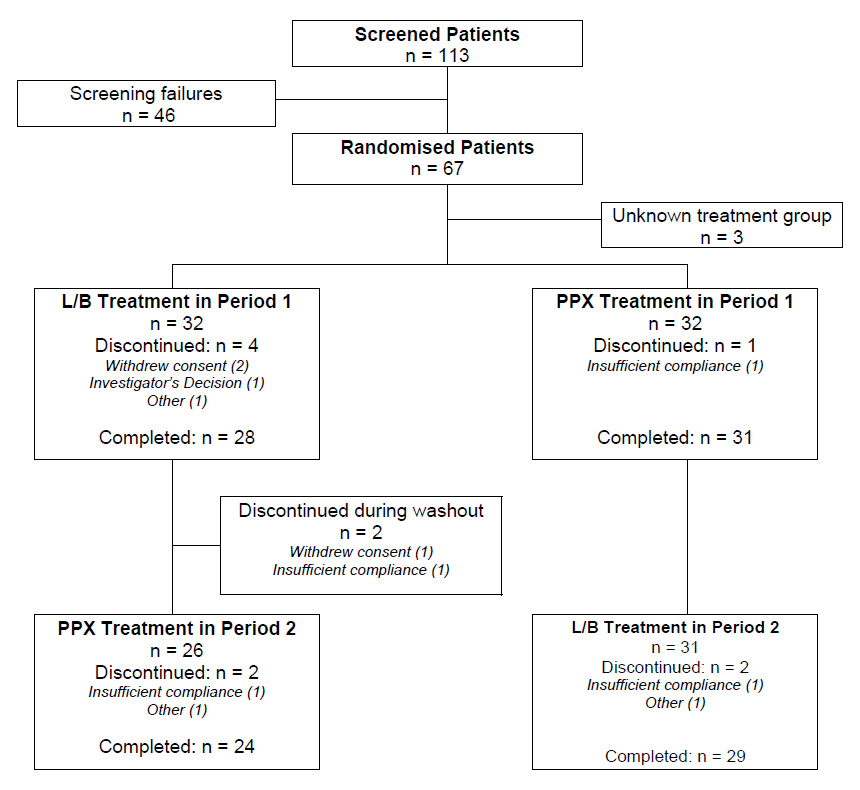
Figure 2
Patient disposition.
Treatment was initiated with one capsule of study drug (0.25 mg PPX or 100/25 mg L/B). The algorithm for titrating was to increase to 3 capsules per day if needed and tolerated, and to decrease the number of capsules for side effects. The recommended time period for any dose adjustments was 3 to 5 days.
Due to insufficient efficacy, the dosage of PPX and L/B was increased from 1 to 2 capsules at day 3 or 4 in 70% and 78% of the patients, respectively, and again increased to 3 capsules in approximately 50% of all patients at day 7 or 8. At day 13 to 15, the dosages were reduced in about 10% of the patients, for L/B (from 3 to 2 capsules) as well as for PPX (from 2 to 1 capsule). During the maintenance period (2 weeks) the dosages were kept constant. The mean daily dosage for PPX was 0.49 mg and for L/B 192/48 mg. The duration of drug exposure was 29 days for both treatments.
Domperidone was administered to 13 patients during treatment with PPX and to 4 patients (safety population) during treatment with L/B, and the number of tablets ranged from 1 to 5 tablets overall in most cases. Two patients administered more than 5 tablets during treatment with levodopa and three patients took more than 5 tablets during pramipexole treatment. As an outlier, one single patient took 66 and 59 domperidone tablets during L/B and PPX treatment periods.
Baseline PLMI scores were comparable between treatment groups (PP population): Mean values were 21.5 (SD 14.9) for PPX and 21.1 (SD 17.0) for L/B. Combining both study periods, the PLM index at week 4 showed mean (SD) values for PPX and L/B of 10.0 (10.9) and 13.4 (12.8), resulting in a mean reduction of 11.5 (13.9) and 7.7 (9.5) respectively (table 1). Comparing reduction from baseline PPX was non-inferior in comparison to L/B on the primary endpoint PLMI (p = 0.00015).
Baseline IRLS scores were comparable between treatment groups; 20.8 (8.2) for PPX and 21.1 (6.9) for L/B. Combining both study periods, the IRLS score at week 4 exhibited a mean reduction from baseline of 7.2 (9.5) and 4.0 (7.5), respectively. Statistical analysis of the treatment effect using an ANCOVA model revealed a p-value of 0.054 and indicated a trend in favour of PPX. A baseline IRLS score ≤20 was found in 18% of the patients, 44% were “mixed” (one score ≤20, one score >20), and 38% had an IRLS score >20. The subgroup analysis in patients with IRLS score >20 (severe to very severe) at baseline showed a significantly (p = 0.047) greater reduction of IRLS score at week 4 for PPX (8.5 (7.7)) as compared to L/B (4.3 (5.4)), shown in table 1, together with median and ranges.
An additional exploratory analysis performed to investigate any potential correlation between PLMI and total IRLS score at baseline and end of treatment and between the corresponding changes, revealed correlation factors of –0.0937 (baseline), 0.0538 (end) and 0.0566 (change), respectively, indicating no correlation between PLMI and total IRLS score.
Comparison of RLS symptoms by means of VAS (100 millimetre scale) during different phases of the day revealed different changes (SD) under PPX compared to L/B treatment: During the day –8.5 (18.7) versus +1.8 (23.3) with a trend in favour of PPX (p = 0.05), at sleep onset –9.3 (25.4) versus –8.6 (32.1) with p = 0.67, and during the night –14.1 (32.4) versus –18.5 (28.3) with p = 0.65. For results on the other secondary efficacy parameters see table 1.
Analysing the safety population (67 patients), 87 adverse drug reactions (ADR) were observed during L/B (39 patients) and 116 ADR were observed during PPX treatment (38 patients). For PPX, a higher incidence of nausea (31%), headache (17%), constipation (9%) and vomiting (8%) was observed compared to L/B treatment (17%, 11%, 5% and 3%, respectively). For L/B, a higher number of events associated with RLS syndrome (22%) and dizziness (17%) was recorded compared to PPX treatment (9% and 13%). The incidence of nightmares was more frequent with PPX compared to L/B treatment (6% and 3%, respectively). In the other organ system classes, adverse events showed a comparably low incidence for both treatment groups. No serious adverse event occurred during the treatment periods. One adverse event occurred in the PPX group, and was classified as significant, described as a “short absence during car driving” assigned to the preferred term “sleep attack”, and judged by the investigator as not related.
Results of the potential adverse reactions (PARS) checklist are shown in table 2.
The majority of patients had clinical laboratory values and vital sign within normal range, clinically acceptable ranges, or with values essentially unchanged between baseline and end of study measurements.
| Table 1:Treatment effects on primary and secondary outcome measures in PP population (n = 39). | |||||
| L/B | PPX | ||||
| Baseline | Week 4 | Baseline | Week 4 | ||
| PLMI | |||||
| Mean (SD) Median (Range) Change from Baseline Mean (SD) | 21.1 (17.0) 14.4 (1.6–69.5) | 13.4 (12.8) 10.1 (0.5–49.3) –7.7 (9.5) | 21.5 (14.9) 18.5 (1.5–55.2) | 10.0 (10.9) 5.4 (0.4–45.0) –11.5 (13.9) | |
| IRLS score | |||||
| Mean (SD) Median (Range) Change from Baseline Mean (SD) | 21.1 (6.9) 22 (7–35) | 17.1 (7.8) 19 (1–30) –4.0 (7.5) | 20.8 (8.2) 22 (6–37) | 13.6 (8.0) 14 (0–31) –7.2 (9.5) | |
| IRLS scores >20 “severe and very severe” (n = 15) | |||||
| Mean (SD) Median (Range) Change from Baseline Mean (SD) | 26.5 (4.3) 27 (21 to 35) | 22.3 (4.3) 23 (10–27) –4.3 (5.4) | 26.3 (3.8) 25 (22–37) | 17.8 (7.7) 19 (0–31) –8.5 (7.7)* | |
| Epworth Sleepiness Scale (ESS) | |||||
| Mean (SD) Median (Range) | 8.7 (3.7) 9 (3–16) | 8.2 (3.7) 8 (2–18) | 8.2 (4.0) 8 (1–17) | 7.9 (3.4) 8 (2–17) | |
| Quality of Life (SF-36) | |||||
| Physical component Mean (SD) Median (Range) Mental Component Mean (SD) Median (Range) | 44.9 (5.3) 45.1(33.0–60.3) 42.2 (6.0) 43.1(20.4–49.4) | 45.0 (5.5) 44.4 (25.5–58.4) 42.5 (6.5) 44.8(20.8–53.0) | 44.8 (5.2) 44.6(32.3–57.6) 42.5 (6.0) 44.2(26.3–51.1) | 43.5 (4.8) 43.5 (34.4–54.0) 43.1 (5.4) 44.1(25.6–52.9) | |
| Hospital Anxiety and Depression Score (HADS) | |||||
| Anxiety score Mean (SD) Median (Range) Depression score Mean (SD) Median (Range) | 8.3 (2.4) 8.0 (3–17) 11.2 (1.6) 11.0 (7–14) | 8.3 (1.8) 8.0 (5–13) 11.2 (1.9) 12.0 (6–15) | 8.6 (2.2) 8.0 (4–15) 11.4 (1.4) 11.5 (9–14) | 8.0 (1.6) 8.0 (5–12) 11.6 (1.4) 12.0 (8–15) | |
| Statistics:PLMI: No carry-over effect and no period effect were observed. The difference in PLM index was not significant (p = 0.065). IRLS-Score: Treatment effect was p = 0.054. Subpopulation severity “low to moderate” was p = 0.68 and“severe to very severe” was p = 0.047*. | |||||
| Table 2: Total number of Potential Adverse Drug Reactions (PARS) in PP population (n = 39). | ||||||
| L/B | PPX | |||||
| PARS | BL | Week 2 | Week 4 | BL | Week 2 | Week 4 |
| Asthenia | 11 | 6 | 5 | 8 | 8 | 8 |
| Augmentation* | 8 | 7 | 3 | 2 | ||
| Constipation | 5 | 4 | 5 | 3 | 9 | 7 |
| Oedema | 1 | 1 | 3 | 2 | 3 | |
| Hallucinations or visual disturbances | 1 | 3 | 2 | 1 | 2 | 4 |
| Hypotension with dizziness | 1 | 9 | 7 | 6 | 11 | 9 |
| Involuntary movements | 12 | 9 | 10 | 15 | 7 | 7 |
| Nausea or vomiting | 9 | 4 | 4 | 20 | 10 | |
| Rebound** | 7 | 4 | 3 | 1 | 2 | 3 |
| Sleep attacks*** | 1 | |||||
| Other adverse event(s) | 7 | 29 | 16 | 3 | 21 | 18 |
| Total | 46 | 77 | 60 | 44 | 85 | 71 |
| * Augmentation:Increased intensity of RLS symptoms OR shorter onset time OR involvement of the other limb. ** Rebound:RLS symptoms in the early morning. *** Sleep attack:Falling asleep, possibly without warning signs, during “active” activities, such as eating, driving a car or making a phone call. | ||||||
The Swiss RLS study is the first investigation directly comparing a non-ergot dopamine agonist with levodopa in a crossover design and overall is the third head-to-head study in RLS. The choice of PLMI as the primary endpoint reflected the standard approach at the time the study was planned. In addition, PLM actigraphy is an objective assessment of leg motor activity. The IRLS score – one of the secondary endpoints in the current study – is currently the preferred endpoint in large-scale RLS trials. The absence of a significant correlation between PLMS and IRLS in this and in previous studies, underscore however the utility of assessing both variables in RLS treatment studies [10, 20, 22, 23].
The current study proves that PPX (mean dose of 0.49 mg/d) and dual release L/B (mean dose of 192/48 mg/d) are comparably effective in the treatment of de novo RLS patients. A significantly greater reduction of the IRLS score in the subpopulation “severe and very severe” (p = 0.047) was found with PPX when compared to dual release L/B. The overall improvement of both PLMS and IRLS was relatively modest, although comparable with data from other studies referring to a 6-week administration of pergolide [22], a 4-week administration of ropinirole [23] and a 12-week treatment with pramipexole [24]. The limited improvement of PLMS/RLS may be related to different factors. Firstly, only de novo patients were included, and secondly, only 38% of our patients had a baseline IRLS >20. Most recently published large-scale treatment studies in RLS included patients with mostly moderate-severe disease and only few de novo patients [14, 22, 23, 25].
Baseline evaluations of the other secondary efficacy parameters including quality of life, ESS and HADS scores reflected moderate disease characteristics, and as a consequence changes at week 4 were of minor magnitude.
Overall, treatment with PPX and dual release L/B was well tolerated; the overall frequency of AEs was similar. No severe AEs assigned to treatment intervals occurred, and only one significant AE was reported. The vast majority of clinical laboratory parameters and vital signs were within normal ranges. Treatment-emerged adverse drug reactions were observed in 59% and 61% of the patients during PPX and L/B treatment, respectively and were comparable with results of studies with pergolide (67%), ropinirole (45%) and cabergoline (55–67%)[22, 23, 26]. During treatment with L/B more patients reported restless legs symptoms (22%) and dizziness (17%). The incidence of nausea with PPX reported in this study was higher (31%) compared to results with PPX in other studies (15% and 19%) [12, 24]. This may in part be related to the higher initial dose of 0.25 mg, compared to the usual starting dose of 0.125 mg, but also to the short titration time from 0.25 mg to 0.75 mg in two steps within 7 to 8 days. The higher incidence and severity of the reported PARS “augmentation” associated with the comparator drug is a well documented effect of L/B, although a treatment duration of 4 weeks may be too short to assess this complication [26, 27].
In conclusion, this study demonstrates pramipexole and dual-release levodopa to be comparably effective in the short-term treatment of patients with mild to moderate idiopathic RLS.
1 Allen RP, Earley CJ. Restless legs syndrome: a review of clinical and pathophysiologic features. J Clin Neurophysiol. 2001;18:128–47.
2 Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–32.
3 Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lespperance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997;12:61–5.
4 Clavadetscher SC, Gugger M, Bassetti CL. Restless legs syndrome: clinical experience with long-term treatment. Sleep Med. 2004;5:495–500.
5 Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the dopaminergic treatment of restless legs syndrome and periodic limb movement disorder. Sleep. 2004;27:557–9.
6 Vignatelli L, Billiard M, Clarenbach P, Garcia-Borreguero D, Kaynak D, Liesiene V, Trenkwalder C, Montagna P. EFNS guidelines on management of restless legs syndrome and periodic limb movement disorder in sleep. Eur J Neurol. 2006;13:1049–65.
7 Benes H, Kurella B, Kummer J, Kazenwadel J, Selzer R, Kohnen R. Rapid onset of action of levodopa in restless legs syndrome: a double-blind, randomized, multicenter, crossover trial. Sleep. 1999;22:1073–81.
8 Collado-Seidel V, Kazenwadel J, Wetter TC, Kohnen R, Winkelmann J, Selzer R, et al. A controlled study of additional sr-L-dopa in L-dopa-responsive restless legs syndrome with late-night symptoms. Neurology. 1999;52(2):285–90.
9 Becker PM, Ondo W, Sharon D. Encouraging initial response of restless legs syndrome to pramipexole. Neurology. 1998;51:1221–3.
10 Montplaisir J, Nicolas A, Denesle R, Gomez-Mancilla B. Restless legs syndrome improved by PPX: a double-blind randomized trial. Neurology. 1999;52(5):938–43.
11 Montplaisir J, Denesle R, Petit D. PPX in the treatment of restless legs syndrome: a follow-up study. Eur J Neurol. 2000;7(Suppl 1):27–31.
12 Partinen M, Hirvonen K, Jama L, Alakuijala A, Hublin C, Tamminen I, et al. Efficacy and safety of pramipexole in idiopathic restless legs syndrome: a polysomnographic dose-finding study – the PRELUDE study. Sleep Med. 2006;7:407–17.
13 Staedt J, Wassmuth F, Ziemann U, Hajak G, Rüther E, Stoppe G. Pergolide: treatment of choice in restless legs syndrome (RLS) and nocturnal myoclonus syndrome (NMS). A double-blind randomized crossover trial of pergolide versus L-Dopa. J Neurol Transm. 1997;104:461–8.
14 Trenkwalder C, Benes H, Grote L, Happe S, Hoegl B, Mathis J, et al. Cabergoline compared to levodopa in the treatment of patients with severe restless legs syndrome: Results from a multi-center, randomized, active controlled trial. Mov Disord. 2007;22:696–703.
15 Descombes S, Bonnet AM, Gasser UE, Thalamas C, Dingemanse J, Arnulf I, et al. Dual-Release Formulation, a novel principle in the Levodopa treatment of Parkinson’s Disease. Neurology. 2001; 56:1239–42.
16 Bonnet M, Carley D, Carskadon M, Easton P, Guilleminault C, Harper R, et al. Atlas Task Force. Recording and scoring leg movements. APSS Natl Mtg, 1992 Sleep. 1993;16:749–59.
17 Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5.
18 Zigmond AS, Snaith RP. The Hospital Anxiety and Depression scale. Acta Psychiatr Scand. 1983;67:361–70.
19 Gorny SW, Allen RP, Krausman DT, Earley CJ. Evaluation of the PAM-RL system for the detection of periodic leg movements during sleep. 12th Ann Mtg of the Associated Professional Sleep Societies, New Orleans, 18–23 June 1998.
20 Sforza E, Mathis J, Bassetti C. The PAM-RL ambulatory device for detection of periodic leg movements: a validation study. Sleep Medicine. 2005;6:407–13.
21 Koch GG. The use of non-parametric methods in the statistical analysis of the two-period change-over design. Biometrics. 1972;28(2):577–84.
22 Trenkwalder C, Hundemer H-P, Lledo A, Swieca J, Polo O, Wetter TC, et al. Efficacy of pergolide in the treatment of restless legs syndrome: The PEARLS Study. Neurology. 2004;62:1391–7.
23 Adler CA, Hauser RA, Sethi K, Cavines JN, Marlor L, Anderson WM, Hentz JG. Ropinirole for restless legs syndrome: A Placebo controlled crossover trial. Neurology. 2004;62:1405–7.
24 Winkelman JW, Sethi KD, Kushida CA, Becker PM, Koester J, Cappola JJ and Reess J. Efficacy and safety of pramipexole in restless legs syndrome. Neurology. 2006; 67:1034–9.
25 Wetter TC, Stiasny K, Winkelmann J, Buhlinger A, Brandenburg U, Penzel T, et al. A randomized controlled study with pergolide in patients with restless legs syndrome. Neurology. 1999;52:944–50.
26 Stiansny-Kolster K, Benes H, Peglau I, Hornyak M, Holinka B, Wessel K, et al. Effective carbergoline treatment in idiopathic restless legs syndrome. Neurology. 2004;63:2272–9.
27 Benes H, Kurella B, Kummer J, Kazenwadel J, Selzer R, Kohnen R. Rapid onset of action of levodopa in restless legs syndrome: a double-blind, randomized, multicenter, crossover trial. Sleep. 1999;22:1073–81.
Trial registration number: ClinicalTrials.gov Identifier: NCT00144209
Acknowledgements:We would like to thank Alex Cueni, Barbara Haefelfinger, and Dr. Philip Barth of Boehringer-Ingelheim (Schweiz) GmbH for the coordination of the study; Marina Tetyusheva of Dr. M. Köhler GmbH and Dr. Urs E. Gasser of ClinResearch GmbH for statistical analysis and support in the preparation of the manuscript, and Mechtild Uhl (study nurse, Zurich), Antoinette Mosbacher and Heidi Mani (Bern) for their support in the acquisition and processing of data.
Funding / potential competing interests: Boehringer-Ingelheim (Schweiz) GmbH was the sponsor of the study, which was conducted under financial contract at six Swiss clinical sites. Role of the Sponsor: Boehringer- Ingelheim (BI) participated in the design and conduct of the study and in the management of the data. BI provided pramipexole and dual-release levodopa for this trial. In addition to their roles as clinical investigators, academic centers of Drs. Bassetti and Mathis have received unrestricted educational grants from Boehringer- Ingelheim (Schweiz) of less than 5,000 $/ year. F. Bornatico, P. Fuhr, J. Schwander, and U. Kallweit declare no financial conflict of interest.