
Figure 1
Body temperature of patients according to their length of stay at infirmary.
DOI: https://doi.org/10.4414/smw.2011.13307
A(H1N1)pdm09 is a highly contagious pathogen which made headlines in 2009, as the so called swine flu, by causing a worldwide influenza pandemic. The pandemic started in Mexico in April 2009 [1], and lasted until August 2010 [2]. It was not the first time that swine influenza had caused a worldwide pandemic. The 1918 “Spanish flu” also contained swine influenza genome. Since then no pandemics have occurred [3].
A(H1N1)pdm09 is a novel pathogen, resulting from a quadruple reassortment of two swine strains, one avian strain and one human strain, allowing this virus to infect humans [4, 5].
During the pandemic of 2009, data from the American Centers for Disease Control and Prevention (CDC) showed, that the A(H1N1)pdm09 strain differed from seasonal influenza in that it mostly infected individuals younger than 65 years old. 90% of A(H1N1)pdm09 related hospitalisations and 87% of estimated deaths occurred in patients younger than 65 years, compared to 40% of hospitalisations and 10% of deaths related to seasonal influenza in the same age group [6]. However, with an estimated 274,000 hospital admissions (0.45%) and 12,470 deaths (0.02%), the rate of serious complications was quite low in comparison to seasonal influenza [6].
The virus is transmitted by sneezing and coughing via droplets [7]. Other bodily fluids (stool) can be infectious as well [8]. Infected patients usually spread the virus at least one day prior to the onset of symptoms [7].
Common symptoms include coughing, fever, sore throat, malaise, chills, myalgias and headache. Vomiting and diarrhoea have also been reported in many patients [9].
Viral pneumonia represents the most common cause of hospital admission [7, 10].
Due to its highly contagious nature A(H1N1)pdm09 is expected to spread rapidly in crowded living quarters, such as those found in military barracks. However, there are few reported epidemics in military bases. In 1976, a swine influenza outbreak in Fort Dix, New Jersey, resulted in the death of one of the 13 soldiers infected [11].
We report the onset and dynamics of an A(H1N1)pdm09 outbreak, which occurred in December 2010 in four Swiss military barracks in Herisau and Gossau, Switzerland, affecting more than 100 recruits. In the influenza season 2010/11, the A(H1N1)pdm09 strain came back as a “seasonal” strain and the outbreak we describe here was the first noticed in the 2010/11 season in Switzerland.
The aim of the analysis was to share the experience of an A(H1N1)pdm09 outbreak and to initiate a discussion about further preventive measures in the Swiss Armed Forces and in similar settings with crowded living conditions.
We performed a retrospective chart analysis of a flu outbreak in an infantry boot camp in the Swiss mountains, occurring between 14 December and 23 December 2010 (week 50 and 51). The camp’s infirmary is responsible for 750 recruits from 4 different barracks located in separate villages within a 30 kilometer radius. The recruits were organised in 5 different companies which were not in close contact with each other. During the investigation period, no female recruits were present in the above mentioned barracks.
Cases were defined as recruits suffering from acute respiratory infection (ARI) with fever and at least one of the following symptoms on admission: myalgia, coughing, sore throat, malaise, chills, and headache.
Nasopharyngeal swabs were obtained from 16 patients with severe ARI (fever >39.5 and severely reduced general condition) among patients admitted to the infirmary within first two days of the outbreak. Blood samples were obtained from 10 patients in severely reduced general condition, within 24 hours after admission. Legionella antigen was tested in the urine of 5 randomly selected patients as part of a military doctrine to exclude other respiratory infections at the beginning of an outbreak. Additionally, 8 paramedics without ARI symptoms had been tested for A(H1N1)pdm09 influenza RNA after discharge of the last patient.
Blood analyses were performed at the infirmary; Legionella antigen was tested at Unilabs AG, CH-9000 St. Gallen; nasopharyngeal swabs were tested for viral A(H1N1)pdm09 RNA by RT-PCR at the ABC-Labs of the Swiss armed forces, Spiez, Switzerland and at the Institute of Clinicial Microbiology and Immunology St. Gallen, Switzerland. The body temperature was measured with an in-ear-thermometer (ThermoScan 6021; Braun, Kronberg, Germany).
Symptomatic therapy was the same for almost all recruits (paracetamol 4 x 1 g/d, ibuprofen 3 x 600 mg/d). Ibuprofen was replaced by Mefenamic acid in two and by metamizole in one case. No antiviral therapy was administered. Criteria for discharge were: Body temperature of less than 38 °C during 48 h and less than 37.5 °C during the last 12 h.
Immediately after the admission of the first five cases, preventive measures were implemented in the infirmary. The preventive measures are standardised within the Swiss Armed Forces and follow mainly the recommendations of the Center for Disease Control (CDC) [7] (table 1).
All cases and all recruits without ARI symptoms (negative controls) of the most-affected barack were interviewed about their vaccination status.
Ethical approval for the study was given by the Command of the Swiss Military Medical Corps.
Prism 5 for Mac (Graphpad Software Inc., La Jolla, CA, USA) was used to analyze and plot the data. Two-tailed student’s t-test was used to compare unpaired samples. Data are presented as means (± standard deviation, SD).
| Table 1: Preventive measures implemented at the infirmary. Based on recommendations of the Center for Disease Control (CDC) [7]. |
| The isolation of affected patients from other patients without A(H1N1)pdm09 and healthy personnel through the establishment of isolation rooms. |
| – Healthcare personnel should protect themselves with surgical facemasks when entering a room with infected patients (droplet isolation). |
| – Healthcare personnel and troops at risk should frequently perform hand hygiene with alcohol-based sanitizers. |
| – Visitors should be made aware of the precautions and the number of visitors should be limited. |
| – Patients should stay in their rooms as long as possible and wear surgical facemasks on leaving the room. |
| – Patients should avoid prolonged contact with healthy individuals. |
| – Potentially contaminated surfaces are to be disinfected daily. |
| – Patients are only to be discharged after 48 fever-free hours, to avoid infecting others through prolonged viral shedding. |
All cases were male recruits in good physical condition prior to the outbreak. Their median age was 20 years (range 18–23 y). All recruits had a body mass index (BMI) below 25 as this is one of the recruitment requirements in the infantry. Overall, 105 of the 750 recruits (attack rate: 14%) had been admitted with ARI symptoms to the infirmary. One company was hit in the first week and especially hard with an attack rate of 42% (69 of 165 recruits). In the beginning, only a few rooms (up to 20 recruits per room) in the dormitory were affected. Two days after the onset of the epidemic recruits from the whole dormitory were treated. Recruits from the same company shared the same mess hall. None of the patients and 1 of the 103 (<1%) interrogated recruits without ARI symptoms had been previously vaccinated against influenza.

Figure 1
Body temperature of patients according to their length of stay at infirmary.
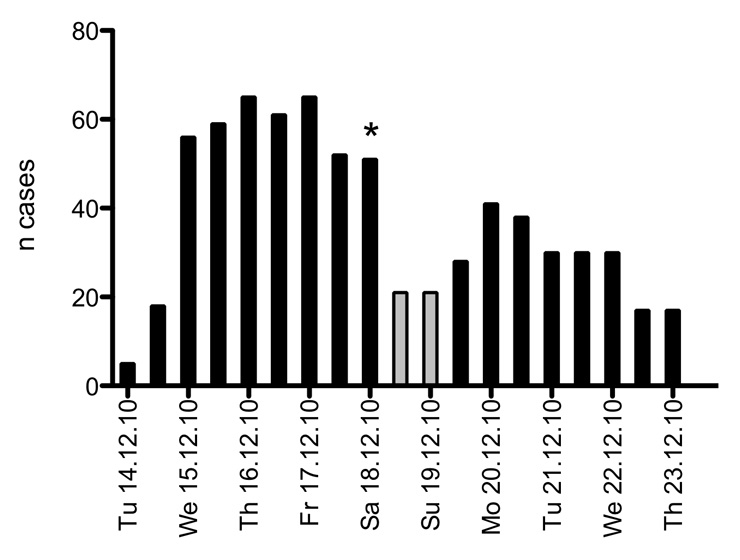
Figure 2
Epidemic curve, representing all cases at infirmary every 12 hours. The 32 patients, who stayed at home after the weekend break, were not included because the exact day of disease onset was not known. The gray bars indicate a 36 hour weekend-leave for healthy recruits. * = hospitalisation of two recruits.
Common clinical symptoms included high fever (39–40 °C), myalgia (including back aches and joint pain) and bronchitis with persistent cough and throat aches. Some patients presented with gastrointestinal symptoms including nausea, vomiting and diarrhoea. Fever progression typically occurred as two peaks within three days. However, some patients experienced several peaks over more than 4 days (fig. 1). Fever was usually highest in the morning.
Two patients with clinical suspicion of viral pneumonia were transferred to a hospital as their oxygen saturation had dropped to a SpO2 of 85%. Both patients were transferred to the hospital 5 days after onset of disease and they recovered fast under oxygen administration.
Fifty-nine patients (56.2%) were admitted to the infirmary within the first two days of the outbreak. Due to the approaching weekend, when healthy recruits are allowed to return home, the number of new admissions dropped on Thursday and Friday and rose again on Sunday evening when the recruits returned to base. Recruits with ARI were kept isolated at the infirmary over the weekend. After the weekend, 32 recruits stayed at home with ARI diagnosis from their general practitioner. No hospitalisations were reported. Including these patients, the total number of patients would have been 137 (attack rate 18%). However, due to the fact, that the exact day of disease onset was not known, they were not included in the analysis.
With the start of the second week, recruits from 4 out of 5 companies were admitted to the infirmary with an influenza diagnosis. During day 7, new admissions declined steadily. The median length of stay at the infirmary was 3 days (range 12 hours to 9 days). The epidemic curve (fig. 2) illustrates the pattern of cases, especially the plateau whilst many recruits were on home leave.
The clinical influenza A(H1N1)pdm09 diagnosis was confirmed in all 16 patients tested. Legionella antigen was not detected in five randomly selected urine samples.
Blood samples from 10 patients with high fever (>39.5 °C) were tested: Only one patient had a moderate leucocytosis with 11.1×103/mm3 (normal range: 4 – 10×103/mm3). Granulocytes were elevated in 3 patients (range 89.7–71.2%, normal range: 50–87%). C-reactive protein (CRP) was elevated in 7 out of 10 samples with a pathologic CRP range of 35–88 mg/L. Hemoglobin and platelet count were within the normal ranges (table 2).
There was no difference in fever peaks nor inpatient days between the subpopulation tested and the remaining patients (table 3).
8 of the 11 paramedics who were most exposed to patients were tested negative for A(H1N1)pdm09. They protected themselves with surgical face masks and did not show any flu symptoms.
| Table 2: Laboratory results of 10 patients with high fever (>39.5 °C). Seven patients were among the 16 patients tested for A(H1N1)pdm09. | ||||||
| Patient | Leucocytes (103/mm3) | Granulocytes (%) | Hemoglobin (g/dL) | Platelets (103/mm3) | C-Reactive Protein (mg/L) | Influenza A(H1N1)pdm09 RNA |
| 1 | 11.1 | 86.0 | 15.1 | 175.0 | 46.0 | positive |
| 2 | 7.2 | 89.8 | 16.1 | 187.0 | 48.0 | positive |
| 3 | 7.7 | 89.7 | 14.5 | 131.0 | 64.0 | positive |
| 4 | 3.0 | 83.0 | 14.3 | 133.0 | 35.0 | positive |
| 5 | 7.6 | 81.4 | 15.2 | 134.0 | 88.0 | positive |
| 6 | 6.0 | 71.2 | 13.7 | 178.0 | 67.0 | positive |
| 7 | 5.0 | 80.3 | 15.2 | 193.0 | 51.0 | positive |
| 8 | 5.6 | 83.1 | 14.7 | 140.0 | <8 | not tested |
| 9 | 3.9 | 85.5 | 13.7 | 161.0 | <8 | not tested |
| 10 | 7.1 | 80.0 | 15.7 | 183.0 | <8 | not tested |
| Mean | 6.4 | 83.0 | 14.8 | 161.5 | 57.0 | |
| SD | 2.3 | 5.4 | 0.8 | 24.8 | 17.5 | |
| Normal range | 4–10 | 71.2–89.7 | 14–18 | 150–400 | <8 | |
| Table 3: Comparison of the subpopulation tested for A(H1N1)pdm09 in comparison to the remaining patients. Data sets are presented as mean (± SD). Student’s t-Test for unpaired samples has been used to compare the two groups. p <0.05 was considered significant. | |||
| A(H1N1)pdm09 positive | not tested | p <0.05 | |
| n | 16 | 89 | – |
| inpatient days | 3.1 (±1.9) | 2.8 (±1.4) | n.s. |
| max body temp. (°C) | 39.0 (±1.0) | 38.7 (±0.9) | n.s. |
| mean body temp (°C) | 37.3 (±0.5) | 37.2 (±0.4) | n.s. |
We describe an A(H1N1)pdm09 epidemic occurring under crowded living conditions. Common exposure, clinical presentation and a 100% confirmation rate of A(H1N1)pdm09 in the subpopulation tested suggest that most of the 105 individuals with ARI suffered from A(H1N1)pdm09. The symptoms including gastrointestinal complaints in a minority of affected individuals were identical with those described by studies on the 2009 A(H1N1)pdm09 pandemic [9]. However, the subpopulation of previously healthy, young and athletic recruits can not be compared with the general population. Especially the healthy worker effect has to be mentioned, which describes the observation, that the working population is healthier than the general population [12, 13]. Other studies with populations comparable to our study did not register any deaths or serious complications either [14].
Except for elevated CRP values, we did not observe laboratory pathologies in our patients. In a cohort of 272 hospitalised patients with influenza A(H1N1)pdm09, Jain et al. found leukopenia in 20%, leukocytosis in 18%, thrombocytopenia in 14%, thrombocytosis in 9% and anemia in 37% of individuals [15]. However these patients covered all age groups and 73% had at least one underlying condition.
The recruits were closely observed by their superiors, and every recruit with suspected ARI was immediately transferred to the infirmary and isolated. However, the analysis of disease dynamics is biased by the recruits’ fear of being kept in the infirmary during the weekend as well as over the upcoming Christmas holidays and by not knowing the exact time of symptom onset in infected patients over the weekend. Sick recruits may not have presented on days 3 and 4, while patients presenting in the infirmary on Sunday night or Monday may actually have been sick for up to 3 days prior. The number of new admissions in the second week declined sharply to zero after Tuesday (day 8). The epidemic curve illustrates the pattern of cases more in detail (fig. 2). It can help to identify the characteristics of a disease outbreak such as the source of the infection, modes of transmission or exposure and incubation period [16].
The fact that we obtained nasopharyngeal swabs for only 16 of 105 patients with ARI represents a scientific limitation of this study, since we cannot prove conclusively that all of the 105 patients were influenza A(H1N1)pdm09 positive. However, due to the fact that 100% of the samples we did take were positive, we are reasonably certain that the remaining patients would have tested positive as well. In any case, clinically, further testing would not have influenced treatment. The moderate cost effectiveness of RT-PCR testing, as well as the relationship between flu-like symptoms and positive RT-PCR results has been shown by Nickel et al., who described the first wave of A(H1N1)v in 2009 in northwestern Switzerland [17].
The return of the influenza A(H1N1)pdm09 strain as a “seasonal” strain had been expected by the Swiss Federal Office of Public Health in the influenza season 2010/11 [18]. “Sentinella”, a network of Swiss general practitioners throughout the country, is responsible for influenza monitoring, and detected some early cases in weeks 49 and 50 and a steep increase of cases in week 51 until its peak in week 52 of the year 2010 [19]. This supports our observations of a locally limited spread in the first week of the reported outbreak (week 50) and a wider distribution of the strain in the general population in the second week (51).
Still, several points merit consideration.
The considerable high attack rate of a new influenza strain represents a serious threat for the functioning of military troops kept under crowded conditions [13, 14]. This is particularly true for young recruits who do not benefit from cross protection of earlier flue episodes and/or vaccinations. The same observations have been made during the pandemic of 2009, where the majority of the infected and sick patients were young (age <64 years) and that older populations were less affected, either because of previous exposure to the virus or because of prior vaccination during small epidemics and sporadic events since 1974 [3, 20, 21].
The quick control of the epidemic after two days suggests that the duration of asymptomatic viral shedding is no longer than one day. Which is consistent with an estimated incubation time of A(H1N1)pdm09 of 1.5 to 3 days [22–26]. However, the low shedding time is not indicative of the population in general, and for the elderly or immunosupressed in particular. Upon return, recruits were either uninfected, had remained at home or presented at the infirmary before causing secondary infections.
The benefit of both the higher awareness of the need for protecting airways when coughing or sneezing and droplet isolation of identified cases have protected the care team as well as recruits in week two from secondary infection.
Considering the ease of transmission due to the close living quarters, measures must be taken if future epidemics are to be avoided in Swiss military barracks:
The 2009 monovalent Influenza A(H1N1)pdm09 vaccine was 95% effective in provoking sufficient antibody titres [27]. This method was found to be cost effective, even if the vaccine was only effective in 75% of cases [28]. In 2010/11, the A(H1N1)pdm09 had been added to the seasonal vaccine into a trivalent influenza vaccine [18]. However, in Switzerland, vaccination against “seasonal” influenza is mainly recommended for people >65 years, patients with comorbidities, pregnant women and healthcare workers and is not part of the current vaccination strategy of the Swiss Armed Forces [18]. Vaccination may also be difficult to implement, since it is not mandatory in Switzerland and there is a high percentage of vaccine skeptics, especially in regard to influenza vaccines. Better education about the subject might increase compliance.
An alternative to the vaccine is the prophylactic administration of antiviral drugs such as oseltamivir. A study performed in a military unit in Singapore lowered the infection rate from 6.4% to 0.6% after such an intervention [29]. The administration of oseltamivir was also found to be cost-effective [30]. However, the risk of oseltamivir resistance generation and its side effects should be considered [31, 32]. However, almost all oseltamivir resistance has been demonstrated among the immunosupressed, as this is the drug of choice for these patients at infection onset [33]. Additionally, the low complication rate of the disease does not support the implementation of antiviral prophylaxis in a training setting.
In case such methods are not implemented, hygienic measures must be strictly followed to contain the spread of the virus and perhaps to avoid an epidemic (table 1). Our experience showed that if these measures are implemented quickly and correctly, even non-vaccinated individuals are able to effectively protect themselves, as shown by 11 paramedics who took care of the patients over the course of two weeks. This had been confirmed by negative test results of the 8 most exposed paramedics. The 4 physicians in charge had been vaccinated at least 3–4 weeks prior to the influenza outbreak and did not show any ARI symptoms.
In conclusion, the A(H1N1)pdm09 virus is still present one year after the 2009 pandemic. In fact, it has become a ubiquitous virus in the region. Complications were uncommon and non-life threatening, as the population was young and previously healthy. However, the virus is still highly contagious, especially in confined living conditions such as those found in military barracks.
To avoid new epidemics, complications and long interruptions in the training schedule of new recruits, vaccination should be considered at the beginning of winter-boot-camps in early November. Similar actions should be discussed for comparable living conditions of other professional corps such as police, firefighters etc. In the event of new influenza outbreaks, hygienic and containment measures must be quickly and correctly implemented, in order to avoid an epidemic. This should also be considered in non-military settings such as school camps or retirement homes. Further studies on a larger scale should address the question of influenza prevention in confined living conditions.
1 Influenza-like illness in the United States and Mexico [Internet]. Geneva: World Health Organization; c2011 [updated 2009 Apr 24; cited 2011 Apr 6]. Available from: http://www.who.int/csr/don/2009_04_24/en/
2 In focus. H1N1 now in the post-pandemic period [Internet]. Geneva: World Health Organization; c2011 [updated 2010 Aug 10; cited 2011 Apr 6]. Available from: http://www.who.int/csr/disease/swineflu/en/index.html
3 Myers KP, Olsen CW, Gray GC. Cases of swine influenza in humans: a review of the literature. Clin Infect Dis.2007;44(8):1084–8.
4 Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science.2009;325(5937):197–201.
5 Trifonov V, Khiabanian H, Rabadan R. Geographic dependence, surveillance, and origins of the 2009 influenza A (H1N1) virus. N Engl J Med. 2009;361(2):115–9.
6 Updated CDC estimates of 2009 H1N1 influenza cases, hospitalizations and deaths in the United States, April 2009 – April 10, 2010 [Internet]. Atlanta: United States Centers for Disease Control and Prevention; c2010 [updated 2010 May 14; cited 2011 Apr 6]. Available from: http://www.cdc.gov/h1n1flu/estimates_2009_h1n1.htm
7 Interim guidance on infection control measures for 2009 h1n1 influenza in healthcare settings, including protection of healthcare personnel [Internet]. Atlanta: United States Centers for Disease Control and Prevention; c2010 [updated 2010 Jul 15; cited 2011 Apr 6]. Available from: http://www.cdc.gov/h1n1flu/guidelines_infection_control.htm
8 Yoo SJ, Moon SJ, Kuak EY, Yoo HM, Kim CK, Chey MJ et al. Frequent detection of pandemic (H1N1) 2009 virus in stools of hospitalized patients. J Clin Microbiol. 2010;48(6):2314–5.
9 Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360(25):2605–15.
10 Louie JK, Acosta M, Winter K, Jean C, Gavali S, Schechter R, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302(17):1896–902.
11 Gaydos JC, Top FH Jr, Hodder RA, Russell PK. Swine influenza a outbreak, Fort Dix, New Jersey, 1976. Emerg Infect Dis. 2006;12(1):23–8.
12 Heederik D. Micro-epidemiology of the healthy worker effect? Occup Environ Med. 2006;63(2):83.
13 Mayet A, Pommier de Santi V, Manet G, Nivoix P, Ligier C, Faure N, et al. A(H1N1) influenza surveillance in the French armed forces: adapting the surveillance systems to the pandemic setting. Med Mal Infect. 2010;40(7):404–11.
14 Witkop CT, Duffy MR, Macias EA, Gibbons TF, Escobar JD, Burwell KN, et al. Novel Influenza A (H1N1) outbreak at the U.S. Air Force Academy: epidemiology and viral shedding duration. Am J Prev Med. 2010;38(2):121–6.
15 Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009;361(20):1935–44.
16 Gail MH, Benichou J. Encyclopedia of epidemiologic methods. New York: Wiley; 2000.
17 Nickel CH, Stephan FP, Dangel M, Blume K, Gehrisch R, Dumoulin A, et al. First wave of the influenza A/H1N1v pandemic in Switzerland. Swiss Med Wkly. 2009;139(51-52):731–7.
18 Empfehlungen zur Impfung gegen die saisonale Grippe (2010–2011). Bull BAG. 2010;25:624–27.
19 Influenza activity and virology in Switzerland [Internet]. Geneva: HUG – Laboratoire central de virologie; c2011 [updated 2011 Apr 04; cited 2011 Jun 7]. Available from: http://virologie.hug-ge.ch/centres_reference/CNI_surveillance_en.html
20 Reichert T, Chowell G, Nishiura H, Christensen RA, McCullers JA. Does Glycosylation as a modifier of Original Antigenic Sin explain the case age distribution and unusual toxicity in pandemic novel H1N1 influenza? BMC Infect Dis. 2010;10:5.
21 McCullers JA, Van De Velde LA, Allison KJ, Branum KC, Webby RJ, Flynn PM. Recipients of vaccine against the 1976 “swine flu” have enhanced neutralization responses to the 2009 novel H1N1 influenza virus. Clin Infect Dis. 2010;50(11):1487–92.
22 Cao B, Li XW, Mao Y, Wang J, Lu HZ, Chen YS, et al. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N Engl J Med.2009;361(26):2507–17.
23 Bautista E, Chotpitayasunondh T, Gao Z, Harper SA, Shaw M, Uyeki TM, et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362(18):1708–19.
24 Lessler J, Reich NG, Cummings DA, Nair HP, Jordan HT, Thompson N. Outbreak of 2009 pandemic influenza A (H1N1) at a New York City school. N Engl J Med. 2009;361(27):2628–36.
25 Yang Y, Sugimoto JD, Halloran ME, Basta NE, Chao DL, Matrajt L et al. The transmissibility and control of pandemic influenza A (H1N1) virus. Science.2009;326(5953):729–33.
26 Cauchemez S, Donnelly CA, Reed C, Ghani AC, Fraser C, Kent CK, et al. Household transmission of 2009 pandemic influenza A (H1N1) virus in the United States. N Engl J Med.2009;361(27):2619–27.
27 Greenberg ME, Lai MH, Hartel GF, Wichems CH, Gittleson C, Bennet J, et al. Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med. 2009;361(25):2405–13.
28 Khazeni N, Hutton DW, Garber AM, Hupert N, Owens DK. Effectiveness and cost-effectiveness of vaccination against pandemic influenza (H1N1) 2009. Ann Intern Med. 2009;151(12):829–39.
29 Lee VJ, Yap J, Cook AR, Chen MI, Tay JK, Tan BH, et al. Oseltamivir ring prophylaxis for containment of 2009 H1N1 influenza outbreaks. N Engl J Med.2010;362(23):2166–74.
30 Khazeni N, Hutton DW, Garber AM, Owens DK. Effectiveness and cost-effectiveness of expanded antiviral prophylaxis and adjuvanted vaccination strategies for an influenza A (H5N1) pandemic. Ann Intern Med. 2009;151(12):840–53.
31 2009-2010 influenza season week 20 ending May 22, 2010 [Internet]. Atlanta: United States Centers for Disease Control and Prevention; c2010 [updated 2010 May 28; cited 2011 Apr 6]. Available from: http://www.cdc.gov/flu/weekly/weeklyarchives2009-2010/weekly20.htm
32 Oseltamivir-resistant pandemic (H1N1) 2009 influenza virus [Internet]. Stockholm: European Center for Disease Prevention and Control; c2005 - 2011 [updated 2009 Nov 18; cited 2011 Apr 6]. Available from: http://www.ecdc.europa.eu/en/activities/sciadvice/Lists/ECDC%20Reviews/ECDC_DispForm.aspx?List=512ff74f-77d4-4ad8-b6d6-bf0f23083f30&ID=683
33 Graitcer SB, Gubareva L, Kamimoto L, Doshi S, Vandermeer M, Louie J, et al. Characteristics of patients with oseltamivir-resistant pandemic (H1N1) 2009, United States. Emerg Infect Dis. 2011;17(2):255–7.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.