DOI: https://doi.org/10.4414/smw.2011.13297
A position paper on the situation in Switzerland
Teriparatide (Forsteo®) is a recombinant formulation of endogenous parathyroid hormone (PTH), containing a 34-amino-acid sequence which is identical to the N-terminal portion of the human hormone [rhPTH(1-34)]. It has been shown to increase bone mineral density, and to reduce both vertebral and non-vertebral fracture risk in a phase III pivotal randomised trial which lasted for a mean period of 19 months [17]. The tolerance and safety were considered as excellent. The bone structural changes under therapy are in favour of higher strength. Indeed, teriparatide treatment has been shown to increase trabecular thickness and connectivity, as assessed by micro computerised tomography of iliac crest bone biopsy specimens [47]. In addition, the efficacy of teriparatide in increasing BMD was also demonstrated in patients under glucocorticoid therapy [22, 23]. In a head-to-head trial, it was shown to be even superior to alendronate in terms of BMD changes. Though the trial, which lasted 3 years, was not designed to demonstrate a superiority for fracture risk reduction, it turned out that patients with GIOP receiving teriparatide had fewer vertebral fractures than those in the alendronate group.
In Switzerland teriparatide is approved for the treatment of postmenopausal women with osteoporosis and increased fracture risk, for the treatment of primary or hypogonadism-induced osteoporosis in men, with increased fracture risk, and for the treatment of adults with glucocorticoid-induced osteoporosis (GIOP) and increased fracture risk.
Currently, the reimbursement of teriparatide in Switzerland is limited to a second-line treatment of postmenopausal women and of men, with an x-ray examination detecting new osteoporotic vertebral fracture after a treatment with calcitonine, SERM (selective estrogen receptor modulator), denosumab or bisphosphonates for at least 6 months. In men and women with established GIOP, its use is restricted to cases with a lack of efficacy or intolerance to previous bisphosphonate therapy. The maximum accepted treatment duration is 24 months. Therefore, patients with multiple fracture and low bone mineral density, hence at a very high risk of subsequent fracture, can unfortunately not benefit from this bone anabolic agent as a first line therapy. In the surrounding countries, prescription policy includes women and men with 2 vertebral fractures (France), women and men at increased risk of fracture (Germany), or progression of osteoporosis with vertebral fracture despite antiresorptive therapy for 2 years (Austria).
In March 2011, a panel of Swiss internists, endocrinologists and rheumatologists specialised in the treatment of osteoporosis met to establish a consensus on the treatment indications of teriparatide in osteoporotic women and men. Prior to the meeting, a search of the literature was conducted using online databases. The search was limited to relevant literature on bone mineral density (BMD) and fracture history as predictors of future fractures (vertebral and non-vertebral), in publications addressing evidence on the need for anabolic treatment in patients with severe osteoporosis and in publications focusing on the question of sequential or combination therapy of teriparatide with bisphosphonates or other antiresorptive agents. The panel presented an overview of the current evidence supporting the use of teriparatide in the treatment of osteoporosis, independent of the limitation, and reached a consensus on how the current limitations should be adapted to meet this evidence and fit the needs of the suffering osteoporotic patients.
In a meta-analysis on prospective cohort studies published between 1985 and the end of 1994 with a baseline measurement of bone density in women and subsequent follow up for fractures, 11 separate study populations with about 90,000 person-year of observation time and over 2000 fractures were identified [1]. All measured anatomic sites had similar predictive abilities for decrease in bone mineral density except for measurement at the spine for predicting vertebral fractures and measurement at the hip for predicting hip fractures which displayed a higher increase of relative risk for each standard deviation decrease in BMD. Another meta-analysis (9,891 men, 29,082 women) showed that BMD measurement at the femoral neck with DXA was a strong predictor of hip fractures, both in men and women [2].

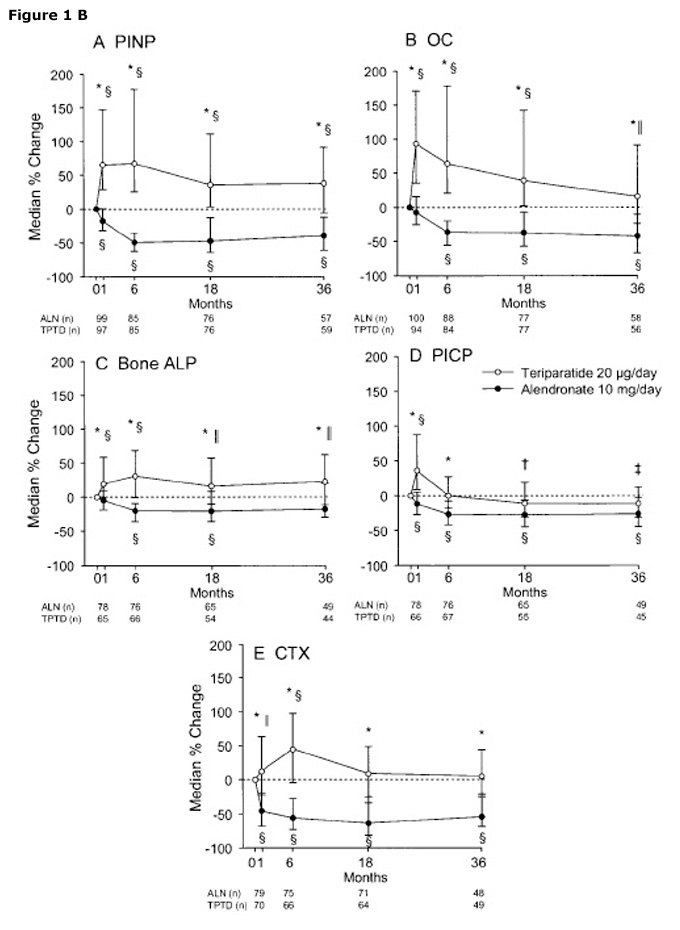
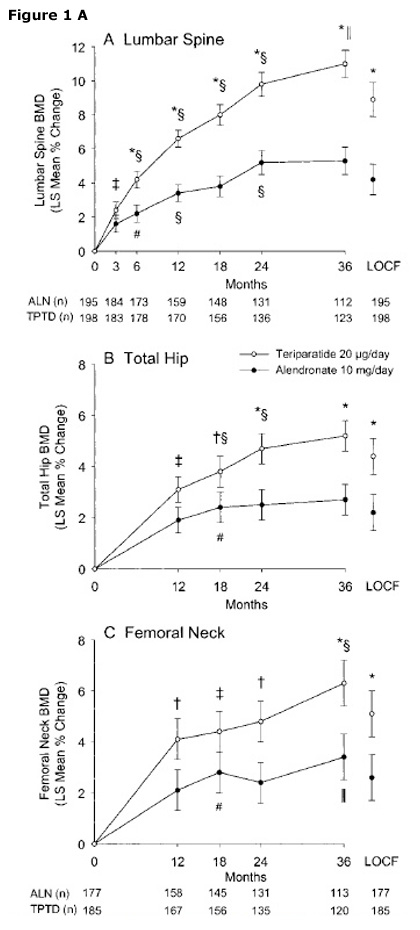
Figure 1
Effects of Teriparatide or alendonate on BMD (A) or bone turnover markers (B) in patients with GIOP. The figures are from Saag et al. [23] with the permission of the publisher.
For any given T-score the risk is higher with increasing age [3]. At any age low BMD similarly predicted fracture in men and women. The pattern of 10-year probability with age varied according to fracture type. A study conducted in Switzerland also found that fracture probability increased with age and decreasing BMD T-score [4]. Risk also increased with lower body mass index (BMI) and clinical risk factors used alone or in combination. An additional study determined remaining lifetime and absolute 10-year probabilities for osteoporotic fractures by gender, age, and BMD values [5]. The absolute 10-year probability of osteoporotic fracture increased with advancing age and decreasing BMD and was higher in women than in men. A longitudinal cohort study with a long term follow up of 15 years showed that the absolute risk of an incident morphometric vertebral fracture increased with decreasing BMD measured at the total hip, the femoral neck, and the lumbar spine [7].
Regarding the influence of prevalent fractures on the risk for future fractures, it seems that more than half of the women with five or more fractures at baseline developed new vertebral fractures, compared to only 3.8% of women without prior vertebral fractures. The presence of one or more vertebral fractures at baseline increased the risk of sustaining a new vertebral fracture by 5-fold during the initial year of the study compared with the incidence in subjects without prevalent vertebral fractures at baseline (RR 5.1; 95% CI 3.1–8.4; P <0.001) [9]. The risk of vertebral fracture was greatest among women with a prevalent vertebral fracture at baseline, irrespective of their BMD. The absolute risk of vertebral fractures was more than 50% among women with both a prevalent vertebral fracture and BMD in the osteoporotic range.
In addition, baseline vertebral fracture severity was shown to be predictive for new vertebral (and non-vertebral) fracture risk [10]. Baseline severity of prevalent vertebral fractures was the only predictor of non-vertebral fracture risk and remained a significant predictor even after adjustment for baseline characteristics, including baseline BMD [10]. Similar observations were reported by Roux et al. in 2007 [11].
A systematic review on the role of prevalent fracture for the risk of subsequent fracture [12], showed that women with pre-existing vertebral fractures (identified at baseline by vertebral morphometry) had approximately 4 times greater risk of subsequent vertebral fractures than those without prior fractures. Similar data were reported by Kanis et al. [13]. This risk increased with the number of prior vertebral fractures (table 1).
In conclusion, low bone mineral density and age are important and independent predictors of vertebral and non-vertebral fracture risk. BMD measured at any site predicts fracture risk at this site and for all typical osteoporotic fracture sites. Prevalent vertebral fractures are an independent short- and long-term predictor of future vertebral fractures. Vertebral fracture severity and/or vertebral fracture number are independent predictors of vertebral and non-vertebral fracture risk, with a clear fracture number dependent risk. History of any fracture during adulthood is a predictor of vertebral and non-vertebral fracture risk.
In conclusion, the risk of fragility fractures markedly increases with decreasing BMD, with increasing number of prevalent fractures and with the severity of vertebral fractures.
| Table 1: Risk ratio for fracture according to the number of prior morphometric vertebral fractures. | |||||||
| Number of prior fractures | |||||||
| Outcome fracture | Numberof fractures a | Sex | 1 | 2 | 3+ | ||
| Black [31] | Vertebral | 389 | F | 1.0 | 3.2 | 5.4 | 10.6 |
| Lunt [32] | Vertebral | 679 | M+F | 1.0 | 3.2 | 9.8 | 23.3 |
| Delmas [10] | Vertebral | 157 | F | 1.0 | 3.1 | 4.4 | 8.4 |
| Siris [33] | Vertebral | 217 | F | 1.0 | 3.1 | 5.5 | 8.6 |
| Puisto [34] | Hip | 182 | M+F | 1.0 | 1.2 | 1.5 | |
| Black [31] | Non-vertebral | 2433 | F | 1.0 | 1.6 | 1.9 | 2.2 |
| Black [31] | Hip | 464 | F | 1.0 | 2.0 | 2.2 | 2.8 |
| Delmas [10] | Non-vertebral | 31 | F | 1.0 | 1.3* | 1.8* | 1.4* |
| Black [31] | Forearm | 574 | F | 1.0 | 1.4 | 1.5 | 1.4* |
| a Number of incident fractures b Includes patients in Delmas et al. 2003 [10] * Not significant Adapted from Kanis et al. (Interpretation and use of FRAX in clinical practice, Osteoporos Int. 2011;22:2395–411. | |||||||
A series of well conducted trials with fracture incidence as endpoint, has demonstrated the antifracture efficacy of antiresorptive treatments such as bisphosphonates, SERMs, Denosumab and hormone replacement therapy. Similar fracture risk reductions were obtained with strontium ranelate [13–15]. The effects of a bone anabolic agent like teriparatide was evaluated in the Fracture Prevention Trial (FPT) which recruited postmenopausal women (mean age 69 ± 7 years) with osteoporosis, with a mean T-score of –2.6 SD and 2.3 ± 1.8 prevalent vertebral fractures. They were treated with teriparatide (20 or 40 µg) [17]. During a mean 19 months duration of the trial, 20 μg and 40 μg doses of teriparatide reduced the risk of one or more new vertebral fractures by 65 and 69 percent, respectively, as compared with placebo. The risk of two or more fractures was reduced by 77 and 86 percent, respectively, and the risk of at least one moderate or severe vertebral fracture was reduced by 90 and 78 percent, respectively. The risk for new non-vertebral fragility fractures was reduced as well by 54%. For comparison, the absolute risk reduction recorded in the various trials with antiresorptives and teriparatide over a similar time period, i.e., 19 months to 2 years are summarised in table 2. In contrast the highly significant reduction of non-vertebral fracture risk with teriparatide in 19 months of treatment, such a reduction does not reach a level of significance for most other agents after 2 years.
Eighteen months after discontinuation of teriparatide treatment, the reduction in fracture risk associated with previous treatment with teriparatide, 20 and 40 µg, was 41% (p = 0.004) and 45% (p = 0.001), respectively, vs. placebo [18]. In addition, Prince et al documented that 30 months after teriparatide discontinuation the risk for new non-vertebral fragility fractures was reduced significantly (Hazard Ratio 0.62, 95% CI 0.41–0.93; p = 0.022) [19]. Furthermore, it has been demonstrated that teriparatide-treated patients had a reduced incidence of back pain versus those receiving a comparator during an interval including the clinical trials plus 30 months of post-treatment follow-up [20].
Glucocorticoids impair the replication, differentiation and function of osteoblasts and induce the apoptosis of mature osteoblasts and osteocytes [21]. These effects lead to a suppression of bone formation which is a landmark of Glucocorticoid-induced osteoporosis (GIOP). In patients with GIOP, after 18 months of teriparatide treatment there was a significant increase in mean lumbar spine BMD and mean hip BMD vs. alendronate [22]. The incidence of new vertebral fractures was lower in the teriparatide group compared to alendronate. After 36 months, increases in BMD from baseline were significantly greater in the teriparatide group than in the alendronate group (p <0.001) (fig. 1 A B). The final analysis of the fracture incidence showed that fewer subjects had vertebral fractures in the teriparatide group than in the alendronate group (3 [1.7%] of 173 versus 13 [7.7%] of 169; p = 0.007), with most occurring during the first 18 months [22].
Teriparatide has a high efficacy in women with severe osteoporosis (based on T-score) and at least 2 vertebral fractures. Ancillary benefits are that the efficacy of teriparatide in fracture risk reduction persists after discontinuation (vertebral and non-vertebral fractures) and that, during and after treatment, back pain decreases. Furthermore, considering the pathogenesis of GIOP, teriparatide is a well suited treatment for GIOP as its effects on bone formation counteract those of the glucocorticoids.
The issue of cost-effectiveness has been addressed. The cost per QALY gained by a treatment with teriparatide in a population of 69-years-old with a T-score at the femoral neck of -3 and lower was in the base case estimated to be between EUR (euro) 20,000 and 64,000 for patients with a recent or a history of vertebral fracture respectively. With a recent vertebral fracture, the cost per Qaly for women >60 years old with a T-score at the femoral neck of –2.5 or lower was always lower than EUR 60,000 [46].
In conclusion, the bone anabolic effect of teriparatide is associated with a large reduction of vertebral and non-vertebral fracture risk. In a head-to-head comparative trial, teriparatide was shown to be superior to alendronate in increasing BMD.
| Table 2: Two-year estimated absolute risk reduction for vertebral and non-vertebral fracture. | |||||
| Agent | Author | 2-year a vertebral fracture risk in the placebo group (%) | 2-year a ARR (%) | 2 year non-a vertebral fracture risk in the placebo group (%) | 2 year a ARR (%) |
| Alendronate | Liberman 1995 [35] | 4.1 | 2.0 | 6.4 | 3.9 |
| Black 1996 [36] | 10 | 4.7 | 9.8 | 1.9 | |
| Cummings 1998 [37] | 1.9 | 0.8 | 6.7 | 0.8 | |
| Risedronate | Harris 1999 [38] | 10.9 | 3.4 | 5.6 | 2.1 |
| Reginster 2000 [39] | 19.3 | 7.2 | 10.7* | 3.4 | |
| Ibandronate | Chesnut 2004 [40] | 6.4 | 3.3 | 5.5 | –0.6 |
| Zoledronate | Black 2007 [41] | 7.7 | 5.5 | 7.1 | 1.8 |
| Lyles 2007 [42] | 2.5 | 1.4 | 7.1 | 2.0 | |
| Denosumab | Cummings 2009 [43] | 4.8 | 3.3 | 5.3 | 1.0 |
| Raloxifene | Ettinger 1999 [44] | 6.7* | 2.3 | 6.2 | 0.4 |
| Bazedoxifene | Silvermann 2008 [45] | 2.8 | 1.2 | 4.2 | 0.4 |
| Teriparatide | Neer 2001 [17] | 14.3 | 9.3 | 5.5 | 2.9 |
| a: 2-year cumulative fracture risk were derived from the 3-year data assuming a linear relationship with time, except for the value with *, for which results are available in the publication. ARR = Absolute fracture Risk Reduction | |||||
Regarding the option of combined or sequential therapy with antiresorbers and bone anabolics, no trial exists with fracture as primary end point, and we are thus referring to surrogate parameters such as BMD and/or bone turnover markers.
Investigating the combined use of alendronate and teriparatide in men with osteoporosis, Finkelstein et al. found that alendronate started 6 months before introducing PTH blunted the ability of the latter to increase bone mineral density at the lumbar spine and the femoral neck in men with osteoporosis [24]. In 2010, they showed that the same could be observed in osteoporotic women [25]. In another trial, the effect of teriparatide on BMD following the use of raloxifene or alendronate was tested [28]. Teriparatide treatment stimulated bone turnover in patients pretreated with raloxifene or alendronate. However, prior treatment with alendronate retarded increases in BMD, whereas raloxifene allowed for the expected teriparatide-induced BMD increases comparable with those previously reported in treatment-naïve patients. On the other hand, investigations on the effect of prior antiresorptive therapy on the BMD response to teriparatide showed that teriparatide treatment for 24 months was associated with a significant increase in spine BMD in patients with and without previous antiresorptive use [29]. In these trials, prior antiresorptive treatment modestly blunted the BMD response to teriparatide, especially during the first 6 months. At 12 months, the BMD of all groups had reached about the same level. For hip BMD, there was a tendency to lose some BMD in the first year of treatment, independently of prior antiresorptive therapy [30]. After this period, the effect of teriparatide on BMD was the same with our without previous antiresorptive use.
In contrast, Cosman et al. showed that the concomitant administration of intravenous zoledronic acid (5 mg) and daily subcutaneous teriparatide (20 µg) did not blunt the effect of the latter on spine BMD but resulted in a significantly greater increment in total hip BMD than teriparatide alone [26]. It should be noted that both therapies were initiated concomitantly. The combination of teriparatide and raloxifene increased bone formation to a similar degree as teriparatide alone [27]. However, the increase in bone resorption was significantly less and total hip BMD significantly increased for combination therapy compared with teriparatide alone.
The use of alendronate after parathyroid hormone (rhPTH 1-84) was investigated by Black et al. [31]. They found that after one year of parathyroid hormone, gains in BMD seemed to be maintained or increased with alendronate. But the gains were lost if parathyroid hormone was not followed by an antiresorptive agent.
Altogether, it appears that the response to teriparatide is impaired if the bone anabolic agent is introduced in patients treated with a bisphosphonate for several months. Thus, in regard to sequential therapy, pre-treatment with a bisphosphonate results in some delay of BMD response to teriparatide, followed by a catch-up. The combination of teriparatide with SERM’s or hormone replacement therapy appears to not impair the response to the parathyroid hormone. Finally, treatment with an antiresorptive drug after cessation of treatment with parathyroid hormone further improves BMD and could represent a suitable option.
In conclusion, the administration of teriparatide to patients on bisphosphonates appeared to be associated with a slightly blunted or retarded BMD response.
Based on the literature summarised above, the expert panel reached the consensus that the current limitation for prescription of teriparatide should be adapted, to allow the patients to benefit from the advantages of PTH given as a first line therapy in specifically selected patients characterised by a very high risk of fracture, thus deserving the deposition of new bone of optimal quality instead of the maintenance of bone mass as it is achieved with antiresorbtive drugs.
The following adaptations for postmenopausal women, for men and for patients with idiopathic or glucocorticoid-induced osteoporosis are recommended.
Postmenopausal women: Teriparatide should be indicated and prescribed as first-line treatment for postmenopausal women with two or more evident (Genant grade 2 or 3) vertebral fractures and a T-score ≤ -2.5 SD as well as in postmenopausal women with one evident (Genant grade 2 or 3) vertebral fracture and a T-score ≤ -3.5 SD.
Men:Teriparatide should be indicated and reimbursed as first-line treatment in men with two or more evident (Genant grade 2 or 3) vertebral fractures and a T-score ≤ -2.5 SD as well as in men with one evident (Genant grade 2 or 3) vertebral fracture and a T-score ≤ -3.5 SD.
GIOP:Teriparatide should be indicated and reimbursed as first-line treatment in patients with Glucocorticoid-induced osteoporosis with one evident (Genant grade 2 or 3) vertebral fracture or a T-score ≤ -2.5 SD.
This adaptation will help to select the most severely affected cases of osteoporosis for first line treatment with teriparatide. Therefore, it is unlikely that the rate of prescriptions will rise markedly.
Acknowledgments: Dr. Therese Schwender is acknowledged for editorial assistance. We thank Ms Katy Giroux for her expert secretarial help.
1 Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–9.
2 Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20:1185–94.
3 Kanis JA, Johnell O, Oden A, Dawson A, De Laet C, Jonsson B. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001;12:989–95.
4 Lippuner K, Johansson H, Kanis JA, Rizzoli R. FRAX assessment of osteoporotic fracture probability in Switzerland. Osteoporos Int. 2010;21:381–9.
5 Lippuner K, Johansson H, Kanis JA, Rizzoli R. Remaining lifetime and absolute 10-year probabilities of osteoporotic fracture in Swiss men and women. Osteoporos Int. 2009;20:1131–40.
6 Cummings SR, Bates D, Black DM. Clinical use of bone densitometry: scientific review. JAMA. 2002;288:1889–97.
7 Cauley JA, Hochberg MC, Lui LY, Palermo L, Ensrud KE, Hillier TA, et al. Long-term risk of incident vertebral fractures. JAMA. 2007;298:2761–7.
8 Nevitt MC, Ross PD, Palermo L, Musliner T, Genant HK, Thompson DE. Association of prevalent vertebral fractures, bone density, and alendronate treatment with incident vertebral fractures: effect of number and spinal location of fractures. The Fracture Intervention Trial Research Group. Bone. 1999;25:613–9.
9 Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320–3.
10 Delmas PD, Genant HK, Crans GG, Stock JL, Wong M, Siris E, Adachi JD. Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: results from the MORE trial. Bone. 2003;33:522–32.
11 Roux C, Fechtenbaum J, Kolta S, Briot K, Girard M. Mild prevalent and incident vertebral fractures are risk factors for new fractures. Osteoporos Int. 2007;18:1617–24.
12 Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–39.
13 Kanis JA, Burlet N, Cooper C, Delmas PD, Reginster JY, Borgstrom F, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2008;19(4):399–428.
14 Rizzoli R, Bruyere O, Cannata-Andia JB, Devogelaer JP, Lyritis G, Ringe JD, et al. Management of osteoporosis in the elderly. Curr Med Res Opin. 2009;25(10):2373–87.
15 Rizzoli R. Bisphosphonates for post-menopausal osteoporosis: are they all the same? QJM. 2011;104(4):281–300.
16 Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35(2):375–82.
17 Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41.
18 Lindsay R, Scheele WH, Neer R, Pohl G, Adami S, Mautalen C, et al. Sustained vertebral fracture risk reduction after withdrawal of teriparatide in postmenopausal women with osteoporosis. Arch Intern Med. 2004;164(18):2024–30.
19 Prince R, Sipos A, Hossain A, Syversen U, Ish-Shalom S, Marcinowska E, et al. Sustained nonvertebral fragility fracture risk reduction after discontinuation of teriparatide treatment. J Bone Miner Res. 2005;20:1507–13.
20 Nevitt MC, Chen P, Kiel DP, Reginster JY, Dore RK, Zanchetta JR, et al. Reduction in the risk of developing back pain persists at least 30 months after discontinuation of teriparatide treatment: a meta-analysis. Osteoporos Int. 2006;17:1630–7.
21 Compston J, et al. Management of glucocorticoid-induced osteoporosis. Nat Rev Rheumatol. 2010;6:82–8.
22 Saag KG, Shane E, Boonen S, Marín F, Donley DW, Taylor KA, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007;357:2028–39.
23 Saag KG, Zanchetta JR, Devogelaer JP, Adler RA, Eastell R, See K, et al. Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum. 2009;60:3346–55.
24 Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. New Engl J Med. 2003;349:1216-26.
25 Finkelstein JS, Wyland JJ, Lee H, Neer RM. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab. 2010;95:1838–45.
26 Cosman F, Eriksen EF, Recknor C, Miller PD, Guanabens N, Kasperk C, et al. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1-34)] in postmenopausal osteoporosis. J Bone Miner Res 2011;26(3):503-11.
27 Deal C, Omizo M, Schwartz EN, Eriksen EF, Cantor P, Wang J, et al. Combination teriparatide and raloxifene therapy for postmenopausal osteoporosis: results from a 6-month double-blind placebo-controlled trial. J Bone Miner Res. 2005;20:1905–11.
28 Ettinger B, San Martin J, Crans G, Pavo I. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res. 2004;19:745–51.
29 Obermayer-Pietsch BM, Marin F, McCloskey EV, Hadji P, Farrerons J, Boonen S, et al.; EUROFORS Investigators. Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J Bone Miner Res. 2008;23:1591–600.
30 Boonen S, Marin F, Obermayer-Pietsch B, Simões ME, Barker C, Glass EV, et al.; EUROFORS Investigators. Effects of previous antiresorptive therapy on the bone mineral density response to two years of teriparatide treatment in postmenopausal women with osteoporosis.J Clin Endocrinol Metab. 2008;93:852–60.
31 Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, et al.; PaTH Study Investigators. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med. 2005;353:555–65.
32 Lunt M, O’Neill TW, Felsenberg D, Reeve J, Kanis JA, Cooper C, et al. Characteristics of a prevalent vertebral deformity predict subsequent vertebral fracture: results from the European Prospective Osteoporosis Study (EPOS). Bone. 2003;33(4):505–13.
33 Siris ES, Genant HK, Laster AJ, Chen P, Misurski DA, Krege JH. Enhanced prediction of fracture risk combining vertebral fracture status and BMD. Osteoporos Int. 2007;18(6):761–70.
34 Puisto V, Heliovaara M, Impivaara O, Jalanko T, Kroger H, Knekt P, et al. Severity of vertebral fracture and risk of hip fracture: a nested case-control study. Osteoporos Int. 2011;22(1):63–8.
35 Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333(22):1437–43.
36 Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348(9041):1535–41.
37 Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280(24):2077–82.
38 Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282(14):1344–52.
39 Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11(1):83–91.
40 Chesnut III CH, Skag A, Christiansen C, Recker R, Stakkestad JA, Hoiseth A, et al; Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19(8):1241-9.
41 Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–22.
42 Lyles KW, Colon-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799–809.
43 Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–65.
44 Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282(7):637–45.
45 Silverman S, Christiansen C, Genant H, et al. Efficacy of Bazedoxifene in Reducing New Vertebral Fracture Risk in Postmenopausal Women With Osteoporosis: Results From a 3-Year, Randomized, Placebo-, and Active-Controlled Clinical Trial J Bone Miner Res. 2008;23:1923–34.
46 Lundkvist J, Johnell O, Cooper C, Sykes D. Economic evaluation of Parathyroid Hormone (PTH) in the treatment of Osteoporosis in Postmenopausal Women. Osteoporosis International, 2006;17(2):201–11.
47 Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF. Recombinant human parathyroid hormone (1-34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res. 2003;18(11):1932–41.
Funding / potential competing interests: This paper is based on discussions held during a meeting sponsored by the Eli Lilly company. None of the authors have received any financial support or paid fee for the writing of this review.