Figure 1
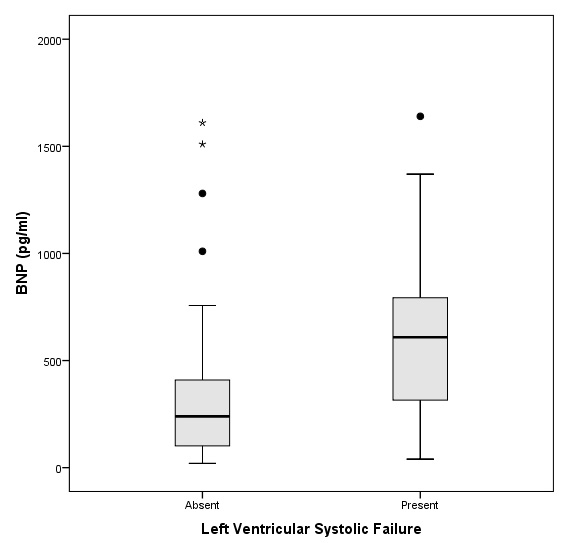
Box-plot B-type Natriuretic Peptide (BNP) values with diagnosis of Left Ventricular Systolic and Diastolic Dysfunction in 57 adults with acute exacerbation of COPD.
DOI: https://doi.org/10.4414/smw.2011.13298
Chronic obstructive pulmonary disease (COPD) is an important cause of morbidity and mortality. It is now considered as the fifth leading cause of death worldwide and could become the third leading cause for both men and women around 2020 [1]. Up to 21 to 30% of COPD patients simultaneously suffer from often-unrecognised chronic heart failure, probably because smoking and chronic inflammation are common risk factors for both conditions [2–6]. The correct identification of patients with co-existing diseases is critical as specific treatment of heart failure and its underlying cause may have a positive impact on mortality, morbidity, and hospital readmission rate [7].
Recognising heart failure in COPD patients, particularly in acute settings, remains a clinical challenge. Symptoms and physical signs may be very similar and chest radiography does not contribute to the diagnosis, as well the hyperinflation and right ventricular enlargement present in COPD can mask the classic radiologic signs of chronic heart failure [8–10]. Cardiac biomarkers, particularly Brain Natriuretic Peptide (BNP) and N-terminal prohormone of Brain Natriuretic Peptide (NT-proBNP), have been increasingly used and validated to help distinguish congestive heart failure from pulmonary disease in acute care settings [11–15]. However, the performance of cardiac biomarkers for the detection of co-existing heart failure in COPD patients is less well known. Two studies have assessed the diagnostic performances of BNP or NT-pro-BNP among patients with a history or asthma or COPD who presented with dyspnea in tertiary hospitals [16, 17]. A third study focused on patients with severe exacerbation of COPD admitted in the intensive care unit and requiring non-invasive or conventional ventilation [18]. These three studies showed encouraging results, yet among a selected population of COPD patients admitted in tertiary settings. Whether these results can be extrapolated to general secondary setting is unknown.
In this study, our objective was to assess the performance of BNP in detecting left ventricular systolic dysfunction in patients with no previous history of heart failure admitted for acute exacerbation of COPD in a regional second-care hospital.
We conducted a retrospective cohort analysis of medical records of individual patients hospitalised at the regional hospital of Morges, Switzerland, a 180-bed regional hospital with an admission rate in medical wards of 2,500 patients per year. We included all adult patients (>18 years old) hospitalised between January 2003 and May 2009 with the final diagnosis of acute exacerbation of COPD, who had undergone BNP dosage at the admission followed by an echocardiocardiography. Patients with known history of heart failure were excluded. BNP dosage was routinely required for all patients with acute dyspnea in Morges regional hospital, based on local guidelines. All patients were included in institutional registries, whose creation was approved by local ethics committee, as was the study protocol.
The main outcome variable was left systolic ventricular dysfunction, defined as a left ventricular ejection fraction inferior to 50% assessed by echocardiography. The test was performed by one of the two cardiologists affiliated at the hospital, using an M-mode, two-dimensional and color Doppler imaging with commercial instruments operating at 2.0 to 3.5 MHZ (Vivid, GE, Health Care Systems).
The main explanatory variable was BNP level at admission, determined by fluorescent polarisation immunoassays (Triage-BNP, Biosite Inc., San Diego, CA, USA). Other co-variables extracted from medical record included age, sex, history of coronary heart disease, hypertension, hypercholesterolemia or diabetes, electrocardiogram abnormalities (defined by the presence of Q wave, ST depression, ST elevation or T wave inversion), blood gas analyses, and chest radiography. The latter was interpreted by independent radiologists who were blinded to the BNP levels Signs of heart dysfunction (defined by cardiomegaly, pleural effusions or vascular redistribution) and radiologic signs of COPD (defined by hyperinflation and flattened diaphragms) were recorded.
We first examined the distribution of patients’ characteristics and BNP levels, which were analysed first as a continuous variable and then divided into three categories: <100 pg/ml, 100–499 pg/ml and ≥500 pg/ml [7]. In univariate analysis, we compared the characteristics and BNP levels of patients presenting a left ventricular systolic dysfunction (i.e., the primary outcome) and patients with normal ventricular function. We used the Mann- Whitney test for continuous variables and Fisher’s exact test for categorical variables. In multivariate analysis, we built two multiple logistic regression models to identify patients’ characteristics that were independently associated with left ventricular systolic dysfunction. We used a forward modeling procedure, guided by the analyst, where only statistically significant variables were included in the model.
Finally we obtained Receiver Operating Characteristic (ROC) curves for the BNP levels as diagnostic test for left ventricular systolic and diastolic dysfunction. We estimated the areas under the ROC curves and their 95% confidences intervals, and computed the sensitivity, specificity, positive and negative predictive value for the two predefined thresholds of BNP level (100 and 500 pg/ml). Data were analysed using SPSS software version 18.0 (SPSS Inc., Chicago, Illinois, USA).
Half of the patients were men with a mean age of 75 years, and many patients had one or more comorbidities, such as diabetes or hypertension (table 1).

Figure 1
Box-plot B-type Natriuretic Peptide (BNP) values with diagnosis of Left Ventricular Systolic and Diastolic Dysfunction in 57 adults with acute exacerbation of COPD.
The left ventricular ejection fraction was 56.5% on average and 13 patients had values below 50%. The mean BNP was 420 pg/ml, with about half of the patients having a value between 100 and 499, and 30% with a BNP value of 500 or more (table 1). There was a statistically significant difference of mean BNP values between patients with or without left ventricular systolic dysfunction (mean 689 pg/ml vs. 340 pg/ml, p = 0.007). The distributions of BNP values are shown in figure 1.
In a univariate analysis cardiovascular comorbid conditions were associated with the frequency of left ventricular systolic dysfunction, but these differences were not statistically significant (table 2). Patients with systolic dysfunction were slightly older (mean 78.2 years) than those with normal ventricular function (mean 74.7 years), yet the difference was not significant (p = 0.180). There was no notable association with hypercapnia or with other signs of heart failure or COPD on chest X-ray.
In a multivariate logistic regression analysis, only a BNP value ≥500 (odds ratio 8.5, 95% confidence interval 1.9 to 38.2, p = 0.005) and history of coronary heart disease (odds ratio 5.9, 95% confidence interval 1.01 to 34.7, p = 0.048) remained as independent and mutually adjusted predictors of left ventricular systolic dysfunction.
We obtained a Receiver Operating Characteristic curve for the BNP levels as diagnostic test for left ventricular systolic dysfunction (fig. 2, A). The area under the ROC curve was 0.75 (95% confidence interval 0.59 to 0.90). The diagnostic performances of two thresholds of BNP values are shown in table 3. In particular, a BNP lower than 100 pg/ml yielded a sensitivity of 92% that resulted in a negative predictive value of 91%. Conversely a BNP higher than 500 yielded a sensitivity of 80% that resulted in a positive predictive value of only 47%.
| Table 1: Patients characteristics (n = 57). | |
| Male | 29 (50.9%) |
| Age (years) – mean (SD) | 75.5 (7.9) |
| Hypertension | 37 (68.5%) |
| Diabetes | 15 (27.3%) |
| Hypercholesterolemia | 18 (36.7%) |
| History of coronary heart disease | 9 (15.8%) |
| Abnormality on ECG* | 22 (38.6%) |
| Radiologic sign of heart failure | 26 (47.3%) |
| Radiologic sign of COPD | 21 (36.8%) |
| Hypoxemia on blood gas analysis† | 53 (93.0%) |
| Hypercapnia on blood gas analysis‡ | 27 (48.2%) |
| Atrial fibrillation | 16 (28.1%) |
| BNP (pg/ml) – mean (SD) | 419.7 (426.2) |
| BNP | |
| <100 pg/ml | 11 (19.3%) |
| 100–499 pg/ml | 29 (50.9%) |
| ≥500 pg/ml | 17 (29.8%) |
| Left ventricular ejection fraction – mean (SD) | 56.7 (14.0) |
| Left ventricular systolic failure | 13 (22.8%) |
| Left ventricular diastolic failure | 15 (26.3%) |
| Pulmonary pressure | |
| ≤35 mm Hg | 13 (28.3%) |
| 35–60 mm Hg | 26 (56.5%) |
| >60 mm Hg | 7 (15.2%) |
| * Presence of Q wave, ST depression, ST elevation or T wave inversed † PaO2 <80 mm Hg (<11 kPa) ‡ PCO2 >45 mm Hg (>5.98 kPa) | |
| Table 2: Univariate associations between patients’ characteristics and left ventricular systolic and diastolic failure. | ||
| Patients characteristics | Left ventricular systolic failure N (row %) | p-value |
| Sex | 0.21 | |
| Male | 9 (31.0) | |
| Female | 4 (14.3) | |
| Hypertension | 1.00 | |
| Yes | 8 (21.6) | |
| No | 3 (17.6) | |
| Diabetes | 1.00 | |
| Yes | 3 (20.0) | |
| No | 9 (22.5) | |
| Hypercholesterolemia | 0.90 | |
| Yes | 7 (38.9) | |
| No | 5 (16.1) | |
| History of coronary heart disease | 0.19 | |
| Yes | 4 (44.4) | |
| No | 9 (18.9) | |
| Abnormality on ECG* | 0.22 | |
| Yes | 7 (31.8) | |
| No | 6 (17.1) | |
| Radiologic sign of heart failure | 0.75 | |
| Yes | 7 (26.9) | |
| No | 6 (20.7) | |
| Radiologic sign of COPD | 1.00 | |
| Yes | 5 (23.8) | |
| No | 8 (23.5) | |
| Hypoxemia on blood gas analysis† | 0.22 | |
| Yes | 11 (20.8) | |
| No | 2 (50.0) | |
| Hypercapnia on blood gas analysis‡ | 0.53 | |
| Yes | 5 (18.5) | |
| No | 8 (27.6) | |
| Atrial fibrillation | 0.10 | |
| Yes | 6 (37.5) | |
| No | 7 (17.1) | |
| BNP | 0.017 | |
| <100 pg/ml | 1 (9.1) | |
| 100–499 pg/ml | 4 (13.8) | |
| ≥500 pg/ml | 8 (47.1) | |
| Table 3: Diagnostic performances of 2 different cut-off values of B-type natriuretic peptide (BNP) for the diagnosis of left ventricular systolic and diastolic failure (proportion and 95% confidence interval). | |||||
| BNP cut-off | Sensitivity | Specificity | Positive predictive value | Negative predictive value | |
| LV Systolic failure | 500 pg/ml | 0.62 (0.36–0.82) | 0.80 (0.66–0.89) | 0.47 (0.26–0.69) | 0.88 (0.74–0.95) |
| 100 pg/ml | 0.92 (0.67–0.99) | 0.23 (0.13–0.37) | 0.26 (0.16–0.40) | 0.91 (0.62–0.98) | |
| LV Diastolic failure | 500 pg/ml | 0.47 (0.25–0.70) | 0.76 (0.62–0.87) | 0.41 (0.22–0.64) | 0.80 (0.65–0.90) |
| 100 pg/ml | 0.93 (0.70–0.99) | 0.24 (0.14–0.39) | 0.30 (0.19–0.45) | 0.91 (0.62–0.98) | |
In patients admitted to a regional secondary-care hospital for acute exacerbation of COPD and with no history of heart failure, BNP measurement showed interesting diagnostic performances in identifying co-existent left ventricular dysfunction. For the detection of systolic dysfunction, a BNP level inferior to 100 pg/ml yielded a sensitivity of 92% and a negative predictive value of 91%, whereas BNP higher than 500 yielded a sensitivity of 80% and a positive predictive value of 47%. In multivariate regression analysis only a BNP ≥500 and a history of coronary heart disease remained as independent predictors of left ventricular systolic function dysfunction.
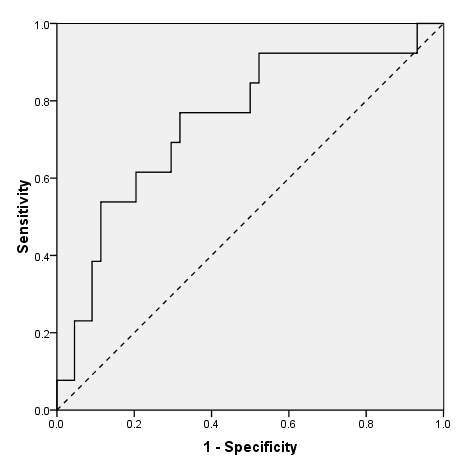
Figure 2
Receiver Operating Characteristics (ROC) curves of B-type Natriuretic Peptide (BNP) for the diagnosis of Left Ventricular Systolic Dysfunction in 57 adults with acute exacerbation of COPD. AUC = 0.75, p = 0.007.
In a subgroup study from the Breathing Not Properly study, McCullough & al. assessed the diagnostic performance of BNP among 417 patients with a history COPD or asthma presenting with acute dyspnea [16]. For a cut-off of 100 pg/ml, they found a sensitivity of 93.1% corresponding to negative predictive value of 97.7%, and a specificity of 77.3%, corresponding to a positive predictive value of 51.9%. In our study, we found a similar sensitivity, but a much lower specificity, namely 23% for the same cut-off value of BNP. The Breathing Not Properly study was designed to distinguish heart failure from pulmonary disease, and thus included patients who presented with acute dyspnea at admission. Their final diagnosis was not always acute exacerbation of COPD, whereas we retrospectively included patients based on this diagnosis. Thus the likelihood of co-existing heart failure in our sample is lower, which may explain the lower specificity and positive predictive value.
The same remarks can be made for another post-hoc analysis of a single-center prospective study, which included 164 dyspneic COPD patients, without previous history of heart failure [17]. For an almost equivalent cut-off of NT-pro-BNP (300 pg/ml), Tung & al. found a sensitivity of 90%, and a specificity of 66%. We assessed BNP instead of NT-pro-BNP, however both markers seem to have very similar diagnostic performance to rule out heart failure in the acute clinical setting of dyspnea [11].
Finally, a third study focused on 148 patients with severe acute exacerbation of COPD admitted in the intensive care unit and requiring non-invasive or conventional ventilation [18]. A NT-pro-BNP cut-off of 1000 pg/ml was an accurate marker to rule out left-heart involvement with a sensitivity of 94%, a negative predictive value of 94%. Although these patients differed from our sample, as they tended to be 10 years younger on average and presented more severe symptoms, the result of this study is still comparable to ours.
Our results, along with previous work, confirm that cardiac biomarkers such as BNP or NT-pro-BNP, that are readily available in most hospital settings, can be useful tools to identify co-existing heart failure in acute exacerbation of COPD patients. Does BNP dosage perform better than clinical appraisal? We did not investigate this question, but another important finding of the study of Tung & al. discussed above, is that high clinical suspicion of heart failure detected only 23% of patients with new onset heart failure among COPD patients, whereas 82% had elevated NT-pro-BNP levels [17].
The rationale for trying to identify co-existing heart failure in acute exacerbation of COPD patients is that it can translate to specific treatment of heart failure, such as diuretics, ACE inhibitors or beta-blockers (after stabilisation) [7]. It can also stimulate specific investigations to find the etiology of heart failure, such as ischemia. Our results suggest that echocardiography could be recommended when BNP level is superior to 100 pg/ml, while an inferior value would have an acceptable, albeit imperfect, negative predictive value. However, the clinical utility and usefulness of such a strategy, in terms of mortality, morbidity, or hospital readmission rate remains unknown. NT-pro-BNP guided management of chronic heart failure has somehow proven disappointing on clinical improvement [19]. COPD patients with unknown co-existent heart failure might better benefit from such BNP guided strategies, since clinical appraisal is less reliable that in non-COPD patients.
This study has several limitations inherent to its retrospective design. First it may suffer from selection bias. The identification of patients based on final diagnosis in their medical record may have missed some eligible patients. Furthermore, although BNP dosage was routinely required in patients with acute dyspnea in our regional hospital (based on local guidelines), it was sometimes omitted, which excluded several patients from the present study. Another limitation is potential information bias, since left ventricular function was assessed by cardiologists who were not blinded to the BNP value. Finally the small sample size of our study limits the precision of our estimates. Yet we did not lack the statistical power to detect positive association between BNP levels and left ventricular failure. We also found an independent association of a history of coronary heart failure and LV systolic dysfunction.
The main strength of our study is that we assessed the diagnostic performance of BNP level among patients with acute exacerbation of COPD admitted in a regional secondary-care hospital, while the few previous studies were realised in tertiary settings [16, 17] or even in intensive care settings [18]. Thus our results may be more representative of ‘real-life’ daily practice secondary-care settings and more generalisable to patients with less severe exacerbation (since patients with more severe symptoms were either redirected or transferred to tertiary centers).
Our study confirms the utility of BNP dosage in detecting left ventricular dysfunction in patients with acute exacerbation of COPD, without history of heart failure. A large randomised controlled trial is needed to assess whether management of acute exacerbation of COPD guided by BNP (or NT-pro-BNP) targets would improve the identification and appropriate treatment of co-existing heart failure. This may hopefully impact on clinically relevant outcomes such as mortality, morbidity, readmissions, or the length of hospital stay.
Acknowledgements: The authors thank Dr Fivaz-Arbanne who performed part of the echocardiographies of the patients included in this study.
1 Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349:1498–504.
2 Rutten FH, Cramer MJ, Lammers JW, Grobbee DE, Hoes AW. Heart failure and chronic obstructive pulmonary disease: An ignored combination? Eur J Heart Fail. 2006;8:706–11.
3 Rutten FH, Moons KG, Cramer MJ, et al. Recognising heart failure in elderly patients with stable chronic obstructive pulmonary disease in primary care: cross sectional diagnostic study. BMJ. 2005;331:1379.
4 Havranek EP, Masoudi FA, Westfall KA, Wolfe P, Ordin DL, Krumholz HM. Spectrum of heart failure in older patients: results from the National Heart Failure project. Am Heart J. 2002;143:412–7.
5 Curkendall SM, DeLuise C, Jones JK, et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol. 2006;16:63–70.
6 Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increase preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42:1226–33.
7 Le Jemtel TH, Padeletti M, Jelic S. Diagnostic and therapeutic challenges in patients with coexistent chronic obstructive pulmonary disease and chronic heart failure. J Am Coll Cardiol. 2007;49:171–80.
8 Rutten FH, Cramer MJ, Zuithoff NP, et al. Comparison of B-type natriuretic peptide assays for identifying heart failure in stable elderly patients with a clinical diagnosis of chronic obstructive pulmonary disease. Eur J Heart Fail. 2007;9:651–9.
9 Render ML, Weinstein AS, Blaustein AS. Left ventricular dysfunction in deteriorating patients with chronic obstructive pulmonary disease. Chest. 1995;107;162–8.
10 Rutten FH, Cramer MJ, Grobbee DE, et al. Unrecognized heart failure in elderly patients with stable chronic obstructive pulmonary disease. Eur Heart J. 2005;26:1887–94.
11 Worster A, Balion CM, Hill SA, et al. Diagnostic accuracy of BNP and NT-proBNP in patients presenting to acute care settings with dyspnea: a systematic review. Clin Biochem. 2008;41:250–9.
12 Mueller C, Laule-Kilian K, Schindler C, et al. Cost-effectiveness of B-type natriuretic peptides testing in patients with acute dyspnea. Arch Intern Med. 2006;166:1081–7.
13 Mueller C, Laule-Kilian K, Frana B, et al. Use of B-type natriuretic peptides in the management of acute dyspnea in patients with pulmonary disease. Am Heart J. 2006;151:471–7.
14 Abroug F, Ouanes-Bebes L. Detection of acute heart failure in chronic obstructive pulmonary disease patients: role of B-type natriuretic peptide. Current Opin Crit Care. 2008;14:340–7.
15 Mueller C, Breidthardt T, Laule-Kilian K, Christ M, Perruchoud AP. The integration of BNP and NT-proBNP into clinical medicine. Swiss Med Wkly. 2007;137(1-2):4–12.
16 McCullough PA, Duc P, Omland T, et al. Breathing Not Properly Multinational Study Investigators. Uncovering heart failure in patients with a history of pulmonary disease: rationale for the early use of B-type natriuretic peptide in the emergency department. Acad Emerg Med. 2003;10:198–204.
17 Tung RH, Camargo CA Jr, Krauser D, Anwaruddin S, Baggish A, Chen A, et al. Amino-terminal pro-brain natriuretic peptide for the diagnosis of acute heart failure in patients with previous obstructive airway disease. Ann Emerg Med. 2006;48:66–74.
18 Abroug F, Ouanes-Besbes L, Nciri N, et al. Association of left-heart dysfunction with severe exacerbation of chronic obstructive pulmonary disease: diagnostic performance of cardiac biomarkers. Am J Respir Crit Care Med. 2006;174:990–6.
19 Eurlings LW, van Pol PE, Kok WE, et al. Management of chronic heart failure guided by individual N-terminal pro-B-type natriuretic peptide targets: results of the PRIMA (Can PRo-brain-natriuretic peptide guided therapy of chronic heart failure IMprove heart fAilure morbidity and mortality?) study. J Am Coll Cardiol. 2010;56:2090–100.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.