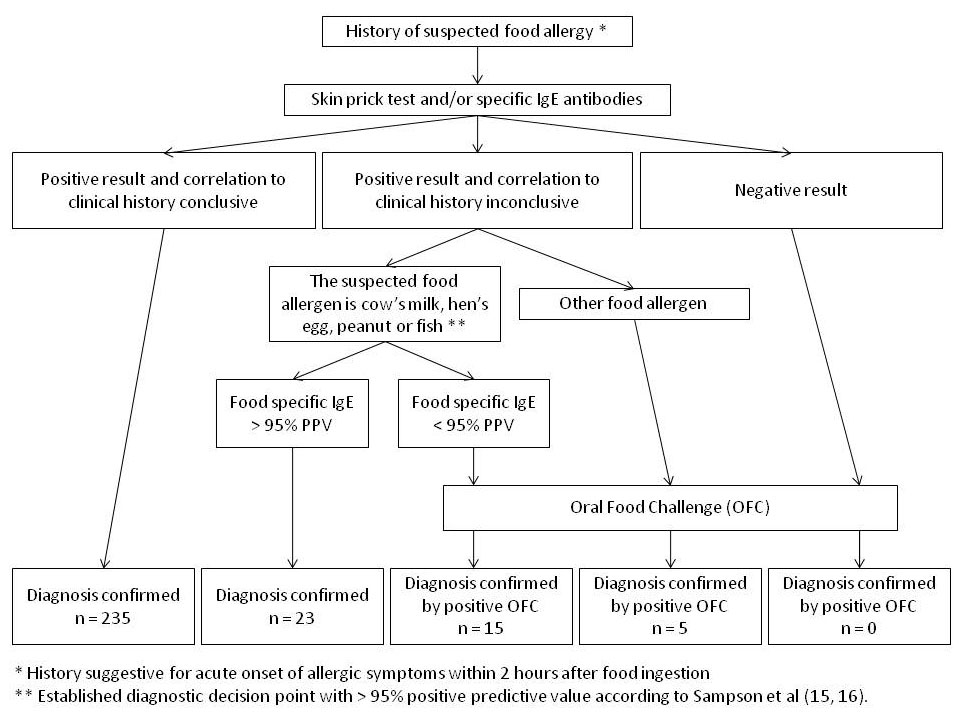
Figure 1
Flow chart for the diagnosis of a food allergy.
DOI: https://doi.org/10.4414/smw.2011.13269
Food allergies are most prevalent in children, particularly in infancy and pre-school age. A number of clinical signs are involved in food allergies ranging from mild cutaneous symptoms to life-threatening anaphylactic reactions. In young children, food allergies are usually acquired through contact of the allergen with the gastrointestinal mucosa. This can be regarded as a failure of oral tolerance induction. In adolescents and adults however, food allergies mainly develop as a consequence of sensitisation to inhalant allergens, for example birch pollen in Europe (pollen-food-related syndrome). The immunological basis of this phenomenon is IgE cross-reactivity due to a high homology in structure and amino acid sequence between certain foods and inhalant allergens [1, 2].
Although any food protein can induce an allergic reaction, just a relatively limited number of foods are responsible for the majority of food hypersensitivity reactions in children worldwide [3, 4]. The prevalence of food allergies and the distribution of allergens depend on nutritional habits as well as methods of food preparation in individual populations studied and on the introduction of new allergenic foods early in life. In the United States, there is a high prevalence of peanut allergy [5], whereas soy, cow’s milk and hen’s egg are the most frequent food allergens in Japan [6, 7], and in France, there are more allergies to mustard than in other countries [8]. In Portugal and Spain frequent food allergens are fish [9], in Italy seafood and cow’s milk [10], and in Scandinavia tree nuts [11]. In Singapore the bird’s nest allergy is the most common [12], whereas sesame seed allergy is frequent in Israel [13].
In this prospective study, we aimed to describe the most common foods that are responsible for allergic reactions in Swiss children of different age groups. Furthermore we investigated the clinical manifestations of IgE-mediated traditional type I food allergies in occurrence with sensitisation to food allergens through the gastrointestinal tract.
The current study was a prospective analysis of 151 patients (101 boys, 50 girls). Individuals included were consecutively referred from 2004 to 2006 for assessment of suspected immediate type allergic reactions to foods. Three age groups were defined: group 1 included infants from 0 to 12 months, group 2 included young children from 13 to 36 months and group 3 included children above 36 months to 16 years of age. The study was performed at the Allergy Units of the Children’s Hospitals of Aarau and Lucerne, Switzerland.
All patients included in the study had a persuasive history of immediate allergic reaction occurring within 2 hours after food ingestion. We did not include children with atopic dermatitis who either had no history of acute onset of allergic symptoms after food ingestion, or a history of delayed allergic reaction to the suspected food, regardless of whether or not they were sensitised to food protein in a skin prick test and/or ImmunoCAP. However, atopic dermatitis per se was not an exclusion criterion. We included children sensitised to food allergens and with evidence of IgE-mediated allergic reaction, such as exacerbation of atopic dermatitis with rash or severe itching, within 2 hours after food ingestion. Since this paper was centred on the evaluation of traditional type I food allergies, we excluded individuals with pollen associated oral allergy syndrome to fruit and vegetables (localised to the oral mucosa as oral irritation, tongue swelling and itching, palatal itching and lip tightness or swelling) occurring within a few minutes after eating fruit or vegetables concomitant to pollen sensitisation.
Diagnosis of a food allergy was based on a careful clinical history including possible causal foods, quantity ingested, time course of reaction and reaction consistency. Skin prick tests (SPT) with a panel of commercial extracts were performed in the majority of the cases, but were avoided in cases with severe atopic dermatitis or cases treated with antihistamines or steroids. In addition, prick-to-prick tests were carried out with the suspected allergen using native food. Positive results were confirmed with in vitrodetermination of specific IgE antibodies to food proteins. The diagnosis of food allergy was confirmed when the clinical history clearly correlated to test results. Oral food challenges (OFC) were performed in patients with inconclusive correlation between history and test results. A positive OFC confirmed a diagnosis of food allergy [14]. In children with suggestive IgE-mediated food allergies to cow’s milk, hen’s egg, peanut and fish, OFC were only performed if the food-specific IgE level was below the diagnostic decision point of 95% probability of symptomatic food allergy (95% positive predictive value, PPV) established by Sampson et al for these four food allergens [15, 16]. In case of suspected allergies to other food allergens without established diagnostic decision points of 95% PPV, OFC were performed regardless of the food-specific IgE level. OFC were not performed in children with a suggestive history of anaphylactic reaction linked to the food and in cases with sensitisation to peanut and nuts, when children were younger than 3 years of age. Figure 1 illustrates the diagnostic work-up of suspected food allergies in a flow chart.

Figure 1
Flow chart for the diagnosis of a food allergy.
Diagnosis of anaphylaxis was based on a history of food ingestion followed by a grade III or IV systemic allergic reaction affecting the respiratory tract and/or cardiovascular system according to the criteria of Müller [17], in addition to cutaneous symptoms, positive SPT and/or an increase in specific IgE antibodies to the suspected food allergen.
Patients were tested with a panel of commercial allergens (Soluprick SQ, ALK-Abello, Hoersholm, Denmark). This included cow’s milk, hen’s egg, hazelnut, peanut, soy, cod’s fish, wheat, grass pollen, birch pollen, cat dander and house dust mites. Other foods were used for skin prick tests, as appropriate. Native foods were used for prick-to-prick testing according to the child’s history. In prick tests, a drop of an allergen was placed on the skin over the flexor aspect of the forearm. A calibrated lancet was used to prick the skin through the drop. After 10 to 15 minutes the size of the observed wheal was compared with that induced by histamine. The child was regarded as being sensitised if the wheal size was more than 3 mm in diameter, compared to the negative normal saline control. The prick-to-prick technique was used for testing with native foods. Here, the lancet was passed through the native food and then pricked to the skin.
Specific IgE was measured using the ImmunoCAP specific IgE test (Phadia, Uppsala, Sweden). A child was regarded as being sensitised to a particular food allergen if the amount of specific IgE antibodies was more than 0.7 kU/I (CAP-class 2 and more) [3, 18].
The food to be tested was avoided for at least two weeks before OFC and antihistamines/steroids were withdrawn. A small amount of the food allergen was given to the patient. Approximately every 20 minutes, an increasing concentration of the food allergen was given. If a significant reaction occurred, including cutaneous symptoms (urticaria, angioedema), gastrointestinal symptoms (nausea, abdominal pain, vomiting, diarrhoea), respiratory symptoms (cough, wheezing, dyspnea), upper airway symptoms (rhinoconjunctivitis, laryngeal oedema, stridor) or cardiovascular symptoms (hypotonia, tachycardia), the challenge was aborted and the patient was considered allergic to the specific food allergen. If the patient could tolerate a full serving of the food allergen, the challenge was finished and the child was considered not allergic to that food. After the last dose of food, patients were observed for any reaction for two hours. If necessary, allergic reactions occurring during the challenge were treated with oral antihistamines and corticosteroids. Only symptoms that developed within a few minutes to two hours from the last dose of the food challenge were used for the diagnosis [19, 20].
A total of 278 IgE-mediated food allergies were diagnosed in 151 children according to the flow chart shown in figure 1. The majority of patients (68%) included in the study were evaluated by both SPT and ImmunoCAP, with 12% only evaluated by SPT and 20% only by ImmunoCAP. Most children (58.3%) presented with allergy to a single food, where 41.7% of individuals had multiple food hypersensitivities (table 1).
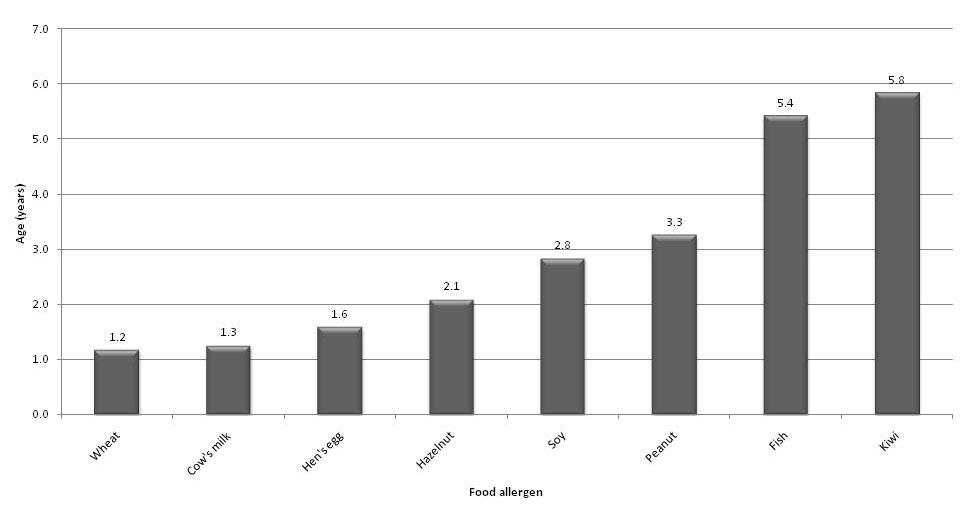
Figure 2
Median age (years) at diagnosis of IgE-mediated allergy to most common foods.
In the majority of the cases (n = 235, 85%), diagnosis was based on a clear correlation between history of immediate allergic reaction and positive SPT and/or increased specific IgE levels to the suspected food. In 20 cases (7%), diagnosis was confirmed by a positive OFC, because the correlation between history and test results was inconclusive. Furthermore, in 23 cases (8%) with questionable correlation between history and test results, the diagnosis of food allergy was based on the high specific IgE levels to either cow’s milk, hen’s egg, peanut or fish, where diagnostic decision points were established (>95% PPV) to predict symptomatic food allergy.
Overall, the most prevalent food allergen was hen’s egg, followed by cow’s milk, peanut, hazelnut, wheat, fish, kiwi and soy in descending order. These eight allergens accounted for 83.1% of food allergies in Swiss infants and children. Table 2a) summarises the top 8 food allergens in frequency of allergic reactions for the entire study population. In table 2b) a wide variety of other food allergens is described which accounted for the other 16.9% of allergic reactions.
There were differences however in the order of most common food allergens among the three individual age groups. In infancy (group 1), cow’s milk was number one, followed by hen’s egg and wheat. Interestingly, peanut and hazelnut are already common allergens triggering hypersensitivity reactions in the first 12 months of life. In the second and third year of life (group 2), cow’s milk is just second to hen’s egg, and other food allergens like fish and sesame seed newly appear in the list of top 8 food allergens. Beyond the age of 3 years (group 3), peanut is the most frequent food allergen, and fish rises to number 3 after hen’s egg (table 3).
There were significantly more boys (n = 101) than girls (n = 50) in the study population. Overall, the median age at diagnosis of food allergy was 1.9 years (mean age 3.0) with a range from 3 months to 15 years. In boys however, diagnosis of food allergy was made at a younger age (1.8 years) than in girls (2.4 years). Figure 2 shows the median age at diagnosis of allergy to individual foods. Allergic reactions to wheat, cow’s milk and hen’s egg occurred early in life. In the older age groups, these three allergens were less frequently implicated in food hypersensitivity reactions. Other allergens like peanut, hazelnut and fish accounted for an increasing relative number of allergic reactions to foods as children got older (fig. 3).
Skin symptoms like urticaria and angioedema were the most common clinical manifestations of food allergy in children of each age group. Gastrointestinal symptoms such as diarrhoea, vomiting and abdominal pain, and respiratory symptoms like cough, dyspnoea and wheezing, were other frequent symptoms in our study population (table 4). There were no significant differences in the pattern of clinical reactions neither between children of different age groups nor to individual allergens (data not shown).
Overall, anaphylaxis as a clinical manifestation of food allergy occurred in 13 (4.7%) of the 278 cases included in this study. Of the 13 anaphylactic reactions, 3 each occurred after ingestion of peanut and fish, 2 each after ingestion of cow’s milk, hen’s egg and wheat and 1 after ingestion of shrimps.
| Table 1: Single and multiple food hypersensitivity in 151 children with food allergy. | ||
| Number of patients | % | |
| One-food hypersensitivity | 88 | 58.3% |
| Two-food hypersensitivities | 28 | 18.5% |
| Three-food hypersensitivities | 20 | 13.2% |
| Four-food hypersensitivities | 11 | 7.3% |
| Five and more food hypersensitivities | 4 | 2.7% |
| Table 2: Frequency of food hypersensitivity to individual foods in 151 children with 278 IgE-mediated food allergies. | |||
| a) Most prevalent 8 food allergens. | |||
| Food allergen | Number of allergy diagnoses | % (of 278 food allergies) | % (of 151 patients) |
| Hen's egg | 66 | 23.7% | 43.7% |
| Cow's milk | 56 | 20.1% | 37.1% |
| Peanut | 39 | 14.0% | 25.8% |
| Hazelnut | 29 | 10.4% | 19.2% |
| Wheat | 17 | 6.1% | 11.3% |
| Fish | 12 | 4.3% | 8.0% |
| Kiwi | 6 | 2.2% | 4.0% |
| Soy | 6 | 2.2% | 4.0% |
| Total | 231 | 83.1% | |
| b) Various other food allergens. | |||
| Food allergen | Number of allergy diagnoses | % (of 278 food allergies) | % (of 151 patients) |
| Walnut | 5 | 1.8% | 3.3% |
| Pistachio nut | 4 | 1.4% | 2.6% |
| Kidney bean | 3 | 1.1% | 2.0% |
| Lentil | 3 | 1.1% | 2.0% |
| Potato | 3 | 1.1% | 2.0% |
| Sesame seed | 3 | 1.1% | 2.0% |
| Shrimp | 3 | 1.1% | 2.0% |
| Almond | 2 | 0.7% | 1.3% |
| Cashew nut | 2 | 0.7% | 1.3% |
| Celery | 2 | 0.7% | 1.3% |
| Coconut | 2 | 0.7% | 1.3% |
| Pea | 2 | 0.7% | 1.3% |
| Rice | 2 | 0.7% | 1.3% |
| Others * | 11 | 4.0% | 7.3% |
| Total | 47 | 16.9% | |
| * One case of allergy was reported for each of the following foods: curry, carrot, chickpea, pumpkin, mango, melon, para nut, pine seed, rye flour, tomato and courgette. | |||
| Table 3: Frequency of the eight most prevalent foods implicated in food allergies according to age. | ||||||||
| 0 to 12 months | 13 to 36 months | Above 36 months | ||||||
| Food allergen | Number of food allergy diagnoses | % (58 food allergies) | Food allergen | Number of food allergy diagnoses | % (122 food allergies) | Food allergen | Number of food allergy diagnoses | % (98 food allergies) |
| Cow’s milk | 22 | 37.9% | Hen’s egg | 34 | 27.9% | Peanut | 21 | 21.4% |
| Hen’s egg | 18 | 31.0% | Cow’s milk | 25 | 20.5% | Hen’s egg | 14 | 14.3% |
| Wheat | 6 | 10.3% | Hazelnut | 16 | 13.1% | Fish | 10 | 10.2% |
| Peanut | 5 | 8.6% | Peanut | 13 | 10.7% | Hazelnut | 10 | 10.2% |
| Hazelnut | 3 | 5.2% | Wheat | 8 | 6.6% | Cow’s milk | 9 | 9.2% |
| Potato | 2 | 3.4% | Sesame seed | 3 | 2.5% | Kiwi | 4 | 4.1% |
| Kiwi | 1 | 1.8% | Soy | 3 | 2.5% | Walnut | 4 | 4.1% |
| Courgette | 1 | 1.8% | Fish | 2 | 1.6% | Soy | 3 | 3.1% |
| Total | 58 | 100.0% | Total | 104 | 85.4% | Total | 75 | 76.6% |
| Table 4:Clinical manifestation (%) of the 278 IgE-mediated food allergies according to the subject’s age. | ||||
| Symptoms | 0 to 12 months (58 food allergies) | 13 to 36 months (122 food allergies) | Above 36 months (98 food allergies) | Overall (278 food allergies) |
| Urticaria | 70.7% | 67.2% | 43.9% | 59.0% (n = 164) |
| Angioedema | 22.4% | 28.7% | 36.7% | 30.2%(n = 84) |
| Gastrointestinal symptoms | 20.7% | 23.8% | 35.7% | 25.9% (n = 72) |
| Respiratory distress | 10.3% | 34.4% | 21.4% | 25.2% (n = 70) |
| Perioral erythema | 0.0% | 11.5% | 15.3% | 11.2% (n = 31) |
| Laryngospasm | 0.0% | 8.2% | 6.1% | 5.4% (n = 15) |
| Anaphylaxis | 10.3% | 0.8% | 6.1% | 4.7% (n = 13) |
| Rhinoconjunctivitis | 0.0% | 5.7% | 1.0% | 2.5% (n = 7) |
This study was a prospective analysis of 278 IgE-mediated food allergies in children of three different age groups. The study focused on traditional type I food allergies in occurrence with IgE-mediated sensitisation to food allergens through the gastrointestinal tract. We excluded individuals with only sensitisation (positive SPT and/or increased food-specific IgE levels) and without evidence of clinical relevance, as assessed by a conclusive history or positive OFC.
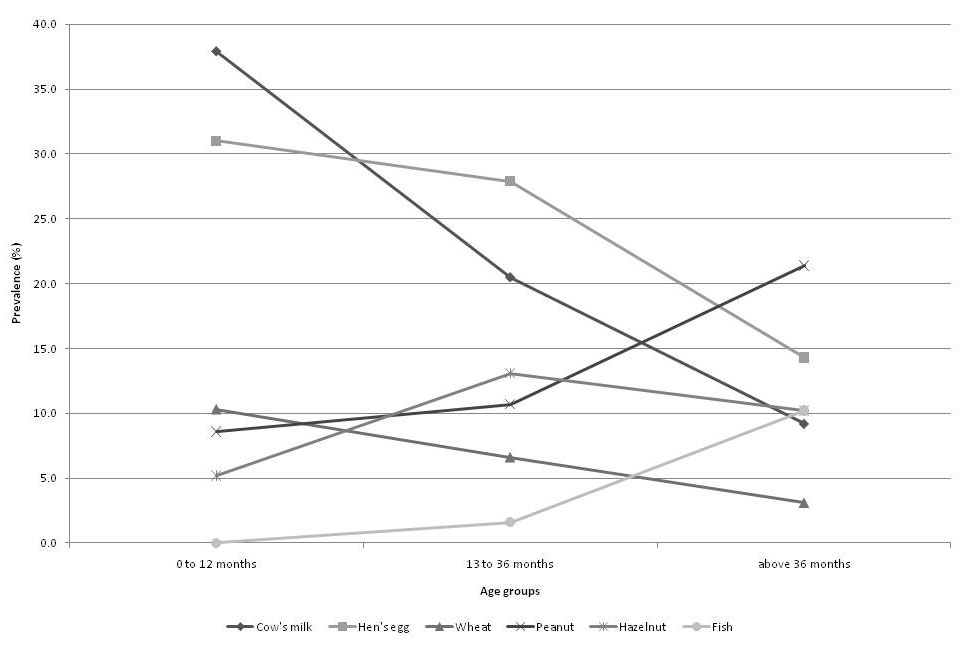
Figure 3
Course of relative prevalence (%) of the predominant allergens implicated in food allergies according to age.
Overall, eight allergens accounted for 83.1% of 278 confirmed food allergies in Swiss infants and children. In each age group however, there was an individual order of the top eight food allergens causing IgE-mediated hypersensitivity reactions. Our data demonstrates that food allergies in young children represent a dynamic process: the frequency of most allergenic food proteins inducing hypersensitivity reactions changes in relation to the children’s age.
A number of studies has shown that the distribution of allergens involved in food allergies in children vary from country to country according to individual dietary practices [5–13]. In Mediterranean countries, fish and seafood are already common allergens in very young subjects [9, 10]. In the United States a peanut allergy is frequent [5], and in France there are more allergies to mustard than in other countries [8]. A recurrent finding of these papers was the limited number of allergens accounting for the majority of food allergies.This needs to be considered when assessing children with suspected food allergies. Therefore, individual panels of food allergens should be set up for skin testing in children according to the subject’s age and nationality.
In our patients, the diagnosis of food allergy was confirmed at a median age of 1.9 years. This is in accordance with epidemiological studies reporting the highest prevalence of food allergies in children less than 3 years of age [1, 2]. Allergies to cow’s milk, hen’s egg and wheat in our study were most frequent in infants. As demonstrated in fig. 3), the prevalence of these three food allergens decreases in older children, most rapidly for cow’s milk allergy. This can be explained by development of tolerance to these common food allergens in a majority of children by the age of 3 to 5 years [1, 2]. However, other allergens like peanut, tree nuts and fish are becoming more frequent triggers of hypersensitivity reactions in older children due to their introduction later in the child’s life (fig. 3). Furthermore, development of clinical tolerance to these food allergens is rare [21, 22].
In contrast to studies from other countries [5–13], our data shows that wheat is a frequent food allergen in Swiss infants and children below the age of 3 years. Early introduction of wheat-containing cereals is a common dietary practice in Switzerland. In addition to wheat, peanut and hazelnut are other common food allergens in Swiss infants. It can be hypothesised that this might be caused by early introduction of cereals, as well as sweets and spreads containing hazelnut and/or peanut. In addition, the use of peanut oil in medications such as creams or vitamin preparations [23] may be a risk factor for early sensitisation to peanut and subsequent allergy development after ingestion of peanut containing foods such as cereals or peanut butter.
There was a significant gender difference with a 2:1 male predominance in our analysis of 151 affected children. Furthermore, the median onset of food allergies was earlier in boys (1.8 years) than in girls (2.4 years). A number of studies have shown that male sex is a risk factor for early sensitisation to food and aeroallergens [24], for hypersensitivity reactions to foods [8, 9], as well as for food induced anaphylaxis in childhood [25, 26]. The reason for male predominance in early sensitisation and hypersensitivity may be explained by sex-specific differences in immune response profiles in early childhood [27]. Other studies demonstrate that after puberty however, female patients outnumber male subjects in the prevalence of IgE-mediated food allergies and in hospital admissions due to food-induced anaphylaxis [26, 28].
Our analysis of 278 food allergies did not show any significant difference in the pattern of clinical reactions to individual foods. Skin symptoms were the most frequent clinical hypersensitivity reaction in each age group and to all individual food allergens. There were 13 cases (4.7%) of anaphylaxis, and 6 of them (46%) occurred in infants below the age of 12 months. Food-induced anaphylaxis can occur very early in young children as described in other studies [26, 28]. This can be seen as a consequence of a high prevalence of food allergies in the first years of life. In their survey of anaphylaxis, Liew et al. describe an increase in the rate of hospital admissions among children and adults due to food-induced anaphylaxis in the past 11 years. The observed increase was highest in young children between 0–4 years of age and with peanut as a causative allergen [28]. This data demonstrates the importance of early diagnosis, as well as the availability and use of adrenaline as an emergency medication even in the very young age group.
A limitation of the present paper is that the children we studied may not have been completely representative of the local population since patients were referred to our Allergy Unit from paediatricians and general practitioners for evaluation of suspected food allergy. Furthermore, a significant number of children were directly recruited through the hospitals’ emergency departments. Therefore, it can be hypothesised that the children included in this study represent a selected group of infants and young children with predominantly more severe food allergies. Furthermore it has to be emphasised that this study was not designed to define the prevalence of food allergies in Swiss children. The scope of this paper was to investigate the clinical manifestation and distribution of allergens most frequently involved in confirmed type I food allergies.
In summary, IgE-mediated food hypersensitivity presents with a wide spectrum of clinical reactions. Acute urticaria and angioedema are the most frequent symptoms of IgE-mediated food allergies in all paediatric age groups. Life-threatening allergic reactions however may occur even in the very young age group. This study found that eight allergens account for 83% of IgE-mediated food allergies in Swiss infants and children. The hierarchy of most frequently involved allergens changes in relation to an individual’s age, with cow’s milk being number one in the first 12 months of life, hen’s egg in the second and third year, and peanut in children above the age of 3 years.
1 Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125(Suppl. 2):S116–25.
2 Wang J, Sampson HA. Food allergy: recent advances in pathophysiology and treatment. Allergy Asthma Immunol Res. 2009;1(1):19–29.
3 Rancé F, Kanny G, Dutau G, Moneret-Vautrin DA. Food hypersensitivity in children: clinical aspects and distribution of allergens. Pediatr Allergy Immunol. 1999;10(1):33–8.
4 Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120(3):638–46.
5 Sicherer SH, Muñoz-Furlong A, Burks AW, Sampson HA. Prevalence of peanut and tree nut allergy in the US determined by a random digit dial telephone survey. J Allergy Clin Immunol. 1999;103(4):559–62.
6 Shibasaki M, Suzuki S, Tajima S, Nemoto H, Kurume T. Allergenicity of major components of soybeans. Int Arch Allergy Appl Immunol. 1980;61(4):441–8.
7 Imamura T, Kanagawa Y, Ebisawa M. A survey of patients with self-reported severe food allergies in Japan. Pediatr Allergy Immunol. 2008;19(3):270–4.
8 Rancé F, Dutau G, Abbal M. Mustard allergy in children. Allergy. 2000;55(5):496–500.
9 Crespo JF, Pascual C, Burks AW, Helm RM, Esteban MM. Frequency of food allergy in a pediatric population from Spain. Pediatr Allergy Immunol. 1995;6(1):39–43.
10 Novembre E, Cianferoni A, Bernardini R, Mugnaini L, Caffarelli C, Cavagni G, et al. Anaphylaxis in children: clinical and allergologic features. Pediatrics. 1998;101(4):E8.
11 Eriksson NE, Möller C, Werner S, Magnusson J, Bengtsson U, Zolubas M. Self-reported food hypersensitivity in Sweden, Denmark, Estonia, Lithuania, and Russia. J Investig Allergol Clin Immunol. 2004;14(1):70–9.
12 Shek LP, Lee BW. Food allergy in children – the Singapore story. Asian Pac J Allergy Immunol. 1999;17(3):203–6.
13 Dalal I, Binson I, Reifen R, Amitai Z, Shohat T, Rahmani S, et al. Food allergy is a matter of geography after all: sesame as a major cause of severe IgE-mediated food allergic reactions among infants and young children in Israel. Allergy. 2002;57(4):362–5.
14 NIAID-Sponsored Expert Panel, Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(Suppl. 6):S1–58.
15 Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107:891–6.
16 Sampson HA. Improving in-vitro tests for the diagnosis of food hypersensitivity. Curr Opin Allergy Clin Immunol. 2002;2(3):257–61.
17 Mueller HL. Diagnosis and treatment of insect sensitivity. J Asthma Res. 1966;3(4):331–8.
18 Eigenmann PA, Sicherer SH, Borkowski TA, Cohen BA, Sampson HA. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998;101(3):
19 Bindslev-Jensen C, Ballmer-Weber BK, Bengtsson U, Blanco C, Ebner C, Hourihane J, et al. Standardization of food challenges in patients with immediate reactions to foods – position paper from the European Academy of Allergology and Clinical Immunology. Allergy. 2004;59(7):690–7.
20 Niggemann B, Beyer K. Diagnosis of food allergy in children: toward a standardization of food challenge. J Pediatr Gastroenterol Nutr. 2007;45(4):399–404.
21 Hourihane JO, Roberts SA, Warner JO. Resolution of peanut allergy: case-control study. BMJ. 1998;316:1271–5.
22 Skolnick HS, Conover-Walker MK, Koerner CB, Sampson HA, Burks W, Wood RA. The natural history of peanut allergy. J Allergy Clin Immunol. 2001;107 (2):367–74.
23 Lack G, Fox D, Northstone K, Golding J. Factor associated with the development of peanut allergy in childhood. N Engl J Med. 2003;348(11):977–85.
24 Dean T, Venter C, Pereira B, Arshad SH, Grundy J, Clayton CB, et al. Patterns of sensitization to food and aeroallergens in the first 3 years of life. J Allergy Clin Immunol. 2007;120(5):1166–71.
25 Mehl A, Wahn U, Niggemann B. Anaphylactic reactions in children – a questionnaire-based survey in Germany. Allergy. 2005;60(11):1440–5.
26 Hompes S, Beyer K, Köhli A, Nemat K, Scherer K, Lange L, et al. Anaphylaxie im Kindes und Jugendalter – Symptome, Auslöser und Therapie. Kinder und Jugendmedizin. 2009;9:393–9.
27 Uekert SJ, Akan G, Evans MD, Li Z, Roberg K, Tisler C, et al. Sex-related differences in immune development and the expression of atopy in early childhood. J Allergy Clin Immunol. 2006;118(6):1375–81.
28 Liew WK, Williamson E, Tang ML. Anaphylaxis fatalities and admissions in Australia. J Allergy Clin Immunol. 2009;123(2):434–42.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article were reported.